Baroceptors, baroreceptors, mechanoreceptors or even mechanoceptors are receptors responsible for the contraction and dilation of blood vessels.1 They are located mainly in the carotid sinus2 and aortic arch3,4 and have the function of detecting variations in blood pressure and transmitting this information to the central nervous system. This information generates responses from the autonomic nervous system, modulating the functioning of the blood circulation (Baroreceptor reflex).5
The more the baroceptors are activated, the more functional oxygen is transported to the lungs and the brain.6 This functioning with active and constant movement of these mechanoceptors also seems to indicate that there is a good flow in the blood displacement at the level of blood microcirculation, also called hemodynamics.7
Adequate hemodynamics depends on the state of cardiovascular health. According to Houston (2014), the blood vessel has a finite number of responses to an infinite number of insults.8 Finite vascular responses are oxidative stress (Reactive Oxygen Species and Reactive Nitrogen Species),9 inflammation10 and exaggerated immune reaction of the organism11 and are related to the pathophysiology of vascular disease, which lead to an abnormal vascular biology, such as endothelial dysfunction (ED) and vascular smooth muscle dysfunction (VSMD).9
In this infinite estimate of insults, there is hypertension, dyslipidemia,12 diabetes,13 hyperglycemia,14 obesity,15 smoking,16 insulin resistance,17 increased homocysteine,18 among others, which enable vascular responses with cytokines,19 chemokines, 20 adhesion molecules,21 heat shock proteins22 and antibodies,23 leading to inflammation,24 oxidative stress25 and autoimmune dysfunction.26
Atherosclerosis and vascular disease are postprandial phenomena, that is, they arise after eating allergenic and inflammatory foods,27 associated with hyperglycemia and hypertriglyceridemia, with endothelial dysfunction being the earliest stage of vascular disease, which can be detected through the baroreflex index, hemodynamics27 and biomarkers already studied in the literature, linked to cardiovascular diseases.28
In this sense, this study aimed to report the values of the baroreflex and hemodynamic index related to biomarkers that signal obstruction of the blood microcirculation found in the 57-year-old male patient, who died after being affected by COVID-19.
MethodsAssessments were performed by functional neurometry (FN) and laboratory tests. The evaluation with functional neurometry was performed in an air-conditioned room at a temperature of 22±2°C. The capacity, functionality and elasticity of the blood vessels are evaluated indirectly in 3 (three) positions. Thus, the FN was performed in 3 positions: dorsal decubitus, standing up and orthostatic, called DSO analysis by the author, organizer and creator of the method, Nelson Alves Pereira Júnior.27 The sympathetic autonomic nervous system represents the vasoconstrictions measured at the frequencies of 0.01–0.04Hz and from 0.04 to 0.20Hz and the parasympathetic represents the vasodilatations measured at the frequencies of 0.20–0.50Hz.27
The five categories of this evaluation protocol of DSO analysis were named by the author as: anxiety control; physiological response; baroreflex index; hemodynamics (blood flow velocity); and brain neurometry. Detailed descriptions of each category, which captures biological signals by sensors calibrated in Series and/or Fourier Transform algorithms, have been published in the Journal of Psychology and Psychotherapy Research (DOI: https://doi.org/10.12974/2313-1047.2020.07.1).27
In this case report, the categories were approached briefly with an emphasis only on the expected values to facilitate the reader's understanding of the interpretation of the results. In Functional Neurometry, anxiety control is measured through the galvanic skin response.29 Thus, the electrodermal resistance is indirectly measured by means of the sweat gland.30 In this context, as sweat on the skin allows greater electrical conduction,31 it can be estimated that with a greater amount of sweat there seems to be less energy stored in the liver (glycogen) for a person's adrenal to enable the fight and/or flight process or for that adrenal to control anxiety through the hormones aldosterone, cortisol, adrenaline and dehydroepiandrosterone.27
The anxiety control category is calculated using a scale with agreed values from 0 to 100%, in which the level of energy provided by the functional reserve already absorbed by the enterocytes and already stored in the liver can be measured indirectly,32 awaiting the need to pass into the bloodstream by glycogenolysis33 to make the fight and/or flight process feasible.34 The minimum expected in this category is 75%.
The physiological response is measured by varying the peripheral temperature,35 demonstrating whether or not there is adequate functionality of blood vessel elasticity.36 This elasticity refers to an expected variation in vasoconstriction and vasodilation,36 legitimizing the sympathetic (fight and flight) and parasympathetic (relaxation).37
The signal origin is made through the peripheral temperature sensor, which scales the unit of measurement in degrees Celsius. The originated values generate 2 more important results, as a percentage of variability of the sympathetic and parasympathetic systems and the temperature values in the thermoregulation. Both are expressed on a scale ranging from 0 to 100 (%) or (°C). The location of this sensor is in the proximal phalanx of the ring finger.38 The expected values for an ideal thermoregulation are between 31.5°C and 32.5°C.35
Baroreflex behavior is the ability of blood vessels to contract and dilate, which allows the neuroscientist to observe the level of functional oxygen that will better signal red blood cell displacement,39 in the process of transporting oxygen within the blood vessels. In functional neurometry, the balance of these contractions and dilations (baroreflex index) must present a percentage above 90%.40
Some studies have been warning about the importance of this measure of the low baroreflex index found in patients with obstructive sleep apnea,41 with slight respiratory changes42 asymptomatic or imperceptible, but which gradually cause mitochondrial suffering.43 Other studies also demonstrate a reduction in the baroreflex index associated with anemia, inflammation,27 to a lack of vitamin C,44,45 folic acid46 and sedentary lifestyle46 and may contribute to the manifestation of possible negative effects in the prevention of atherosclerosis.
Hemodynamics47 may be directly related to endothelial dysfunction and, consequently, to the risk of cardiovascular disease.48 In functional neurometry, the ideal hemodynamics should be below 10%. When above this percentage, it indicates a reduction in blood flow speed caused by peripheral vascular resistance, cardiac output and/or inflammatory response.27
Case reportA fifty-seven-year-old male patient, assessed on February 10, 2020 at the Brain Institute of Pernambuco – Instituto do Cérebro de Pernambuco (ICerPE). Before the assessment by functional neurometry, the following measurements were taken: weight [100kg], height [1m 78cm], waist circumference [117cm] and body mass index [31.6], fulfilling the classification criteria of mild obesity, with 23.3kg overweight. The patient reported that he did not frequently do physical activity (sedentary). The patient went to ICerPE, taken by his daughter, with the objective of undergoing a complete assessment with functional neurometry and quantitative electroencephalography, after learning that there was treatment for mood disorder and generalized anxiety disorder.
When the evaluation with functional neurometry ended, the ICerPE partner physician and researcher asked him to perform a blood test of the following biomarkers: blood count: [red blood cells (RBCs); hemoglobin (Hb); hematocrit (Hct); mean corpuscular volume (MCV); mean corpuscular hemoglobin (MCH); mean corpuscular hemoglobin concentration (MCHC); total leukocytes; red cell distribution width (RDW)]; serum ferritin (SF); transferrin saturation (TS); vitamin B12; homocysteine; C-reactive protein (CRP); fibrinogen; 25-hydroxy-vitamin-D3 (D3); apolipoprotein A-1 (Apo-A1); apolipoprotein B (Apo-B); glutamic oxalacetic transaminase (GOT); glutamic-pyruvic transaminase (GPT); gamma-glutamyl transpeptidase (Gamma GT); total cholesterol (TC); triglycerides (TG), and; high-density lipoprotein (HDL) and low-density lipoprotein (LDL).
After this stage, the patient went on to confinement due to the COVID-19 pandemic. However, on April 23, 2020, the patient's daughter sent us a message, saying that her father had not respected the confinement recommendations and that, unfortunately, he had died on April 18, 2020.
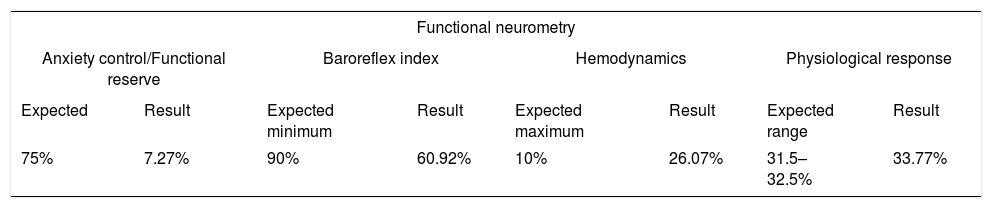
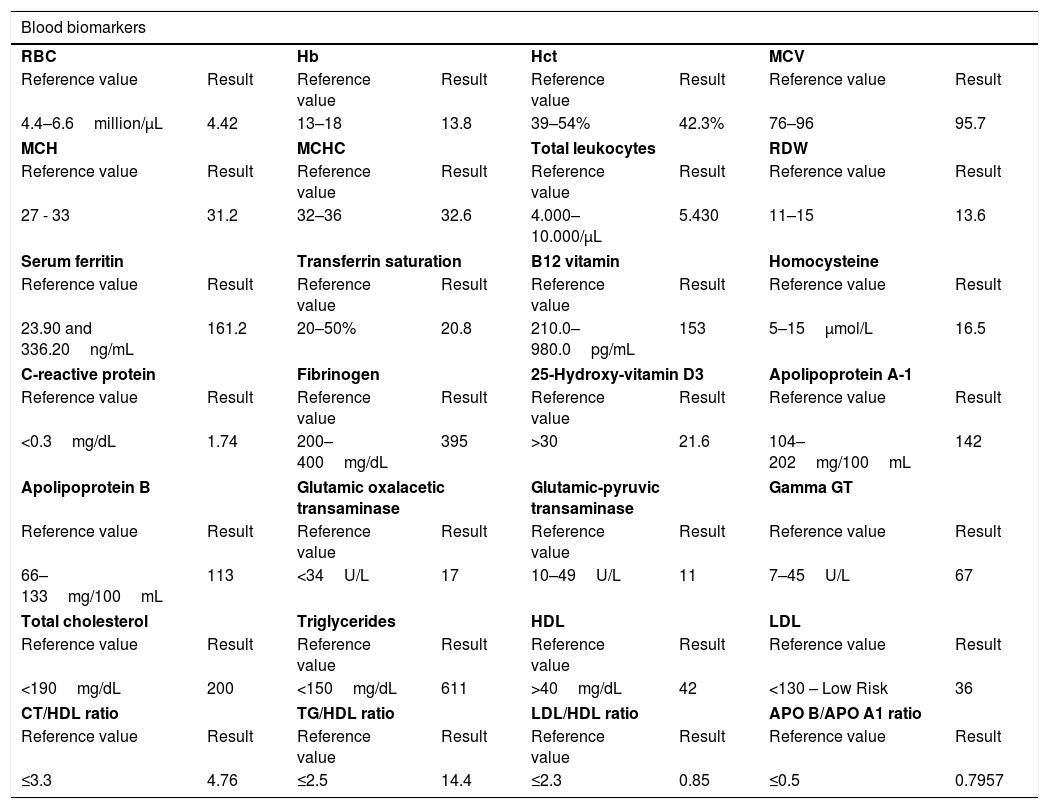
Results of the assessment by functional neurometry and biomarkersThe result of the patient's anxiety control assessed indirectly by the functional reserve, in percentage, was 7.27% [expected value: 75%] (Table 1). This result is usually compatible with exhaustion of the adrenal gland and/or fatty liver. In a semiological analysis, as the patient was 23.3kg overweight and with an abdominal circumference of 117cm, diagnostic compatibility increased in probability. Even though, some thin patients also have hepatic steatosis. The gamma GT level found was 67 [Reference value: 7–45U/L] (Table 2). The high level of gamma GT, exceeding the laboratory's maximum reference value, is related to insulin resistance, preventing diabetes, and can also signal toxic and/or free radical intoxication, in addition to indicating an affected liver.
Synthesis of the results of the functional neurometry exam of the male patient who died after being affected by COVID-19.
| Functional neurometry | |||||||
|---|---|---|---|---|---|---|---|
| Anxiety control/Functional reserve | Baroreflex index | Hemodynamics | Physiological response | ||||
| Expected | Result | Expected minimum | Result | Expected maximum | Result | Expected range | Result |
| 75% | 7.27% | 90% | 60.92% | 10% | 26.07% | 31.5–32.5% | 33.77% |
Synthesis of the results of the biomarkers of the male patient who died after being affected by COVID-19.
| Blood biomarkers | |||||||
|---|---|---|---|---|---|---|---|
| RBC | Hb | Hct | MCV | ||||
| Reference value | Result | Reference value | Result | Reference value | Result | Reference value | Result |
| 4.4–6.6million/μL | 4.42 | 13–18 | 13.8 | 39–54% | 42.3% | 76–96 | 95.7 |
| MCH | MCHC | Total leukocytes | RDW | ||||
| Reference value | Result | Reference value | Result | Reference value | Result | Reference value | Result |
| 27 - 33 | 31.2 | 32–36 | 32.6 | 4.000–10.000/μL | 5.430 | 11–15 | 13.6 |
| Serum ferritin | Transferrin saturation | B12 vitamin | Homocysteine | ||||
| Reference value | Result | Reference value | Result | Reference value | Result | Reference value | Result |
| 23.90 and 336.20ng/mL | 161.2 | 20–50% | 20.8 | 210.0–980.0pg/mL | 153 | 5–15μmol/L | 16.5 |
| C-reactive protein | Fibrinogen | 25-Hydroxy-vitamin D3 | Apolipoprotein A-1 | ||||
| Reference value | Result | Reference value | Result | Reference value | Result | Reference value | Result |
| <0.3mg/dL | 1.74 | 200–400mg/dL | 395 | >30 | 21.6 | 104–202mg/100mL | 142 |
| Apolipoprotein B | Glutamic oxalacetic transaminase | Glutamic-pyruvic transaminase | Gamma GT | ||||
| Reference value | Result | Reference value | Result | Reference value | Result | Reference value | Result |
| 66–133mg/100mL | 113 | <34U/L | 17 | 10–49U/L | 11 | 7–45U/L | 67 |
| Total cholesterol | Triglycerides | HDL | LDL | ||||
| Reference value | Result | Reference value | Result | Reference value | Result | Reference value | Result |
| <190mg/dL | 200 | <150mg/dL | 611 | >40mg/dL | 42 | <130 – Low Risk | 36 |
| CT/HDL ratio | TG/HDL ratio | LDL/HDL ratio | APO B/APO A1 ratio | ||||
| Reference value | Result | Reference value | Result | Reference value | Result | Reference value | Result |
| ≤3.3 | 4.76 | ≤2.5 | 14.4 | ≤2.3 | 0.85 | ≤0.5 | 0.7957 |
The gamma-GT catalyzes a transpeptidation reaction that is involved in the glutathione metabolism. Glutathione is abundant in the epithelial fluid of the lung lining. However, little is known about gamma-GT expression in pulmonary alveoli epithelial cells. There is, however, a relevant study49 showing that the pulmonary alveolar epithelial cell type 2 expresses the gene for gamma-GT. The gamma-GT expression in the type 2 pulmonary alveolar epithelial cell is via mRNA III, a transcript that was initially cloned from the liver. This cell synthesizes the gamma-GT protein and releases enzyme activity in a pool associated with surfactant in the pulmonary alveolus.49 The results of this study49 suggest that the surfactant may play an expanded role in the biology of lung cells as a vehicle for the redistribution of proteins anchored in amphipathic signals across the gas exchange surface of the lung.
The gamma GT transports the glutathione that is outside the cell into the cell and thus keeps the levels of reactive oxygen species low. Some epidemiological studies50,51 have shown that gamma GT levels, within laboratory reference values, closest to the highest reference value, are associated with cardiovascular risks, with predictors of future cardiovascular diseases, hypertension, stroke and type II diabetes mellitus.
There is still much to be researched and studied to better understand the relationship between the liver and the lung during deaths caused by COVID-19. However, lung injuries have been considered as the main damage caused by SARS-CoV-2 infection and there are already findings reporting that liver damage occurred during the course of the disease in severe cases.52
Regarding the baroreflex index, the patient presented 60.92% [expected value: >90%] (Table 1) and hemodynamics of 26.07% [expected value: <10%] (Table 1), values found in patients with endothelial dysfunction usually in outpatient consultations. The endothelial dysfunction in the case of the patient in this study seems to have been confirmed by the level of his gamma GT, reported in the above-mentioned paragraphs, of 67 [reference value: 7–45U/L] (Table 2). There is an epidemiological study in the literature that corroborates this interpretation. This study was carried out with 4.266 participants, demonstrating the association of gamma GT with C-reactive protein in men and women and, mainly, with the stiffening of the brachial artery in men.51
The endothelial dysfunction in this case report can still be identified by several biomarkers, among which are: low 25-hydroxy-vitamin D3 of 21.6 [reference value: >30] (Table 2), high CRP of 1.74 [reference value: <0.3mg/dL] (Table 2) and the physiological response of 33.77% [reference value: 31.5–32.5] (Table 2). The digestive overload gives meaning to the low absorption of vitamin B12, found to measure 153 in the patient's blood test [reference value: 210–980pg/mL] (Table 2), The decrease in vitamin B12 also causes an increase in homocysteine 16.5 [reference value: 5–15μmol/L] (Table 2), which further increases the probability of endothelial dysfunction.
The patient in this study furthermore had a low 25-hydroxy-vitamin D3 and high C-reactive protein. However, an important finding in the literature53 analyzed the relationship of vitamin D3 and C-Reactive protein with asymptomatic individuals supplemented with 21ng/mL vitamin D3, and found no significant reduction specifically in C-reactive protein.
However, in different contexts from the previous study, another very relevant study54 demonstrated that patients supplemented with vitamin D3 of 10ng/mL for 3 months showed improvement in endothelial function, reduction of oxidative stress and improvement in insulin sensitivity. This finding may encourage the scientific community to improve respiratory microcirculation, as obstruction and vascular resistance have been a major concern for scientists during the COVID-19 pandemic.55
And to further encourage researchers in relation to the benefits of vitamin D, an epidemiological study by Melamed et al. (2008) found that low levels of vitamin D3 increase the risk of mortality. This study used NHANES III data, collected from 1988 to 1994, studying 13,331 adult individuals of both genders with an average follow-up of 8.8 years. The result was 1,806 deaths, 43% from cardiovascular disease, 26% from cancer, 6% from infectious diseases and 5% from external causes. It was found that people with serum vitamin 25-OH-vitamin-D3 levels between 40-49ng/mL had a lower mortality rate.56
The indication of digestive overload found in the patient in this study using the functional neurometry27 may be related to parasites, but it may also have been due to sedentary habits, which seems to explain his low vitamin B12 as well. This result seems to reinforce the hypotheses about the obstructions of the respiratory microcirculation.55
Kwok et al. (2012)57 conducted a study using vitamin B12 in 50 vegetarian subjects who had serum B12<150pmol/L (<202pg/mL) and the results showed a significant serum increase in B12, a decrease in homocysteine and a significant improvement in dilation mediated by brachial flow from 6.3±1.8% to 7.4±1.7%. The conclusion of this study was that B12 supplementation improved the arterial function of vegetarians with subnormal B12 levels, thus proposing a new strategy for the prevention of atherosclerosis.
In this context of importance of vitamin B12, another study58 recently published reinforces the importance of vitamin B12 in the treatment of COVID-19, the authors point out that the virus of the Coronaviridae family (COVID-19) has a single-stranded positive RNA genome. This genome encodes the nsp12 protein, which hosts the RNA polymerase-dependent RNA (RdRP) activity responsible for replicating the viral genome.
Thus, the authors58 report that a nsp12 homology model was prepared using the SARS nsp12 (6NUR) framework and that it was used to screen silica to identify molecules among FDA-approved natural products or drugs that could potentially inhibit nsp12 activity. However, this experiment showed that vitamin B12 (methylcobalamin) can bind to the active site as an inhibitor of the nsp12 protein.58
Regarding total cholesterol (TC), triglycerides (TG), HDL and LDL, this study highlighted the results of TG and HDL of the patient who died after acquiring COVID-19. In addition to the TG being well above 611mg/dL (Table 2) of the expected reference value [<150mg/dL], there are findings in the literature demonstrating ideal values expected from CT/HDL ratios ≤3.3, TG/HDL ≤2.5 and LDL/HDL ≤2.3,59 called atherogenicity indexes, functioning as a safe predictor of high lipoprotein density with the risk of coronary disease. Data from the Framingham study show that each 1mg/dL increase in HDL decreases the coronary disease mortality rate by 2% for men and 3% for women.59,60
However, Tian and Fu (2010)59 establish that the greater the result of the relationship between CT/HDL and TG/HDL, the less will be the protective effect of HDL, because it will indicate a greater quantity of small HDLs (HDL Pre 1) and lesser quantities of large HDLs (HDL2b) and add that when these relationships worsen, not only the HDL amount worsens, but the type of HDL as well.59
The HDL is responsible for much more than transporting cholesterol from arteries and peripheral tissues to the liver; it has antioxidant action, anti-inflammatory action, pro-fibrinolytic action, antithrombotic action, and even reverse cholesterol transport.61 Thus, when the relationships based on the above-mentioned study were replicated in this case report, the following results were found: CT/HDL=4.76, TG/HDL=14.4 and LDL/HDL=0.85, indicating that the patient in this case report had a high level of atherogenicity.
It also seems relevant that, if there is a decision to initiate early care with the population to improve the microvascular state in order to have better conditions to face the COVID-19 virus, it will be necessary to better understand other relationships, such as apolipoprotein A1 (APO-A1) and apolipoprotein B (APO-B).
O’Keefe et al. (2008) measured APO-A1 and saw that APO-A1 does not become a risk factor when in high concentrations.62 A large HDL can be an atherogenic HDL because it can donate cholesterol in peripheral tissues through the scavenger receptor class B1 (SR-B1), unless the APO-A1 is also large.62
Thus, it also seems relevant to investigate the relationship between Apo B and Apo A1. In this case report, when the studied patient's APO B [113mg/100mL] was divided by his APO A1 [142mg/100mL], a result of 0.7957 was found. However, a study by Tian and Fu (2010)59 showed that people with an APO B/APO A1 ratio with a value equal to or less than 0.5 die less from cardiovascular disease.
ConclusionThe assessment of the baroreflex and hemodynamic index by functional neurometry by health professionals can anticipate the identification of people who will need to have a blood test to investigate the biomarkers that indicate subclinical inflammation, hepatic steatosis and endothelial dysfunction.
This study found subclinical inflammation and endothelial dysfunction. However, as this study is only a case report, it is suggested to conduct a clinical study with several patients to ensure statistical significance.
Conflicts of interestThe authors declare no conflicts of interest.