The guidelines project is a joint initiative of the Associação Médica Brasileira and the Conselho Federal de Medicina. It aims to bring together information in medicine to standardize conduct in order to help decision-making during treatment. The data contained in this article were prepared by and are recommended by the Associação Brasileira de Hematologia, Hemoterapia e Terapia Celular (ABHH). Even so, all possible medical approaches should be evaluated by the physician responsible for treatment depending on the patient's characteristics and clinical status.
This article presents the guidelines on immune thrombocytopenia in adults.
Description of the method used to gather evidenceThese guidelines are the result of a systematic evidence-based review centered on the Evidence-Based Medicine movement, where clinical experience is integrated with the ability to critically analyze and apply scientific information rationally, thereby improving the quality of medical care.
Initially articles were selected by title, sequentially by summary, and finally by their full text. All works retrieved in the primary and secondary databases were evaluated according to inclusion and exclusion criteria, by study design, PICO and submitted to critical evaluation and the extraction of results related to the outcomes. This evaluation included studies available in Portuguese, English or Spanish.
Studies were included according to the PICO components, language, full text availability and study design. The study designs included varied according to the clinical question, with the greatest strength of the available evidence for each question always being selected.
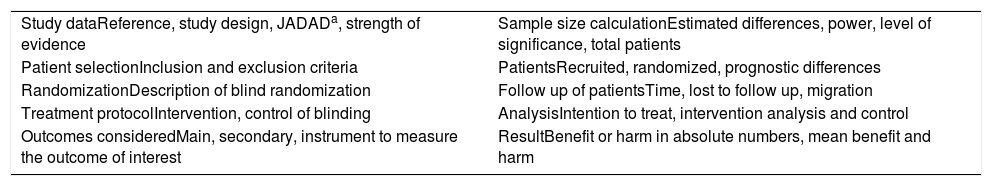
If the evidence selected in the search was defined as a randomized controlled clinical trial (RCT), it was submitted to an appropriate critical assessment checklist (Table 1).
Critical outline of randomized controlled trials (checklist).
| Study dataReference, study design, JADADa, strength of evidence | Sample size calculationEstimated differences, power, level of significance, total patients |
| Patient selectionInclusion and exclusion criteria | PatientsRecruited, randomized, prognostic differences |
| RandomizationDescription of blind randomization | Follow up of patientsTime, lost to follow up, migration |
| Treatment protocolIntervention, control of blinding | AnalysisIntention to treat, intervention analysis and control |
| Outcomes consideredMain, secondary, instrument to measure the outcome of interest | ResultBenefit or harm in absolute numbers, mean benefit and harm |
The questions were structured using the Patient/Problem, Intervention, Comparison and Outcome (PICO) system, allowing the generation of evidence search strategies in the key scientific databases (MEDLINE PubMed, Embase and Cochrane Library). The data recovered were critically analyzed using discriminatory instruments (scores) according to the type of evidence. After identifying studies that potentially substantiate recommendations, the level of evidence and degree of recommendation were calculated using the Oxford Classification.
Using the PICO system, the P corresponds to patients with immune thrombocytopenia and the O to the outcomes (diagnosis, classification, treatment and prognosis).
Degree of recommendation and level of evidenceA: Major experimental and observational studies
B: Minor experimental and observational studies
C: Case reports (non-controlled studies)
D: Opinion without critical evaluation based on consensus, physiological studies or animal models
To identify the best evidence available at the present time related to the diagnosis, classification, treatment and prognosis of patients with immune thrombocytopenia.
When should the diagnosis of immune thrombocytopenia (ITP) be considered in patients with thrombocytopenia? Using which platelet count should ITP be investigated?P: Patients with thrombocytopenia
I: Complete blood count
C:
O: Diagnostic criteria of ITP
A platelet count of less than 100×109/L defines the diagnostic suspicion of ITP. Mild thrombocytopenia, ranging from 100×109/L to 150×109/L, may be within the range of normal values in certain populations1–3 (D). This cutoff point was chosen based on a prospective study in which apparently healthy individuals with platelet counts between 100×109/L and 150×109/L were monitored over the long term; in 64% of cases there was spontaneous normalization or stabilization of the platelet count. The ten-year probability of developing more severe thrombocytopenia (persistently less than 100×109/L platelets) was 6.9%4 (B).
There is a proposal that in addition to a platelet count below 100×109/L to diagnose ITP, there should be a follow-up of between two and six months, or two more consecutive low platelet counts5,6 (D). Additionally, a peripheral blood smear should be assessed to rule out pseudothrombocytopenia and morphological alterations associated with hereditary thrombopathies7 (D).
RecommendationA diagnosis of ITP is suspected when the platelet count is less than 100×109/L. However, low levels should be verified in two further measurements or in a follow-up of two to six months.
Is there evidence of the need to perform a myelogram for the diagnostic confirmation of ITP? When is a myelogram and/or bone marrow biopsy indicated in patients with thrombocytopenia?P: Adult and pediatric patients with thrombocytopenia
I: Myelogram and/or bone marrow biopsy
C: Evaluation of just the blood count
O: Diagnosis of ITP
A two-year follow-up of adult patients with an initial diagnosis of ITP did not modify the established laboratory diagnosis and myelograms of these patients did not contribute to the diagnosis8 (C).
In two years of follow-up of adult patients with platelets below 38×109/L, the initial diagnosis of ITP was confirmed in 98% of cases, regardless of a normal myelogram9 (C).
Evaluations of the bone marrow in adult patients with suspicion of ITP presented normal results in 92% of the cases10 (C).
RecommendationThere is no consistent evidence to justify an evaluation of the bone marrow for the diagnosis of ITP in cases of isolated thrombocytopenia.
What are the etiological factors involved in secondary ITP? Which exams should be part of the ITP work up?P: Patients with ITP
I: Serology of HIV, Hepatitis B and C, cytomegalovirus, investigation of Helicobacter pylori, rheumatologic diseases, antiphospholipid syndrome, thyroid-stimulating hormone (TSH) and neoplastic screening
C: Complete blood count
O: Etiology of secondary ITP and differential diagnosis of ITP
In adult patients diagnosed with ITP, 14% have secondary etiology, with infectious diseases [human immunodeficiency virus (HIV), hepatitis C virus (HCV), cytomegalovirus (CMV) and H. pylori], autoimmune diseases and neoplastic diseases being the main causes11,12 (C).
A bone marrow biopsy confirmed ITP in 14% of HIV-positive adult patients with thrombocytopenia (67% with less than 100×109/L)13 (C).
The incidence of ITP in patients with HCV infection is about twice as high as in the uninfected population14 (B). In patients with ITP, the association with HCV confers some laboratory differences in the presentation of the disease: lower risk of severe thrombocytopenia (less than 10×109/L), less symptoms and the presence of serum cryoglobulins15 (B).
The presence of CMV associated with ITP may lead to treatment refractoriness, but the use of antivirals improves the response16 (C).
The association between ITP and H. pylori seems to be correlated with the epidemiological profile of each population12 (C),17 (D),18 (B). In a study of 95 patients with chronic ITP (platelets <100×109/L persisting for >12 months and with no evidence of other conditions that could be causing thrombocytopenia) carried out at the Universidade Federal de Minas Gerais, 74 (77.9%) were positive for H. pylori infection based on the respiratory test with carbon-13 labeled urea (13C-urea test). Of these, 61 patients received treatment for H. pylori, which was eradicated in 59 (96.7%). Of the patients who eradicated H. pylori, 17 (28.8%) achieved remission of the chronic ITP in a period of three to six months, with partial remission in one case and complete remission in the remainder19 (B).
Antiphospholipid antibodies are present in 15% of adult patients with persistent moderate thrombocytopenia (84–139×109/L platelets); 50% are associated with antiplatelet antibodies20 (C). Antiphospholipid antibodies (anticardiolipin and lupus anticoagulant) are detected in 26% of cases of ITP and, in a follow-up of 30 months, it was shown that there is an increased risk of association with systemic lupus erythematosus or antiphospholipid syndrome. There is no difference in the therapeutic response between patients who are positive and negative for antibodies21,22 (B).
Lymphoproliferative diseases are the most frequent neoplasms associated with ITP. Chronic lymphocytic leukemia (CLL) presents in about 10% of cases of autoimmune cytopenias, and ITP is present in about 1–5% of the cases23 (B),24 (D). It is important to remember that in these cases other causes of thrombocytopenia may be present, including hypersplenism, bone marrow failure due to neoplastic infiltration, myelotoxicity from chemotherapy and myelodysplasia23 (B). In the evaluation of 960 patients with CLL followed up for 28 years in a hospital in Barcelona, 70 (7%) had autoimmune cytopenia, 20 (2.1%) had ITP and one case had Evans syndrome (ITP associated with autoimmune hemolytic anemia)25 (B).
In another population, 878,161 patients diagnosed with autoimmune diseases were followed up for a mean of 9.4 years (maximum follow-up: 47 years) in Sweden with 3096 (0.35%) progressing with non-Hodgkin lymphoma (NHL). Among the cases diagnosed with ITP, the standard incidence rate was 7.5 [95% confidence interval (95% CI): 5.9–9.4]26 (B).
RecommendationConsidering the association between ITP and infectious diseases such as HCV, HIV, CMV and H. pylori, autoimmune diseases and neoplastic diseases, as well as the benefit of response to treatment, investigations of the presence of these clinical situations should be performed using appropriate methods.
When should the diagnosis of ITP be considered in a pregnant patient with thrombocytopenia? What are the differential diagnoses of thrombocytopenia in pregnancy?P: Pregnant women with thrombocytopenia
I: Complete blood count
C:
O: Diagnostic criteria of ITP and differential diagnoses
Pregnant women (n=58) with a mean age of 29.2±4 years and a platelet count <100×109/L, excluding those with prior history of thrombocytopenia, pregnancy-induced hypertension, disseminated intravascular coagulation, systemic lupus erythematosus, hematological or hepatic diseases, drug-induced thrombocytopenia or systemic viral infections, were evaluated and monitored for an average follow up of 105 months (range: 5–225) after receiving diagnostic confirmation a few weeks after delivery. When the platelet count returned to normal within 12 weeks (56.9% of the cases), gestational thrombocytopenia was considered, otherwise the diagnosis of ITP was made (43.1% of cases)27 (B).
Some characteristics during gestation suggest the diagnosis of ITP: ITP occurs earlier than gestational thrombocytopenia and with lower platelet counts (p-value<0.001). Of eight women affected with <50×109/L platelets within the first 28 weeks of gestation, 100% were diagnosed as ITP. However, of 13 women in the same gestational period with counts >50×109/L platelets, 76% were diagnosed with ITP and 24% with gestational thrombocytopenia. Of 11 women affected after 28 weeks of gestation with ≤50×109/L platelets, 54% were diagnosed with ITP and 46% with gestational thrombocytopenia and of 26 with platelet counts >50×109/L, 76% had ITP and 24% had gestational thrombocytopenia27 (B).
The platelet count combined with gestational age had a sensitivity of 96% and a specificity of 75.8%, with positive and negative predictive values of 96.2% and 96.2%, respectively for the diagnosis of ITP27 (B).
Thrombotic thrombocytopenic purpura (TTP) and uremic hemolytic syndrome are among the differential diagnoses of thrombocytopenia during pregnancy but they have specific features such as hemolysis seen in the peripheral blood smear associated with elevated lactate dehydrogenase (LDH) levels, neurological and/or renal symptoms and fever, signs and symptoms not present in the ITP. In addition, HELLP (Hemolysis, Elevated Liver enzyme levels, and low platelet count), sepsis and pre-eclampsia should be excluded28 (C).
A study evaluating 186,602 deliveries performed during a period of 19 years, identified 104 (0.06%) pregnant women with ITP. Some gestational complications were more frequent in patients with ITP such as hypertensive disorders [Odds ratio (OR): 1.89; 95% CI: 1.04–3.41; p-value=0.033], diabetes mellitus (OR: 1.81; CI 95%: 1.00–3.25; p-value=0.048) and preterm delivery (<34 weeks – OR: 3.02; 95% CI: 1.40–6.54; p-value=0.005)29 (B).
RecommendationThe diagnosis of ITP involves the exclusion of other causes of thrombocytopenia during pregnancy, in particular gestational thrombocytopenia, which generally has platelet values >50×109/L and begins after the 28th week of gestation; HELLP syndrome, pre-eclampsia, thrombotic thrombocytopenic purpura and uremic hemolytic syndrome, and infectious conditions that have specific clinical conditions.
When is ITP considered acute, persistent and chronic?P: Patients with ITP
I: Time after diagnosis
C:
O: Definition of acute, persistent and chronic ITP
The term acute ITP should be avoided because it is vague and because it is a post hoc definition. At diagnosis, the term ‘newly diagnosed ITP’ is recommended. Persistent ITP refers to patients who did not obtain remission with treatment or did not sustain their response in a period of three to 12 months after disease diagnosis and chronic ITP is the persistence of thrombocytopenia after 12 months of disease diagnosis1 (D).
RecommendationNewly diagnosed ITP is characterized by thrombocytopenia (<100×109/L). When there is no remission or there is an unsustained response in a period of three months to 12 months, it is called persistent ITP. With the persistence of thrombocytopenia lasting for more than 12 months the disease should be termed chronic.
What criteria define refractory ITP, complete remission and partial remission?P: Patients with ITP
I: Type of response to treatment
C:
O: Definition
The definition of remission or complete response is a platelet count after treatment >100×109/L and no clinically relevant bleeding. Remission or partial response is defined as a platelet count between 30 and 100×109/L, which is twice the baseline count, and no clinically relevant bleeding2,5 (D).
In order for the concept of response not to be exclusively based on platelet counts, resolution of bleeding should be considered. ITP should be considered as refractory when it meets two criteria: failure or relapse after splenectomy and hemorrhagic symptoms or hemorrhagic risk requiring treatment1,2 (D). The idea of refractoriness is based on the expectation of response of splenectomy in 60% of the cases, which, when not achieved, represents resistance to the extreme form of treatment5 (D). Less than 10% of patients with ITP evolve with refractoriness30 (D).
RecommendationThe definition of remission or complete response is a platelet count >100×109/L and no clinically relevant bleeding. Remission or partial response is a platelet count between 30 and 100×109/L, which is double the baseline count, without any clinically relevant bleeding. The definition of refractoriness is related to failure of splenectomy and the persistence of symptoms or hemorrhagic risk requiring treatment.
What is the conduct in patients with ITP and active bleeding?P: Adult and pediatric patients with recent diagnosis of ITP and active bleeding
I: Intravenous human immunoglobulin, corticoid therapy, vincristine, anti-D globulin, transfusion of platelet concentrates
C:
O: Control of bleeding
In adult patients with chronic ITP and platelet count <20×109/L, treatment with intravenous immunoglobulin G (IVIG), associated or not with corticosteroids regardless of the bleeding intensity, defines therapeutic success (improvement of bleeding and/or increase in platelet count) within 48hours in 100% of cases. The use of corticosteroids in patients with a score of less than 8 (mild bleeding) had therapeutic failure in 24% of cases, despite the fact that these patients remained less hospitalized compared to those on IV immunoglobulin treatment31 (B).
Treatment of elderly patients (>60 years) with ITP (77% of cases with bleeding) using corticosteroids (methylprednisolone), splenectomy, danazol, or IV immunoglobulin, produces partial or complete response (platelets >50×109/L) in 61%, 80%, 14% and 13% cases, respectively, after one month of follow-up. At six months follow up, the response rates with corticosteroids, splenectomy and danazol were 33%, 50% and 60%, respectively32 (C).
In patients with chronic refractory ITP who present with severe bleeding or with a high risk of bleeding, a platelet transfusion may stop the bleeding33 (C). The combined use of IV immunoglobulin as a continuous infusion (1g/kg in 24h) and platelet transfusion (one unit of apheresis platelets every 8h) resulted in a 24-h platelet response (>50×109/L) with initial control of the bleeding in 63% of 40 patients with ITP and severe thrombocytopenia (<10×109/L) who presented with active bleeding, or need for surgery, or anticoagulation34 (B).
In a single center study with a five-year follow-up, 75 patients were admitted to the emergency room with bleeding and thrombocytopenia; in 57 (76%) the diagnosis of ITP was reached at that time and the others had already been diagnosed with ITP; 37 (49%) had secondary ITP. The median age was 43 years (range: 20–82) and the median platelet count was 5×109/L (range: 0–22×109/L). The most common symptoms were cutaneous bleeding and petechiae (99%), mucosal bleeding (76%), gingivorrhagia (45%), vaginal bleeding (25%) and epistaxis (17%). Most of the newly diagnosed patients received corticosteroids with 60 being treated with high doses of dexamethasone (20–40mg/day for four days) and six cases receiving methylprednisolone (0.5–2mg/kg/day). The response (>100×109/L) or partial response rate (30–100×109/L) was 68%; 45% of the dexamethasone group achieved complete response versus 17% of patients taking methylprednisolone. Another nine patients received IV immunoglobulin, with a 55% complete or partial response. For the cases that presented response to first-line treatment, the mean response time was four days (range: 3–8 days). Ten patients required associated platelet transfusions because of the severity of the bleeding, but no fatal case due to acute bleeding was reported35 (B).
In a case–control study involving 167 patients (91 adults) with ITP where all patients received IV immunoglobulin due to severe thrombocytopenia with or without bleeding, the patients were divided into three groups according to the IV immunoglobulin dose: Group A (0.2g/kg/day), Group B (0.3g/kg/day) and Group C (0.4g/kg/day). Of the adult patients, partial or complete response was observed in 97% of Group A with a mean time to achieve platelet >30×109/L of 2.5 days, for Group B the response was 97.2% in 3.2 days and for Group C the response was 100% in 2.9 days36 (B).
Treatment of adult patients with ITP and platelet counts <30×109/L using anti-D immunoglobulin compared to conventional treatment (prednisone) did not reduce the number of bleeds37 (B).
RecommendationThe initial therapeutic options for ITP patients with platelet counts generally <30×109/L and active bleeding are corticosteroids and IV immunoglobulin. A combination of these therapies may be appropriate in emergency situations. Refractory patients may require platelet transfusions, which are more effective in combination with an IV immunoglobulin infusion.
Is there evidence that the use of methylprednisolone is faster and/or more effective than the use of oral corticosteroids?P: Patients with ITP
I: Pulse therapy with methylprednisolone
C: Corticosteroid VO (prednisone)
O: Improvement of thrombocytopenia
In a prospective study involving 30 patients, a platelet count >50×109/L was achieved in 27% of adult patients with ITP in response to high doses of methylprednisolone (30mg/kg/day orally for seven days); 90% of these patients presented an increase in the platelet count >50×109/L for two to seven days with peak response (90%) on the seventh day38 (C). In a retrospective case analysis of patients who received corticosteroids before splenectomy, the rates of increase in platelet counts evaluated on Days 3, 5, and 7 after treatment were significantly higher with the use of high-dose corticosteroids (30mg/kg/day of methylprednisolone) when compared to those taking conventional doses (1mg/kg/day methylprednisolone; p-value<0.05), although platelet counts had been similar in both groups39 (C). In an open-label study, 80% of adult patients with ITP responded to the use of high doses of methylprednisolone (30mg/kg/day orally) compared to 53% in patients treated with conventional doses (1mg/kg/day of prednisolone). In addition, the required treatment time was 4.7±1.4 days for high doses and 8.4±2.9 days for conventional doses. However, treatment modalities were similar with respect to the rate of complete remission (52.6% versus 47.2%) and persistent remission rate (33% versus 25%), with relapse in less than six months post-treatment in 22.2% of patients who took high doses of methylprednisolone and 43.7% who took a conventional dose of corticosteroids with no significant difference between the groups40 (B).
The side effects secondary to high doses of methylprednisolone and conventional corticosteroids, such as cushingoid appearance, myopathy, gastrointestinal bleeding, infectious complications, diabetes and hypertension are similar39–41 (B) and without necessity to cease treatment38 (C).
RecommendationThe use of high doses of methylprednisolone is an effective strategy in the treatment of adults with ITP and may lead to a faster increase in platelet counts without differences in maintaining the response.
Is the use of dexamethasone in pulses better than the use of prednisone? What is the most recommended dexamethasone regimen?P: Adult patients with ITP
I: Dexamethasone pulses
C: Prednisone
O: Control of thrombocytopenia
In the treatment of adult patients with ITP, the use of conventional doses of prednisone (1mg/kg/day for three weeks) led to a therapeutic response in 59% of the cases42 (A). On the other hand, the use of high-dose dexamethasone at 40mg/day for four days was associated with overall responses ranging from 85.6% to 100% in four open prospective studies with 10, 37, 95 and 125 patients, respectively41,43,44 (B). However, only 50% of patients maintain platelet responses >50×109/L six months after the start of treatment44 (B). High-dose dexamethasone therapy is well tolerated41,43,44 (B). Adverse effects are reported in 2.1% of cases of ITP adults with transient hypertension being the most common.
In a randomized study of 195 patients, treatment with high doses of dexamethasone (1 or 2 cycles of 40mg/day for four days) compared to prednisone (1mg/kg for four weeks) had higher initial (82.1% vs. 67.4%) and complete responses (50.5% vs. 26.8%), but with no significant difference in maintaining the response (40% vs. 41.2%)45 (B).
RecommendationIn adults with ITP, dexamethasone at a dose of 40mg/day for four days every 14 or 28 days for four to six cycles is effective and well tolerated with immediate results superior to those of conventional treatment using prednisone 1mg/kg/day for four weeks but with no significant difference in maintaining the response.
When is splenectomy indicated for the treatment of ITP in the adult patient?P: Adult patients with ITP
I: Splenectomy
C:
O: Control of thrombocytopenia
Adult patients with a diagnosis of resistant or recurrent ITP who present with thrombocytopenia without any other cause of the thrombocytopenia can be submitted to open or laparoscopic splenectomy. Laparoscopic splenectomy requires a longer surgical time than open splenectomy and there is a 9% conversion rate to open surgery. The hospital stay is shorter with laparoscopy. There is a non-significant increase in complications and mortality using the open surgery technique. The partial responses are similar in laparoscopic and open splenectomy (27% and 29%, respectively) and the complete response is 59% for both. The need for additional therapies (corticosteroids or other immunosuppressants) is also similar for the two forms of treatment46 (B).
In a study of adult patients with ITP, a comparison was made between treatment with prednisone (1mg/kg oral) alone for six to eight weeks (17 patients) and treatment with splenectomy (24 patients) in patients with recurrent thrombocytopenia after treatment with prednisone. The response to treatment was evaluated by platelet kinetics studies evaluating platelet mean life and platelet production. Treatment with prednisone and splenectomy increases platelet half-life and production. However, the mean platelet life was normalized only in patients submitted to splenectomy47 (B).
A randomized study compared the need for splenectomy in Rho (D)-positive patients with newly diagnosed ITP and platelet count <30×109/L treated with prednisone (1mg/kg/day) for 14 days or anti-D (3–5mg/day) associated with prednisone. Splenectomy was indicated if bleeding occurred or thrombocytopenia persisted (<30×109/L). The incidence of splenectomy was similar in treatment with prednisone alone (38%) and in those taking anti-D (42%)37 (A).
Prior to corticosteroid use 50 years ago, splenectomy was considered the primary treatment of ITP but currently it is reserved for cases that do not respond to corticosteroid treatment or for cases where corticosteroids are continuously required to support a safe platelet count. In patients submitted to splenectomy, 88% achieved complete or partial response in a two-year follow-up, and a complete response of 64% in a seven-year follow-up. Relapse in three years of follow-up is 15%. Previous response to corticosteroids or immunoglobulins prior to relapse does not predict response to splenectomy. Complications and mortality in these patients are mainly due to bleeding (occurring mainly if platelet levels <20×109/L), cardiovascular events and infections48 (B).
Patients with ITP, including isolated thrombocytopenia, are indicated for splenectomy when they lack corticosteroid response, have a platelet count <10×109/L, or have active bleeding and platelet count <30×109/L when they have normal findings for peripheral blood, normal bone marrow aspiration and absence of splenomegaly. Considering complete preoperative response to pharmacological therapy, platelet count >100×109/L for three months, absence of response to corticosteroids, platelet count ≤100×109/L independent of optimized treatment and relapse of thrombocytopenia two years after cessation of corticosteroids, the rate of complete response after laparoscopic splenectomy is 64%, with refractoriness in 21% and relapse in 15%49 (B).
In adult ITP patients, treatment with laparoscopic splenectomy requires a longer operative time but shorter hospitalization time compared to open splenectomy. The platelet count obtained in the follow-up is similar in the two techniques with relapse occurring in 28% and 15% of the cases submitted to laparotomy and laparoscopic splenectomy, respectively50 (B).
In adult ITP patients submitted to splenectomy with a mean duration of disease of 26 months, who may have been submitted to several preoperative treatments, the definition of a short-term therapeutic response or failure is usually evaluated within 4–8 weeks with long-term therapeutic failure defined by platelet counts <50×109/L. The short-term and long-term therapeutic failure rates are 8.2% and 43.6%, respectively51 (B).
With the definition of ITP as isolated thrombocytopenia (platelet count <140×109/L) without any other clinical cause of thrombocytopenia, patients presenting with hemorrhagic symptoms (62%) can be submitted to splenectomy if they have been treated with corticosteroids (85%) alone, with a combination of corticosteroids and immunoglobulin (10%), or with pulse therapy of dexamethasone, immunoglobulin, or vinblastine sulfate only (5%) and did not respond or who presented with an allergic reaction or major bleeding. At a mean follow-up of 64 months, 92% of patients submitted to splenectomy presented complete or partial response compared to 30% of patients treated clinically [adjusted risk ratio: 62%; number needed to treat (NNT): 2]52 (B).
In patients with ITP treated with splenectomy based on these criteria: (1) therapeutic failure with corticosteroids or other medications; (2) prolonged use of high doses of steroids to maintain a platelet count >30×109/L; (3) relapse after remission obtained with steroid treatment; and (4) contraindication to steroid use, the mean follow-up of 43 months shows a complete or partial response in 80% of the cases, with the postoperative platelet level predicting the response; 404×109/L vs. 213×109/L (p-value<0.001) in responders and non-responders, respectively53 (B).
Of 167 adult patients with persistent (three to 12 months of diagnosis) or chronic (>12 months) ITP who underwent splenectomy, 148 (88.6%) presented an initial response on average one day after the procedure (range: 1–54 days) and 10 (6.5%) lost response in two months. After a median follow-up of 54.3 months (range: 1–290 months), 115 (68.9%) maintained a response. The median for loss of response after splenectomy was 8.8 months (range: 1–108 months)54 (B).
RecommendationFor adult patients with ITP, splenectomy (open surgery or laparoscopy) is indicated in those who did not respond to pharmacological treatment with corticosteroids or who relapsed after treatment.
Is there evidence that ITP patients benefit with the use of rituximab? In which situation is the use of rituximab recommended?P: Patients with ITP
I: Rituximab (anti CD20)
C: Corticoid and/or splenectomy
O: Control of thrombocytopenia
Dexamethasone therapy, with or without rituximab, was evaluated in treatment-naive adult patients with a diagnosis of ITP and a platelet count ≤20×109/L. A daily dose of 40mg dexamethasone orally administered to all patients for four consecutive days (Days 1–4) was compared to the combination of dexamethasone (regardless of the initial response) with 375mg/m2 rituximab administered intravenously on Days 7, 14, 21, and 28. The results indicate an increase of 52% (NNT: 2) in sustained response (evaluated at six months) with the association of rituximab (63% vs. 36%). During the evaluation of the early response (the first month of treatment), there was no significant difference between patients treated with rituximab or not. Overall, treatments were well tolerated and no grade 5 toxicity, hemorrhage, or deaths occurred. Patients receiving rituximab had a higher incidence of grade 3–4 toxicity (10% vs. 2%) and drug-related adverse events (4% vs. 0%), but no significant increase in severe adverse events (6% vs. 2%)55 (A).
Treatment with or without dexamethasone was compared to rituximab in patients diagnosed with ITP who were resistant to one or three different treatment regimens or untreated. Patients given oral dexamethasone (40mg/day) for four successive days (Days 1–4) and then on the following days changed to oral prednisone were compared with patients receiving associated rituximab with an intravenous infusion of 100mg/week for four consecutive doses (on Days 7, 14, 21 and 28). Patients exhibited non-significant differences in mean platelet counts on Day 14 (205±148×109/L on rituximab treatment and 180±111×109/L on corticosteroid treatment). Overall response rates, complete and partial responses were similar with both treatments (rituximab vs. corticosteroids) on Day 28. A total of 8.1% of patients had mild to moderate short-term toxicity (infusion-related adverse events including chills, fever and angioedema, secondary hypertension and secondary hyperglycemia)56 (A).
A multicenter, randomized, double-blind, placebo-controlled study was conducted to evaluate the efficacy and safety of rituximab in adult patients with primary ITP treated with corticosteroids. In this study, corticosteroid could be maintained in both groups. Overall response rates were 81% in the rituximab arm and 73% in the placebo arm, with complete responses of 58% and 50%, respectively (non-significant difference). Bleeding and infection rates were similar in both groups57 (A).
In a meta-analysis of rituximab treatment in non-splenectomized adult patients with primary ITP, the overall response rate (platelet count >50×109/L) was 57% (95% CI: 48–65) at one year (this rate remained stable). The complete response rate with platelet count >150×109/L was 36% and with a platelet count >100×109/L, it was 45.6%. The mean time to reach a response was 6.34 weeks (range: 2.83–9.85 weeks) and the mean duration of response was 49 weeks (range: 17–60)58 (B).
Rituximab was initially administered at a conventional dose of 375mg/m2 in patients with ITP but recent studies have tested its use at alternative doses. In a prospective study involving 48 patients with primary ITP, rituximab was given at a fixed dose of 100mg weekly for four weeks. Complete response (platelet count >100×109/L) was achieved in 39.5% of treated patients and overall response (platelet count >50×109/L) in 60.5% of treated patients. The mean time to reach response was 35 days (range: 7–112 days). Relapse-free survival at 12 and 24 months was 61% and 45%, respectively59 (B).
In addition to the low-dose regimen, rituximab has also been tested at a fixed dose of 1000mg on Day 1 and Day 15 in a regimen similar to that used in rheumatoid arthritis. In a retrospective study involving 107 patients with primary ITP, a comparison was made between two regimens of rituximab: one group was treated with weekly doses of the conventional dose of 375mg/m2 for four weeks and one group was treated with fixed doses of 1g on Days 1 and 15. The response rates at 12 months were 36% for the conventional regimen and 50% for the fixed dose (non-significant difference). The occurrence of side effects was similar between the two groups60 (B).
In a prospective multicenter registry, 248 patients with primary ITP were treated with either conventional or fixed dose rituximab (1g on Days 1 and 15). The response rate was 61%, with no differences between the two regimens. The sustained response at 24 months was 39%61 (A).
RecommendationThe use of rituximab in patients with primary ITP who are not responsive to the first-line treatment may achieve complete or partial response in the medium term. There are three possible dose regimens for rituximab treatment.
Regarding second-line treatment, is there evidence that adult ITP patients benefit from the use of dapsone, immunoglobulin G (IVIG), azathioprine, cyclosporin A, danazol, vincristine sulfate, or mycophenolate mofetil?P: Adult patients with ITP on second line treatment
I: Dapsone, immunoglobulin G (IVIG), azathioprine, cyclosporin A, cyclophosphamide danazol, vincristine, mycophenolate mofetil
C: Corticoid and/or splenectomy, other drug treatments
O: Control of thrombocytopenia
Dapsone at a dose of 100mg/day was used in 15 adult patients with refractory ITP over a period of 1–31 months. The overall response rate was 40%. Pre-treatment baseline characteristics such as gender, age, platelet count and duration of ITP are not correlated with response to dapsone. The most common adverse effect is dose related: hemolytic anemia62 (C).
Patients with ITP presenting platelet counts <50×109/L were treated with dapsone (75–100mg orally). A total of 66 patients were included in the study with 33 of them responding to the drug. The median duration of treatment required for a response was 21 days (range: 8–90). The use of dapsone remained in 60% of the cases for an average of 12.5 months (range: 1–48). There was relapse of thrombocytopenia in 90% of the cases that stopped taking the drug. Secondary adverse effects determined cessation of treatment in 20% of patients63 (C).
In one study involving eight corticosteroid-dependent patients, dapsone was used at a dose of 100mg/day until response, followed by drug abstinence for at least four weeks, and then re-exposure to the drug. In the initial phase of the study, seven of the eight patients treated responded to the drug, but the response was transient in one. The mean time to maximum drug response was 5.7 weeks. Five patients were re-exposed to the drug; all were responsive with mean platelet counts before and after re-exposure of 32.2×109/L and 83×109/L, respectively64 (C).
Patients with duration of primary ITP of more than six months and platelet counts <50×109/L were treated with dapsone at a dose of 1–2mg/kg/day for at least three months after failure of initial prednisolone therapy. A total of 55 adults were treated with a response rate of 61.8%. The mean response time was 3.5 months (range: 1–9). Serious adverse events were observed in 2% of cases including acute hemolysis and erythematous eruption65 (B).
Treatment with dapsone (100mg/day orally) was performed for at least 30 consecutive days in a retrospective study involving 52 corticosteroid-refractory or corticosteroid-dependent patients. A sustained increase in platelet count after initiating dapsone therapy was observed in 44.2% of patients. The rate of splenectomy or some additional treatment after dapsone was discontinued was 79.3% in non-responders compared to 0% in responders. The most common adverse event was subclinical hemolysis; in 21.16% of the cases, hemoglobin dropped by more than 2g/dL in the first three or four weeks of treatment. Hemolysis reverts after discontinuation of treatment or dose reduction66 (B).
A response (platelet count >30×109/L) and complete response (platelet count >100×109/L) of 55% and 20%, respectively were observed in 20 consecutive adult patients with primary ITP treated at least with steroids and rituximab; the mean time to response was one month. None of the responders lost their response during treatment and none of the patients discontinued treatment due to toxicity67 (C).
Immunoglobulin G (IVIG)In a study of 25 ITP patients treated with IVIG (0.4g/kg) for five days, all patients had a platelet response with the time to peak platelet count ranging from 2 to 120 days. The response was transient in all cases. There were no differences in response rates comparing splenectomized vs. non-splenectomized patients68 (B).
A cohort of 22 patients, including 16 children and six adults, were treated with IVIG at a dose of 0.4g/kg for five days, in repeated cycles according to clinical need (a drop in platelet count <20×109/L) but always with intervals of greater than two weeks between cycles. Of the treated adults, three became refractory (failure to maintain platelet count >20×109/L for a minimum of two weeks)69 (C).
AzathioprineA series of 22 patients with chronic ITP aged between nine and 70 years, with relapse after splenectomy or inadequate response to corticosteroids, were treated with azathioprine (1.0–3.2mg/kg daily) for a mean of six months; 75% presented a drug response70–72 (C).
Azathioprine was used in a study involving 53 adults with chronic ITP. All patients had received at least one form of therapy (including splenectomy) and had platelet counts <50×109/L. All patients initially received 150mg/day azathioprine, with an overall response rate of 64%, including 45% complete remissions. The mean response time was four months. Forty percent of the patients had responses lasting one year or more and 32% had responses lasting two years or more. Adverse events included leukopenia and elevated transaminases73 (C).
Adult ITP patients were treated with vinca alkaloids (vincristine sulfate and vinblastine sulfate), azathioprine and/or danazol. Azathioprine was given at a daily dose of 150mg for an average of six months. The response rate was 45% and remission (maintenance of platelet count >100×109/L for more than three months without further treatment) was 9% in the patients treated with azathioprine74 (C).
Cyclosporin APatients with chronic ITP were treated with cyclosporin alone (Group 1) or in combination with prednisone (Group 2). Ten corticosteroid-refractory patients were included in each group with those in Group 2 being splenectomized. In Group 1, oral cyclosporin was started at a dose of 3mg/kg b.i.d. for at least four weeks. In Group 2, cyclosporin was administered at a dose of 2.5mg/kg b.i.d. in combination with prednisone 0.4mg/kg/day. The response rates were 50% in Group 1 and 60% in Group 2. Among the patients in Group 1, nine had to undergo splenectomy due to treatment failure or intolerance. Treatment was discontinued in 30% of patients due to side effects. The most common side effects were hypertension, severe muscle pain and headaches75 (C).
In 12 patients with ITP, cyclosporin was started at a dose of 5mg/kg/day b.i.d. given orally. The dose was then adjusted to maintain a serum level between 200 and 400ng/mL. Platelet counts generally began to increase in the third or fourth week of treatment. The response rate was 83.3%. The side effects and other complications of cyclosporin are transient and reversible intolerance, small elevation in creatinine, moderate hypertension, fatigue, paresthesias, gingival hyperplasia, myalgias, dyspepsia, hypertrichosis and tremor. These conditions were generally resolved spontaneously or in response to a reduction in cyclosporin dose76 (C).
Treatment with cyclosporin at doses between 200 and 350mg/day with maintenance of the serum level between 150 and 250μg/L was carried out in a study including six patients with refractory ITP. Complete remission was achieved in 50% of the patients and cyclosporin treatment was gradually discontinued. Some of these patients relapsed afterwards, but responded to an additional course of cyclosporin, achieving a second complete response. The most common side effect was painful edema in the lower extremities77 (C).
DanazolData collected from 22 adult patients receiving danazol therapy (200mg 2–4 times/day) for ITP demonstrate that the overall response rate is 61.4%. Among responders, the response duration was 2.7±3 months. Side effects were well tolerated with the most frequent being weight gain78 (C).
In 16 patients with chronic ITP, danazol was given at a daily dose of 100mg t.i.d. In 50% of the cases there was an increase in the platelet count and in 80% of the cases the hemorrhagic symptoms disappeared. Side effects were not significant79 (C).
Danazol (dose of 600mg/day for three months) in the treatment of ten patients previously treated with steroids and/or splenectomy, produced a transient increase in the platelet count in only one patient. Side effects were seen in 60% of cases during treatment80 (C).
A study with 24 patients compared the response to low dose danazol (50mg/day) vs. conventional dose danazol (400–800mg/day). In Group 1, the low dose was initiated 1–24 months after the conventional dose has been discontinued, in Group 2, patients received low doses immediately after the conventional dose and in Group 3, patients were treated with low doses from the start. In Group 1, similar responses were observed in 70% of patients for each dose. All patients in Group 2 had low dose remissions. In Group 3, only 40% had a response. Side effects were generally less common and less severe at low doses81 (C).
Patients with refractory chronic ITP were treated with danazol at a fixed dose of 600mg daily initiated after the discontinuation of other treatments. The study included 57 patients and the treatment was maintained for a mean period of 17.8±7.5 months. The overall response rate (complete or partial) was 67%; 16% of the patients had complete response. The mean duration of remission was 119±45 months (range: 3–182 months) and 46% of the patients remained in remission for a mean of 70 months. Thus, the ten-year response rate (complete or partial response) for danazol therapy was 42%. In 16% of cases, danazol therapy was discontinued because of serious adverse effects including elevated transaminases levels, intracranial hypertension, generalized rash and rhabdomyolysis. The majority of patients tolerated treatment well with 36% having mild or moderate adverse events: weight gain and edema, increase in transaminases, amenorrhea, nausea, hypertension, diabetes mellitus, headache, phlebitis, rash and alopecia82 (C).
Recently a retrospective study including 319 patients with chronic ITP reported the use of danazol at doses of 200–300mg/day alone or in combination with prednisone. The overall response rate for danazol was 65%, 63.1% among those treated with danazol alone. Among patients who received combination therapy (danazol and corticoid) 48.7% were responsive and were able to suspend the use of corticosteroids. Mild or moderate side effects were observed in 21.1% of the patients with 1.2% of patients having their treatment suspended due to adverse effects83 (B).
Vincristine sulfateIn a study of eight patients with ITP, treatment with IV vincristine sulfate at a dose of 1mg/week was completed until a total dose of 4mg was achieved. Complete remission was observed in two patients with duration of five and 20 months after therapy; three patients presented partial remission, which was transient for two. Side effects (finger numbness and constipation) were observed in only one patient84 (C).
Adult patients with refractory chronic ITP were treated with slow weekly infusions of vincristine sulfate (0.02–0.04mg/kg) or vinblastine sulfate (0.1–0.2mg/kg). Response was observed in 60% of patients after 1–8 infusions. These responses were generally short, and lasted only in 20% of patients after discontinuation of therapy. Efficacy was comparable between vincristine sulfate and vinblastine sulfate. Side effects such as peripheral neuropathy, alopecia, gastrointestinal symptoms and leukopenia occurred in 90% of the patients, and required discontinuation of therapy in 20%85 (C).
Mycophenolate mofetilMycophenolate mofetil (500mg b.i.d.) was investigated in a study including six patients with ITP with the dose being increased to 1g b.i.d. after two weeks. There was a response in 80% of cases. Side effects such as headache and back pain were observed in 15% of the cases at the highest dose of 2g/day however symptoms regressed when the dose was reduced to 1g/day86 (C).
Mycophenolate mofetil at a dose of 1.5–2.0g/day was used to treat 21 patients with ITP. The overall response rate was 62%, including 24% in complete response (platelet count >100×109/L). The response rates for non-splenectomized and splenectomized patients with ITP were 64% and 57%, respectively (p-value>0.05). Among respondents, 39% relapsed after reducing or suspension of the drug with 61% having the response maintained for a mean of 24 weeks without the drug. Mycophenolate mofetil was well tolerated with only mild cases of nausea and diarrhea87 (C).
Adult patients with refractory ITP were treated with mycophenolate mofetil at the dose of 1g b.i.d. for three weeks. Of the 18 patients included in the study, a response was observed in 40% of them but none had complete responses. Patients who had ITP for a shorter period of time (<8 years vs. >8 years) showed a non-significant tendency for a better response rate (55% vs. 22%; p-value=0.16)88 (C).
In a retrospective study including 46 patients with primary or secondary ITP, mycophenolate mofetil was prescribed at a dose of 1g/day. The response rate was 52%, with 33% of complete responses (platelet count >100×109/L). There was no difference between responders and non-responders regarding gender, age, previous treatments or disease duration. Treatment was suspended in four patients due to adverse effects, with gastrointestinal intolerance being the most common88 (B).
RecommendationThe use of dapsone, azathioprine, immunoglobulin G, cyclosporin A, danazol, vincristine sulfate or mycophenolate mofetil in adults with ITP has demonstrated sustained response in some cases but with varying efficacy.
In adult patients with refractory ITP, is there a platelet count that indicates treatment even in the asymptomatic patient (without bleeding)?P: Adult patients with refractory ITP without active bleeding
I: Drug treatment based on platelet count
C: Clinical observation
O: Prevention of hemorrhagic events
The risk of bleeding in ITP patients with platelet counts persistently <30×109/L was evaluated in an analysis of 17 case series involving 1817 patients and 49 cases of fatal bleeding at an estimated exposure of 1258–3023 patients-year. The annual rates of age-adjusted fatal bleeding were 0.004, 0.012, and 0.130 for the 40-year-old, 40–60, and 60-year-old groups, respectively. Five-year mortality was estimated at 2.2% for patients under 40 years of age and 47.8% for patients over 60 years of age89 (B).
In a subsequent study of 152 adult patients with ITP, the hemorrhagic risks of ITP were assessed in a consecutive cohort study. At a median of two years after diagnosis, four patients died, two patients were lost to follow-up and 12 were classified as secondary thrombocytopenia. Of the 134 remaining patients, 85% achieved a platelet count response >30×109/L even after treatment discontinuation. This group had a mortality rate similar to that of the general population. In contrast, 12 patients (9%) with refractory ITP, defined as a platelet count <30×109/L despite treatment for ITP, presented a 4.2-fold higher mortality rate (95% CI: 1.7–10.0) with bleeding and infections contributing equally. Finally, in the group of patients requiring maintenance treatment, mortality was very low [relative risk (RR)=1.8] in relation to the normal population and well below the group defined as refractory90 (B).
RecommendationThere is no platelet count that in isolation defines the need for treatment in patients with refractory ITP.
Is there any benefit of thrombopoietin receptor agonists (eltrombopag, romiplostim) in patients with refractory ITP and indication for treatment?P: patients with refractory ITP
I: Thrombopoietin receptor agonists (eltrombopag, romiplostim)
C: Corticoid and/or splenectomy, other drug treatments
O: Prevention of bleeding events and control of thrombocytopenia
In a randomized placebo-controlled study, over 18-year-old patients with a history of six months of ITP, a history of at least one previous treatment and a platelet count <30×109/L were randomized for treatment using eltrombopag (at 30, 50 or 75mg/day) or placebo for up to six weeks. The primary endpoint, a platelet count of ≥50×109/L on the 43rd day, was achieved in 81% of patients with 75mg of eltrombopag, 70% of patients with 50mg, 28% of patients with 30mg and 11% of the patients in the untreated group (p-value<0.001 for the groups receiving 50mg and 75mg of eltrombopag). The mean platelet count in patients of the groups receiving eltrombopag 50mg or 75mg who continued treatment was maintained at ≥50×109/L at each subsequent visit during treatment. Among patients who had a baseline platelet count between 15×109/L and 30×109/L, there was a substantially higher percentage of responders in all groups, except for the group receiving 30mg of eltrombopag. During treatment with eltrombopag (50mg or 75mg), the incidence of bleeding decreased with a frequency of bleeding events regardless of grade and cause of 14% in placebo patients and 17%, 7% and 4% in the groups receiving 30, 50, and 75mg of eltrombopag, respectively. The incidence and severity of adverse reactions were similar for all four groups: mild to moderate headache91 (A).
In a second multicenter placebo-controlled randomized study, over 18-year-old patients with ITP, a history of six months of disease, at least one previous treatment and pre-treatment platelet counts <30×109/L were randomized to conventional treatment of ITP associated with a placebo or eltrombopag 50mg once daily for up to six weeks. The dose could be elevated to 75mg in patients with a platelet count <50×109/L after three weeks of treatment. The primary endpoint (platelet count ≥50×109/L) on the 43rd day was reached by more patients in the eltrombopag group than in the placebo group (59% vs. 16%; p-value<0.0001). The platelet count usually returned to baseline within two weeks after the end of treatment. The response to eltrombopag did not depend on the use of concomitant medications, splenectomy, or baseline platelet count. Significantly less patients in the eltrombopag group presented bleeding symptoms on Day 43 (39% vs. 60%; p-value=0.029) or at any timepoint during the course of treatment (61% vs. 79%; p-value=0.021). The proportions of patients who had one or more adverse events during the treatment phase were 59% for the eltrombopag group and 37% for the untreated patients. Nausea and vomiting were the only two adverse events recorded in 5% or more of the patients in the eltrombopag group. The frequency of Grade 3–4 adverse events during treatment and adverse effects leading to discontinuation of treatment were similar for both groups92 (A).
The efficacy and long-term safety of eltrombopag was evaluated in a placebo-controlled phase III study. Over 18-year-old patients with ITP lasting more than six months, baseline platelet count <30×109/L and at least one previous treatment for ITP were submitted to conventional treatment associated with eltrombopag 50mg daily or placebo for six months. Dose escalation (up to a maximum of 75mg once daily) was allowed after the 22nd day for patients with platelet counts <50×109/L. A significant increase in platelets was observed in the eltrombopag group compared to the placebo group, with a 79% response in at least one follow-up point in the treated group vs. 28% in the placebo group. In the eltrombopag group, the frequency of bleeding was reduced by about 50% from the 15th day but returned to the baseline level after discontinuation of eltrombopag. Nausea and vomiting were reported in at least 5% of patients receiving eltrombopag. Three patients (2%) in the eltrombopag group presented thromboembolic events compared to none in the placebo group. Nine (7%) and two (3%) patients in the eltrombopag and placebo groups, respectively presented mild alanine aminotransferase (ALT) elevations, and five (4%) eltrombopag patients had elevated total bilirubin compared to none in the placebo group. Four (7%) of the placebo patients experienced severe bleeding compared to one (<1%) in the eltrombopag group93 (A).
RomiplostimThe efficacy and safety of romiplostim was evaluated in two controlled studies in patients with ITP. In the first, 63 splenectomized patients and 62 non-splenectomized patients were randomized to treatment with weekly injections of romiplostim or placebo for 24 weeks at a titrated dose targeting a platelet count of 50×109/L. The primary endpoint of the study was to obtain a count >50×109/L for at least six of the last eight weeks of the study. This was achieved by 16 and 25 of the 42 and 41 splenectomized and non-splenectomized patients respectively, compared to only one non-splenectomized patient from the placebo group. The frequency of adverse events was similar in both groups94 (A). In addition, significant improvements in quality of life scores were observed in patients treated with romiplostim95 (A).
The second, open-label, 52-week study randomized 234 adult non-splenectomized patients with ITP for conventional treatment or weekly romiplostim injections. The primary outcome was therapeutic failure and need for splenectomy. Romiplostim was given weekly at an initial dose of 3μg/kg that was increased to a maximum dose of 10μg/kg. Treatment was discontinued if the platelet count remained <20×109/L for four consecutive weeks at the maximum dose. Between two and 52 weeks, the percentage of patients with response (platelet count >50×109/L) ranged from 71% to 92% in the romiplostim group and from 26% to 51% in the conventional treatment group. The median romiplostim dose required to maintain the platelet count within the desired range (50×109/L to 200×109/L) remained stable over time, in particular after the first 12 weeks of treatment. The mean (±standard error) weekly dose was 3.9±2.1μg/kg. The incidence of treatment failure was significantly lower among patients receiving romiplostim (11%) than among those receiving standard treatment (30%; p-value<0.001). Furthermore, the incidence of splenectomy was significantly lower among patients receiving romiplostim (9%) than among those receiving standard treatment (36%; p-value<0.001). Over 90% of patients in both groups had at least one adverse event during the treatment period, with headache and fatigue being the most common. Serious adverse events occurred in 23% of patients receiving romiplostim and in 37% of patients receiving the standard treatment96 (A).
Similar results were demonstrated in a 12-week randomized, double-blind phase III study involving 22 Japanese patients with ITP. The primary endpoint, defined as the number of weeks with a satisfactory response (platelet count >50×109/L) was significantly higher in the romiplostim treated group than in the placebo group (11 vs. 0 weeks; p-value<0.0001). The mean dose of romiplostim immediately before the first weekly platelet response was 3.2 (±0.4)μg/kg, regardless of the status of the splenectomy and any concomitant treatment of ITP. The incidence of serious adverse events was similar. Adverse events that occurred in 5% or more of patients in the treatment group compared to the control group were: nasopharyngitis (41% vs. 17%), headache (32% vs. 17%), peripheral edema (18% vs. 0%), pain (14% vs. 0%), pain in the extremities (14% vs. 0%), nephrocalcinosis (9% vs. 0%), burn lesions (9% vs. 0%), thrombocytopenia (9% vs. 0%), and fatigue (9% vs. 0%)97 (A).
An adverse event of special interest in adults with thrombocytopenia exposed to romiplostim or eltrombopag are thromboembolic events. In a systematic analysis of 15 studies and 3026 adult patients with thrombocytopenia, the estimated frequency of these events was 3.69% (95% CI: 2.95–4.61%) in patients treated with one of these agents and 1.46% (95% CI: 0.89–2.40%) in the control groups. These two agents were associated with a RR of thromboembolism of 1.81 (95% CI: 1.04–3.14) representing a NNH of 4898 (B).
Recent meta-analysis evaluated 13 studies involving 1126 adult patients and children with persistent or chronic ITP and failure or relapse after one or more therapies. In 12 studies, patients with platelet count <30×109/L were evaluated with the other accepting patients with platelet counts <50×109/L. Increased platelet response and durable response were observed in adults with a RR of 3.13 (95% CI: 1.96–4.99) and 7.45 (95% CI: 3.25–17.08), respectively. There was a reduction in the need for rescue therapy compared to the control group (RR: 0.5; CI 95%: 0.42–0.59), the incidence of all bleedings (RR: 0.8; CI 95%: 0.67–0.95) and severe bleedings (RR: 0.52; 95% CI: 0.27–0.99). Regarding adverse events, the rates of any event and serious events observed were similar to those of the control group (RR: 1.01; 95% CI: 0.92–1.10 and RR: 0.74; 95% CI: 0.54–1.01, respectively). The platelet response in splenectomized patients was comparable to that of non-splenectomized patients (RR: 0.84; 95% CI: 0.49–1.42), but the duration of response was shorter in those submitted to surgery (RR: 0.72; 95% CI: 0.54–0.92)99 (A).
RecommendationThe use of thrombopoietin receptor agonists (eltrombopag or romiplostim) in the treatment of adults ITP as second-line therapy reduces bleeding, decreases the need for rescue medication and increases platelet counts with support limited to the period of its use. The duration of response is greater in non-splenectomized patients.
Is there any benefit in combining chemotherapy, or continuous immunosuppression, or alemtuzumab in patients with refractory ITP and indication for treatment?P: Adult patients with refractory ITP
I: Combined chemotherapy, or continuous immunosuppression, or alemtuzumab
C:
O: Prevention of bleeding events and thrombocytopenia control
A chemotherapy combination was used to treat ten adult patients with ITP refractory to a mean of seven previous therapies including corticosteroids and splenectomy. Patients received 3–8 cycles of cyclophosphamide and prednisone combined with vincristine sulfate or vincristine sulfate, and procarbazine or etoposide. Of the treated patients, 60% had complete responses (platelet count >180×109/L), 70% of whom had responses that persisted for more than 11, 30, 54 or 126 months. Moreover, 20% of the patients had partial responses (platelet count >50×109/L) with platelet counts remaining stable for more than nine months after the end of therapy however 20% of the patients had no response. The complete response was associated with a marked disappearance or decrease in the level of antiplatelet autoantibodies100 (C).
Adolescent patients with ITP refractory to previous treatments including immunoglobulin, intravenous corticosteroids, anti-Rh(D) IgG or splenectomy were treated with an immunosuppressive therapy combination. The therapy consisted of weekly doses of vincristine sulphate (1.5mg/m2 IV – maximum dose 2mg), a weekly dose of methylprednisolone (100mg/m2 IV), and oral cyclosporin (5mg/kg b.i.d.). Vincristine sulfate and methylprednisolone were given weekly, until the platelet count was >50×109/L, with a minimum of 2 doses and a maximum of 4 doses. Cyclosporin was maintained until the platelet count was normal for 3–6 months. Of the treated patients, 70% had continuous complete responses (platelet count normal after cyclosporin cessation) for a median of 13 months after completing therapy. Furthermore, 10% of the patients had partial responses (platelet count 80–120×109/L without cyclosporin for three months). However, 20% were non-responders (platelet count <40×109/L), one of whom had therapy discontinued after two weeks due to peripheral neuropathy. The mean time to response was seven days101 (C).
Adult patients with active symptomatic ITP or autoimmune hemolytic anemia, who had already received at least one line of therapy or followed a chronic relapsing course, were treated with alemtuzumab at a fixed dose of 10mg subcutaneously on Days 1–3, and rituximab at the dose of 100mg IV on Days 4, 11, 18, and 25. Responses were evaluated weekly for one month, every two weeks up to six months and then monthly. The criteria for complete response and partial response were defined as platelet counts >150×109/L and>50×109/L, respectively. Of the patients with ITP (mean age: 26 years; range: 16–71 years), the mean duration of ITP was four years and the mean number of previous treatments was 2.5. Of the treated patients, 50% achieved complete response and 50% achieved partial response. The mean duration of complete response was 46 weeks. Grade 1 fever was observed in 90% of patients following alemtuzumab administration; no adverse events were reported with rituximab. The adverse events found were herpes zoster, urinary tract infection and upper airway infection; all patients with adverse events had favorable outcomes after receiving antiviral/oral antibiotic therapy102 (C). Immunosuppressive therapy combination was provided to 19 patients with a platelet count <20×109/L that had persisted for at least 12 months with an inadequate or transient response to multiple therapies. Thus, adults with chronic refractory ITP were treated with a combination of azathioprine, mycophenolate mofetil, and cyclosporin. Mean age of the treated patients was 51 years and the mean baseline platelet count was 7×109/L. The mean duration of ITP was eight years and the mean number of previous treatments was six, including splenectomy, prednisone, IV immunoglobulin, danazol, cyclophosphamide, vincristine sulfate, azathioprine and cyclosporin. Most of the patients (89.5%) had previous bleeding episodes, the most serious being intracerebral hemorrhage, vaginal bleeding, epistaxis and mucocutaneous hemorrhage. The immunosuppressive therapy combination was administered for a median of 36 months with a follow-up of 47 months. Of the patients treated, 73.7% had a global response (platelet count >30×109/L and double the baseline), which lasted an average of 24 months. The time to respond was two months with 68.4% having a platelet count >50×109/L and 57.9% achieving a platelet count >100×109/L. Among the responders, the mean platelet count on treatment was 72×109/L. Of the total cases, 57.1% relapsed, of which 70% responded to the addition of different treatments. Only 10% of patients stopped with all medications and remained in remission after four and 20 months of follow-up. Adverse events were reported in 57.9% of the patients including moderate transient leukopenia and infections; none of the infectious episodes were associated with leukopenia. Twenty percent of patients had cyclosporin-related toxicities, including gingival hypertrophy and reversible tremors. Patients with refractory chronic ITP represent less than 10% of patients with ITP however, mortality (from 10% to 30%), bleeding, or perhaps more often, therapeutic toxicity are associated. Single agent immunosuppressive therapy has been used in patients with chronic ITP with moderate success. There is information that azathioprine results in at least a partial response in 66% of cases. Mycophenolate mofetil also improves platelet counts to >50×109/L in 38.9% of refractory patients and in 62% of patients with severe ITP. Cyclosporin has been associated with a platelet response in 44% to 75% of patients. The combination of mycophenolate mofetil, azathioprine and cyclosporin resulted in a platelet response in 73.7% of patients with refractory severe ITP103 (C). A phase I–II study was performed to evaluate autologous bone marrow transplantation with B and T lymphocyte depletion. Fourteen 17- to 54-year-old patients with ITP were evaluated for more than six months with no lasting response after IV immunoglobulin, corticoid and splenectomy. All patients received four days of cyclophosphamide (50mg/kg/day) before the bone marrow infusion. Eight patients (57.1%) responded with a complete response in 6/14 (42.8%) and partial in 2/14 (14.3%). Adverse events included febrile neutropenia responsive to antibiotic therapy, hemorrhagic cystitis in one patient, vaginal bleeding in two patients, bleeding in the gastrointestinal tract in one patient and epistaxis in one patient104 (C).
RecommendationIn adults, the combination of immunosuppressants should be reserved for cases of severe chronic refractory ITP.
In pregnant patients with ITP, is there a platelet count that indicates treatment even in patients without active bleeding?P: Pregnant women with ITP without active bleeding
I: Drug treatment indicated by platelet count
C: Clinical observation
O: Prevention of bleeding in pregnant women and fetuses
Fifty-eight pregnant women with a mean age of 29.2±4 years and a platelet count <100×109/L were followed up for an average of 105 months (range: 5–225). Patients were excluded if they had a history of thrombocytopenia, hypertension induced by pregnancy, disseminated intravascular coagulation, systemic lupus erythematosus, hematological or hepatic diseases, drug-induced thrombocytopenia or systemic viral infection. Oral prednisolone (1mg/kg/day) was prescribed if the platelet count dropped to below 30–40×109/L27 (B).
A retrospective study of 119 pregnancies in 92 pregnant women with mean age of 29 years (range: 26–32 years) and who were diagnosed with immune thrombocytopenic purpura before gestation (69.7%) or during pregnancy (30.3%) after other causes of thrombocytopenia had been excluded, assessed the need for treatment: 68.9% did not require drug therapy with the platelet count being maintained between 32 and 521×109/L. Women with prior diagnosis of ITP were less likely to need treatment for ITP during pregnancy (24.4% vs. 42.1%; p-value=0.047)105 (C).
A total of 31.1% of women required medical treatment to increase platelets because of a low platelet count or the presence of signs and symptoms of bleeding or the need for invasive interventions; 46% of the pregnant women treated with corticosteroid and/or IVIG and/or anti-D responded (without significant difference between treatments) if the diagnosis of ITP was reached before or during pregnancy105 (C).
RecommendationDespite the scarce evidence, treatment for pregnant women with ITP begins with platelet values from 30 to 40×109/L, or due to signs or symptoms of bleeding or the need for surgical interventions.
Is there evidence of relevant differences in hemorrhagic risk across different platelet counts in patients with ITP?P: Patients with ITP
I: Determination of hemorrhagic risk based on platelet count
C:
O: Prevention of hemorrhagic events
Platelet counts and platelet adhesion are significantly associated with the severity of bleeding in patients with ITP106 (B). ITP Patients with a mean platelet count <30×109/L present an increased risk of bleeding107,108 (B). In adult patients with ITP, a platelet count >70×109/L indicates a lower risk of bleeding requiring treatment108 (B).
There is an inverse correlation between platelet count and bleeding score in patients with ITP, especially with platelet counts <30×109/L. Other parameters associated with the bleeding score are the absolute count of the immature platelet fraction and overall thromboelastometry clotting variables109 (B).
In addition to the platelet count, patient-related factors such as comorbidities, occupational activities, and lifestyle should be taken into account when assessing bleeding risk, all of these factors may increase the risk of bleeding2,7 (D). Advanced age (>60 years) and history of hemorrhagic events are considered the two main risk factors for the occurrence of bleeding in ITP89,108 (B).
RecommendationIn adults with ITP, the intensity of bleeding is inversely proportional to the platelet count with the critical level (major hemorrhagic complications) being 30×109/L platelets.
Is there a platelet count below which the patient with ITP should be admitted for treatment? What clinical conditions in patients with ITP require hospitalization?P: Adult and pediatric patients diagnosed with ITP
I: Hospitalization
C: Outpatient follow up
O: Prevention of hemorrhagic events
The most frequent events that lead patients with chronic ITP to seek medical care are: for blood or platelet transfusions and hemorrhagic events (ocular bleeding, hematemesis, ecchymosis, hemoptysis and hematuria) with these conditions not always requiring hospitalization; 50% of patients need hospitalization in about three years of follow-up, accounting for about 90% of annual expenditures with these patients110 (B).
The most common reasons for the hospitalization of patients with ITP are: coagulation disorders, splenectomy, gastrointestinal bleeding, septicemia, intracranial hemorrhage and epistaxis with septicemia and intracranial hemorrhage correlated to the highest mortality rates in these patients111 (B).
There is an increased risk of hospitalization for infection in the first and second years of follow-up in about 10.8% and 3.5% of patients, respectively in particular for pneumonia and sepsis and mainly due to the use of immunosuppressive drugs. However, infection rates at five years are not different for platelet counts > or <30×109/L3 (B). At five years of follow-up, episodes of bleeding with a platelet count <30×109/L are reasons for hospitalization in 80% of cases of ITP, and the increased risk of bleeding is 2.5%. Patients whose platelet count is <30×109/L have a cumulative bleeding episode rate requiring hospitalization (other than intracranial bleeding) of 4.1% compared to 2.1% in patients with platelet counts >30×109/L112 (B). Regarding intracranial hemorrhage, platelet count <30×109/L is a parameter present at hospitalization and the risk of bleeding is 0.8% higher when compared to a population without thrombocytopenia112 (B).
In the two-year follow-up, 80% of hospitalizations of patients with ITP are due to severe forms of the disease. The main reasons were related to the treatment of thrombocytopenia (70% of cases) and the treatment of infections (10% of cases). Severe thrombocytopenia has a 1.5-fold higher mortality rate, patients with hemorrhagic symptoms have a 1.1-fold higher mortality rate and the mortality rate is 4.2 times higher in patients with no response to treatment (platelets <30×109/L)90 (B).
RecommendationFor adults, the presence of severe, life-threatening bleeding with any platelet count is a parameter of hospitalization. There is no platelet level that indicates the need of hospitalization, but severe bleeding is more frequent when the platelet count is <30×109/L. Other clinical conditions that may determine hospitalization in adults regardless of the presence of bleeding are: infections especially sepsis and preparation for splenectomy with prophylaxis for infections and bleeding.
Is there evidence of clinical and/or laboratory criteria predictive of ITP response to splenectomy?P: Patients with ITP before splenectomy
I: Clinical and laboratory evaluation (previous response to immunoglobulin, discard accessory spleen)
C:
O: Control of thrombocytopenia
In ITP patients with indication for splenectomy (no response to high-dose prednisone therapy (60mg/day), adverse effects to prednisone doses of 20–60mg/day or combination with other medications) and preoperative mean platelet count of 21×109/L, the hematologic response to splenectomy is 71%, with non-responders being significantly older than responders, but preoperative platelet count is not a predictor of response to splenectomy113 (B).
ITP patients (aged >17 years) who received IV immunoglobulin (dose of 2g/kg/day) and/or corticosteroids (prednisone 1mg/kg/day VO or methylprednisone 15mg/kg IV), followed by splenectomy, were followed up for an average of 42 months. The time between diagnosis and splenectomy ranged from three to 156 months with more than six months in 87% of the cases. The responses to IV immunoglobulin, corticosteroids and splenectomy were 76%, 69% and 77%, respectively. The positive and negative predictive values of the preoperative IV immunoglobulin response to post-splenectomy response are 81% and 33%, respectively. The positive and negative predictive values of the preoperative corticosteroid response to post-splenectomy response are 81% and 26%, respectively. Taking into account IV immunoglobulin and corticosteroid, the positive and negative predictive values of response after splenectomy are 86% and 35%, respectively2 (B). In this study, there were no differences in the response rate to splenectomy between responders and non-responders to IV immunoglobulin (81% vs. 67%; p-value=0.36). Age, time between diagnosis and splenectomy and response to corticosteroids were not predictive of response to splenectomy too114 (B).
Patients with ITP pre-treated with prednisone at a dose of 1mg/kg/day and IV immunoglobulin at a dose of 400mg/kg/day and second-line treatment (vincristine sulfate or vinblastine sulfate, danazol, azathioprine, cyclophosphamide) with platelets ≤40×109/L, were splenectomized with a complete response in 67.7%, partial response in 10.8% and failure in 21.5%. In the first week after splenectomy, 88% of the patients had a platelet count >100×109/L and 84% had a complete or partial response. Of the patients with platelets <100×109/L in the first week after splenectomy, only 37% evolved with complete or partial responses. The only predictor of the response to splenectomy was the initial response to corticosteroid therapy115 (B).
After therapeutic failure with corticosteroids in adult patients with chronic ITP (platelets <20×109/L or significant mucosal bleeding), patients were treated with preoperative IVIG and subsequently submitted to splenectomy. Of these patients, 73% responded to IV immunoglobulin and 92% responded to splenectomy with a sustained response in 60% of the cases over two years. The relationship between the response to IV immunoglobulin and immediate response (two weeks) to splenectomy was not significant. However, there was a significant association between the sustained response to splenectomy (74%) and the response to IV immunoglobulin (p-value=0.001), as well as the absence of response to IV immunoglobulin and response to splenectomy (25%). The sensitivity, specificity, positive predictive value and negative predictive value of the IV immunoglobulin response as a predictor of response to splenectomy were 88.6%, 52.2%, 73.8% and 75%, respectively. The sustained response to splenectomy had a significantly higher postoperative platelet count compared to non-responders (401×109/L vs. 200×109/L; p-value=0.04)116 (B).
There was no correlation between the response of adult patients with ITP to previous treatment with IV anti-D immunoglobulin and IV immunoglobulin, and the response obtained after splenectomy with differences of 50% between responses117 (B).
Splenectomy performed in patients without response to prednisolone, produced complete and partial responses in 48.1% and 21.1%, respectively. The responders were significantly younger and had a higher pre-operative platelet count. On setting the dose cut-off point for prednisolone at 40mg/day, patients who required higher doses to achieve preoperative platelet counts have a worse prognosis in their response after splenectomy. Similarly, establishing a cutoff point for the platelet count of 50×109/L, patients who presented a higher level in the first two postoperative weeks had a more favorable prognosis118 (B).
Patients experiencing a complete or partial response to splenectomy were significantly younger (mean age: 51 vs. 73 years; p-value<0.001), were more likely to respond to corticosteroids (complete or partial response of 91% vs. 76%; p-value=0.03), had a higher platelet count at the time of splenectomy (59×109/L vs. 27×109/L; p-value=0.008) and had a higher postoperative platelet count (385×109/L vs. 135×109/L; p-value<0.001) than patients who did not respond or had a transient response119 (B).
The diagnosis of ITP was based on the presence of isolated thrombocytopenia and on the exclusion of other causes of thrombocytopenia. In these patients the mean platelet count at diagnosis was 16×109/L. All patients received steroids as initial treatment and 20% received IV immunoglobulin before splenectomy. Patients who had a complete or partial response to splenectomy were significantly younger, did not receive prior treatment or were treated with only one medication, had a greater platelet count before splenectomy and in the immediate postoperative period when compared to patients who did not respond. Patients receiving more than one treatment before splenectomy (≤1 vs. >2 treatments; p-value=0.048), lower platelet count at the time of splenectomy (18×109/L vs. 30×109/L; p-value=0.004) and platelet count after minor splenectomy (125×109/L vs. 448×109/L; p-value<0.001) were more refractory during follow-up after splenectomy120 (B).
In a study of 30 patients with ITP, all patients were treated with prednisone at an initial dose of 1mg/kg/day for one month; the dose was then adjusted to the lowest dose to maintain platelets at >30×109/L. Patients refractory to steroid therapy, who relapsed after dose reduction, were intolerant to treatment, pregnant women, and those who had active bleeding with a platelet count <30×109/L were selected for splenectomy. At a mean follow-up of 24.3 months, the initial response was 73.3% (complete response was 50% and partial response was 23%). The preoperative response to corticosteroids was significantly correlated with response after splenectomy having a 35.1% response rate (NNT: 3) when compared to the response to splenectomy in steroid refractory patients. Patients with disease duration>12 months had an increase in post-splenectomy response of 39.4% (NNT: 3) compared to patients with duration lasting <12 months. Antiplatelet antibody and antinuclear antibody (ANA) tests are not correlated with response after splenectomy121 (B).
Adult patients with ITP and the following indications underwent splenectomy: therapeutic failure after an initial response to oral prednisolone (1mg/kg/day) or after two or more courses of high-dose pulsed dexamethasone VO and/or IV immunoglobulin, steroid-dependent disease, and recurrent thrombocytopenia after prolonged remission and after steroid treatment. The patients were divided into three hematological response groups: ≤20×109/L (Group I), 20×109/L to 50×109/L (Group II) and >50×109/L (Group III). A significant preoperative predictor of postoperative refractoriness was resistance to corticosteroids: 92% in Group I, 28% in Group II and 38% in Group III (p-value=0.002)122 (B).
Splenectomy was performed in ITP patients who were unresponsive to corticosteroids and those who required corticosteroids at a dose of 10–15mg/day to maintain a platelet count >30×109/L. After the 33-month period, the remission rate was 89.3%, with a complete response of 74.8%; 10.7% of the patients did not respond to surgery (platelets <50×109/L). Factors correlated with the response to splenectomy were the corticoid dose required to obtain platelet counts >50×109/L, treatment received (prednisone alone or associated with other treatments) and duration of disease. In multivariate analysis, preoperative platelet count >20×109/L can predict postoperative response123 (B).
Three variables have a prognostic value to predict complete response after splenectomy in patients with ITP: a rapid response, in which platelet counts increase more than twice compared to preoperative counts within seven days after splenectomy, recurrence after preoperative treatment and a platelet count >150×109/L in the postoperative period124 (B).
One month after splenectomy in patients with ITP, 81% of patients achieved complete response, 12% had partial responses and 7% were unresponsive (platelet count <50×109/L). The mean age at the time of splenectomy for the responding patients was 30.31 years compared to 58.00 years in the unresponsive patients (p-value<0.01). The duration of the disease was higher in patients with no response compared to responders (90.80 months vs. 13.73 months; p-value<0.05). The duration of corticosteroid treatment was also higher among non-responders (48.00 months vs. 8.04 months; p-value<0.05). The preoperative platelet count was comparable between responsive and nonresponsive patients. Moreover, platelet counts at 6, 12, and 24 months after surgery were superior in responders compared to unresponsive patients125 (B).
Among the significant factors that influenced the response to splenectomy in 167 adult patients with persistent or chronic ITP, 115 (68.9%) of whom had a sustained response for a median of 8.8 months (range: 1–108 months), were being female (p-value=0.015), corticoresponsive ITP (p-value=0.043) and greater platelet count at the time of splenectomy54 (B).
RecommendationAge, preoperative platelet count, immediate postoperative platelet count, previous response to corticosteroids and preoperative IV immunoglobulin, and the time between diagnosis and splenectomy are possible prognostic factors of response to splenectomy.
Is there any indication for vaccination in patients who will undergo splenectomy? Which vaccines are indicated?P: Patients with ITP before splenectomy
I: Vaccination (meningococcal, pneumococcal)
C:
O: Prevention of infections after splenectomy
Splenectomized adults received the pneumococcal vaccine containing 23 pneumococcal serotypes (1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F and 33F) with significant postoperative serological response in all indications for splenectomy, including lymphoma, autoimmune hemolytic anemia, ITP, and trauma126 (C).
Splenectomized adults (60% lymphomas, 8% hemolytic anemia and 30% ITP) were immunized prior to surgery with a 14-valent pneumococcal vaccine containing 23 pneumococcal serotypes: 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F and 33F. The level of anti-pneumococcal antibodies after splenectomy demonstrated 28% of poor responders (no increase in antibodies at one year). Although the other patients responded to the vaccine, the level of antibodies reduced in one year (<0.7 units) for 10% of the responders, who were then revaccinated. Weak responders had no serological response to vaccination. During the follow-up, pneumococcal infection (pneumonia) occurred in 10% of cases, all of whom had not responded to the vaccine (risk of infection: 3.2% in 156 person-years)127 (B).
The first line treatment for patients with ITP was medication with those who did not respond to steroids with or without IV immunoglobulin for a period of three months being submitted to splenectomy. These adult patients were vaccinated eight days before surgery with the 23-valent polysaccharide vaccine against Streptococcus pneumoniae and the vaccine against Haemophilus influenzae type B. The overall morbidity of these patients was 9%, with a 100% response, without bleeding or infection, over a 427-day follow-up128 (C).
Indications for splenectomy in over 16-year-old patients were: (1) solid neoplasms; (2) hematological malignancies; (3) hematological diseases (hemolytic and aplastic anemias, platelet abnormalities) and (4) trauma. The patients received the valiant-23 pneumococcal vaccine. The response was defined as an increase in specific pneumococcal IgG titers >395U/mL. The proportion of patients that responded was 70% of the cases. No significant difference was found between responders to primary vaccination or revaccination (70.6% vs. 63.6%; p-value=0.73). Non-response to vaccination in immunosuppressed patients compared to non-immunosuppressed patients was 3.19 (95% CI: 1.04–9.73). Non-response to vaccination in patients with hematological malignancies compared to patients with non-malignant hematological diseases was 7.37 (95% CI 1.71–31.7)129 (B). Splenectomized adult subjects who received a dose of serogroup C meningococcal conjugate vaccine compared to vaccinated non-splenectomized patients showed significantly lower protective titers (serogroup C specific IgG) (157.8 vs. 1448.2; p-value=0.001) with an 83% lower titer compared to non-splenectomized patients. However, 80% of splenectomized patients achieved a protective titer ≥8 at follow-up. Patients who did not achieve a titer ≥8 (20%) received a second dose of vaccine with a response in 61% of the cases. In total, 93% of splenectomized patients achieved protective titers ≥8 after vaccination against meningococcus130 (B).
Invasive infections occurred in 3.2% of patients splenectomized for different reasons with an overall mortality of 1.4% in a mean follow-up time of 6.9 years; 48.3% were children (under 16 years of age) and 51.7% were adults. The incidence of infection among children and adults was similar, but mortality was higher in children (1.7%) compared to adults (1.3%; p-value<0.001). The incidence of infection was higher among patients with thalassemia major (8.2%) and sickle cell anemia (7.3%) (p-value<0.01). The lowest incidence of infection occurred in patients submitted to splenectomy for immune thrombocytopenic purpura (2.1%) and trauma (2.3%). The highest mortality rates were observed among patients with thalassemia major (5.1%) and sickle cell anemia (4.8%) (p-value<0.01). The lowest mortality rates were observed among the patients splenectomized for trauma (1.1%), ITP (1.2%) and spherocytosis (1.3%) (p-value<0.00001). The incidence of sepsis was higher among children with thalassemia major (11.6%) and sickle-cell anemia (8.9%) (p-value<0.01) and in adults, 7.4% and 6.4%, respectively (p-value<0.01). Streptococcal pneumonia was responsible for most infections (66%) with 55.3% mortality. The highest mortality, however, was attributed to gram negative bacteria (62%) and Neisseria meningitidis (58.8%; p-value=0.017)131 (B).
Patients without spleens (children and adults) had previously been immunized with pentavalent-23 pneumococcal vaccine (97.3% of cases); 82% received Haemophilus vaccine and 62% were vaccinated against N. meningitidis. All patients received pentavalent-7 pneumococcal vaccine. After splenectomy, invasive pneumococcal infections occurred in 5% of cases. High concentrations of specific serotype IgG were observed prior to the pentavalent-7 pneumococcal vaccine, with significant increases in mean concentrations of pentavalent-7 pneumococcal serotypes after vaccination. No significant increase was observed after the pneumococcal pentavalent-23 vaccine. Individuals without spleens responded well to pentavalent-7 vaccine, however sufficient levels of protection were identified in these patients following pentavalent-23 pneumococcal vaccination132 (C).
Heptavalent pneumococcal conjugate vaccine was initially used and then valiant-23 pneumococcal to immunize patients undergoing splenectomy for ITP, Hodgkin's disease, non-Hodgkin lymphoma, hemolytic anemia, or hypersplenism. The influenza vaccine is trivalent inactivated. Of the patients, 72% were over 18 years old. The most common hematologic diagnosis was hypersplenism (78% children, 44% adults) followed by ITP (11% children, 23% adults). Splenectomy for ITP was the most common indication (4.04 splenectomies/1000 person years). Immunization against S. pneumoniae was performed in 16.5% of splenectomized patients. Immunization against Influenza was performed in 53.1% of these patients. Infectious episodes were more common in splenectomized patients compared to non-splenectomized patients (151 visits/100 person-years vs. 120 visits/100 person-years; p-value<0.0001). Among splenectomized patients, pneumococcal immunization was associated with reduced risk of death when the effect of the influenza vaccine was not considered. However, no benefit from the pneumococcal vaccine was observed when the analysis was adjusted for the effect of the influenza vaccine. The risk of death was reduced among splenectomized patients who received an influenza vaccine133 (B).
The immunological response of splenectomized patients with congenital asplenia (adults and children) to the H. influenzae b-type conjugate vaccine demonstrated that prior to vaccination the mean antibody concentration was 3.21μg/mL and that after vaccination it rose significantly to 6.78μg/mL. Four and a half years after vaccination, the antibody concentration was similar to that of unvaccinated children134 (B).
Splenectomized patients (children and adults) who received the pneumococcal vaccine and who developed severe pneumococcal infections were compared with vaccinated non-splenectomized patients who also developed severe pneumococcal infections. The main indications of splenectomy were: Hodgkin's disease, ITP, hemolytic anemia and trauma. Of the splenectomized patients, 50% had pneumococcal sepsis, 25% pneumococcal meningitis and 25% had both. All patients received vaccines containing the pneumococcal antigen 12 or 14. Of the pneumococci isolated in the patients with infection, 70% were of the types included in the vaccine. The most common serotypes were 6A, 14, 19F and 23F, whereas in the 30% of infections with serotypes not included in the vaccine, the 22F serotype was the most common. The mean interval between vaccination and pneumococcal infection was ten months for the serotypes included in the vaccine and 6.7 months for the non-included serotypes. Comparatively, in non-splenectomized patients who had received pneumococcal vaccines, 60% had pneumonia due to S. pneumoniae, 30% had sepsis and 10% had meningitis. All patients had been vaccinated with a 12 or 14-valent vaccine; vaccine-specific pneumococcus were isolated in 65% of cases and serotypes not included in the vaccine in 35% of cases. The most common vaccine-related serotypes isolated were 6A, 19F and 23F, and the isolates unrelated to the vaccine were 6B and 19A. The mean interval from vaccination to pneumococcal infection was 7.4 months135 (B).
Of the adults who received pneumococcal vaccinations because of splenectomy, 50% were vaccinated for up to five years and 50% between five and ten years. Of the children who received pneumococcal vaccinations because of splenectomy, 30% were vaccinated for up to five years (requiring re-vaccination in 20% of cases) and 70% between five and ten years (requiring re-vaccination in 63% of the cases). Of the patients who received pneumococcal vaccinations, 52% had presumed protective levels of antibodies. The mean interval between splenectomy and pneumococcal vaccination was 23±8 days when given prior to splenectomy and 78±3 days when given after splenectomy136 (B).
RecommendationDue to the increased risk of infections by S. pneumoniae, H. influenzae and N. meningitidis in splenectomized patients (children and adults), vaccination against these infectious agents is indicated and should be performed prior to splenectomy. Vaccination against S. pneumoniae is not 100% effective in protecting against infections by all serotypes.
Is there evidence of the benefit of performing bone marrow transplantation (BMT) in adult patients with refractory ITP? In what situations can BMT be considered as a therapeutic alternative for refractory ITP?P: Patients with refractory ITP
I: Bone marrow transplant
C:
O: Prevention of bleeding events and thrombocytopenia control
Patients with refractory autoimmune cytopenia received autologous or allogeneic transplantation to modify the course of the disease. Patients were followed up for at least three months after transplantation. These patients received 38 transplants, one patient underwent mobilization for autologous transplantation without receiving a transplant after achieving a complete response and three patients underwent an allogeneic transplant after an autologous transplant failed. First transplants were autologous in 26 patients and allogeneic in nine. Indication was for several different types of autoimmune hematological cytopenias including hemolytic anemia (5 autologous; 2 allogeneic), Evans syndrome (2 autologous; 5 allogeneic), ITP (12 autologous2), pure red cell aplasia (4 autologous; 1 allogeneic), pure leukocyte aplasia (1 autologous; 1 allogeneic) and thrombotic thrombocytopenic purpura (3 autologous). There were important differences between the patients selected for autologous and allogeneic transplantations. Autologous transplant recipients were older (mean age: 31 years; range: 4–48 years) than allogeneic transplant recipients (mean: 14 years; range: 2–57 years) and had longer durations of disease [83 months (range: 3–299) vs. 16 months (range: 2–119)]. All patients underwent several previous treatments without response. The results of peripheral blood counts were collected at 1, 3, 6 and 12 months after transplantation and annually thereafter. Complete remission was defined as blood count normalization [hemoglobin (Hb)>12g/dL, neutrophils>1.5×109/L and platelets>150×109/L]; partial remission was an improvement to levels greater than 8g/dL for Hb, 50×109/L for platelets and 0.5×109/L for neutrophils. Progression-free survival was defined as being alive without disease progression. Mortality was defined as death from any cause. Treatment-related mortality was defined as death without disease progression. Because of the difficulty in assessing whether death due to hemorrhagic complications in patients with thrombocytopenia should be attributed to the underlying disease or to treatment, these events were counted as treatment-related mortality. Of the autologous transplant recipients, 10% died of treatment-related complications (patients with ITP died of cerebral hemorrhage and septicemia). Of the patients mobilized for autologous transplants, 30% did not show any evidence of response and 10% died of treatment-related complications; 20% had a transient response and 35% had a continuous response after transplantation. Among recipients of allogeneic transplants, 50% had a continuous response. Of the patients who did not respond or who relapsed after autologous transplantation, 40% underwent an allogeneic transplant. The five-year overall survival rates of the autologous and allogeneic transplant recipients were 84±15% and 78±28%, respectively. Progression-free survival of the autologous and allogeneic transplant recipients were 45±21%, and 78±28%, respectively137 (C).
A phase I–II study was performed to evaluate autologous bone marrow transplantation with B and T lymphocyte depletion. Fourteen 17- to 54-year-old patients with ITP were evaluated for more than six months with no lasting response after IV immunoglobulin, corticoid and splenectomy. All patients received four days of cyclophosphamide (50mg/kg/day) before the bone marrow infusion. Eight patients (57.1%) responded with a complete response in 6/14 (42.8%) and partial in 2/14 (14.3%). Adverse events included febrile neutropenia responsive to antibiotic therapy, hemorrhagic cystitis in one patient, vaginal bleeding in two patients, bleeding in the gastrointestinal tract in one patient and epistaxis in one patient104 (C).
RecommendationAlthough allogeneic or autologous bone marrow transplantation was performed as a therapeutic option in autoimmune cytopenias, its use has not been adequately studied in specific populations with refractory ITP to allow any recommendation.
In pregnant patients with ITP, is there a safe platelet count for vaginal or cesarean delivery?P: Pregnant women with ITP
I: Delivery as indicated by platelet count
C: Delivery as indicated by obstetrics
O: Prevent bleeding during labor
A retrospective study of 119 pregnancies of 92 pregnant women with mean age of 29 years (range: 26–32 years) were diagnosed with immune thrombocytopenic purpura before gestation (69.7%) or during pregnancy (30.3%) after other causes of thrombocytopenia had been excluded. Platelet counts were evaluated at the time of delivery with the mean count being 85×109/L (range: 61–104×109/L); 6.4% of women had a count <20×109/L and 9.1% had a count between 20 and 49×109/L105 (C). Of the 119 pregnancies, 82.4% were delivered vaginally and 17.6% were cesarean sections; the mean platelet counts were 88×109/L (range: 63–105×109/L) and 75×109/L (range: 54–100×109/L), respectively, without statistically significant difference between the interventions (p-value=0.16)105 (C). Hemorrhagic complications were rare and were not related to the degree of thrombocytopenia. The mean platelet count for pregnant women with previous diagnosis of ITP was 94×109/L compared to 69.5×109/L in the group without previous diagnosis (p=0.003)105 (C).
Another retrospective study evaluated the type of delivery performed and postoperative complications in 30 pregnant women with an average age of 28 years (range: 18–41 years) with ITP excluding incidental or gestational thrombocytopenia diagnosed during pregnancy138 (B). Of the 30 deliveries (37% vaginal, 42% elective C-sections for hematological reasons and 21% C-sections justified for obstetric reasons), 26.3% of women who underwent vaginal delivery and 27.2% of those who underwent cesarean delivery, presented severe bleeding with transfusion needs; there was no significant difference between the groups138 (B).
RecommendationThere is no consistent evidence of safe platelet counts for C-section or vaginal delivery. However, there is no significant difference between the complications related to thrombocytopenia in both types of delivery.
In pregnant patients with ITP, is there a platelet count that allows for spinal anesthesia?P: Pregnant women with ITP
I: Epidural anesthesia
C:
O: Prevention of bleeding associated with spinal puncture during childbirth
A group of pregnant non-preeclamptic women with a platelet count <100×109/L were studied. Their platelet counts were analyzed on the day of delivery to choose the anesthetic technique (epidural, subarachnoid, general or absence of anesthesia) and type of delivery (vaginal or C-section)139 (C). Of the 75 patients evaluated, the etiology of thrombocytopenia included 65.3% with ITP, 26.6% with gestational thrombocytopenia and 8.1% with other causes (liver cirrhosis, nocturnal paroxysmal hemoglobinuria, antiphospholipid syndrome, or without diagnosis)139 (C). Of the women with platelet counts of 80–99×109/L, 91.9% performed regional anesthesia (spinal or epidural); those with platelet counts of 50–79×109/L, 48.1% underwent regional anesthesia with no pregnant women with a platelet count <50×109/L performed this type of anesthesia for delivery139 (C). Forty percent of women underwent C-sections and 60% delivered vaginally. There were no complications, such as neurological deficits or paralysis, related to anesthesia. This study suggests that in patients with ITP, without bleeding or other coagulopathies, a platelet count ≥50×109/L can be considered safe139 (C).
Thirty pregnant women with a diagnosis of gestational thrombocytopenia (66%), pre-eclampsia (20%), ITP (10%) or infection (3.4%) and platelet counts at delivery <100×109/L (range: 69–98×109/L) underwent regional anesthesia; none of the women had neurological complications140 (C).
In a cohort of 2929 pregnant women, 24 (0.8%) presented platelet counts <100×109/L (range: 18–99×109/L) due to preeclampsia associated with miscarriage and thrombocytopenia. Two women had thrombocytopenia due to ITP with platelet counts before delivery of 18 and 32×109/L. The patients with ITP were submitted to vaginal delivery and only one received epidural anesthesia. Considering all the diagnoses of thrombocytopenia of the 24 patients, only ten (42%) received platelet transfusions before the surgical procedure141 (C).
RecommendationThere is no consistent evidence of a safe platelet count for spinal anesthesia, but it is suggested that the safe platelet count is >50×109/L for patients with stable counts, no eclampsia and no history of bleeding.
Conflicts of interestDo Nascimento ACKV declares that she participates on an advisory board for Novartis and as a speaker for Amgen and Novartis. Villaça PR declares that she consults for Novartis and Amgen and is a speaker for GSK. The other authors have no conflicts of interest to declare in relation to this review.
(((Pregnancy OR Prenatal Care OR Gestation OR Postnatal Care OR Infant, Newborn))) AND (Purpura, Thrombocytopenic, Idiopathic OR Werlhof Disease OR Autoimmune Thrombocytopenic Purpura OR Purpura, Thrombocytopenic) AND (Therapy/broad[filter] OR Diagnosis/broad[filter] OR Prognosis/broad[filter] OR Etiology/broad[filter])=1173.
((Purpura, Thrombocytopenic, Idiopathic OR Werlhof Disease OR Autoimmune Thrombocytopenic Purpura OR Purpura, Thrombocytopenic)) AND (Prednisolone OR Methylprednisolone OR Prednisone OR Glucocorticoids) AND (Therapy/broad[filter] OR comparative study OR comparative studies OR Random*).
((Purpura, Thrombocytopenic, Idiopathic OR Werlhof Disease OR Autoimmune Thrombocytopenic Purpura OR Purpura, Thrombocytopenic)) AND (Dexamethasone OR Glucocorticoids) AND (Therapy/broad[filter] OR comparative study OR comparative studies OR Random*)
(((((Purpura, Thrombocytopenic, Idiopathic OR Werlhof Disease OR Autoimmune Thrombocytopenic Purpura OR Purpura, Thrombocytopenic))) AND (splenectomy) AND (Therapy/broad[filter] OR comparative study OR comparative studies OR random*))) NOT (((((((Purpura, Thrombocytopenic, Idiopathic OR Werlhof Disease OR Autoimmune Thrombocytopenic Purpura OR Purpura, Thrombocytopenic))) AND (splenectomy)) AND ((Therapy/broad[filter] OR comparative study OR comparative studies OR random*)) AND ((infant[MeSH] OR child[MeSH] OR adolescent[MeSH])))) OR (((((((Purpura, Thrombocytopenic, Idiopathic OR Werlhof Disease OR Autoimmune Thrombocytopenic Purpura OR Purpura, Thrombocytopenic))) AND (splenectomy)) AND ((Therapy/broad[filter] OR comparative study OR comparative studies OR random*)))) AND (Chronic Disease)))=662.
(Purpura, Thrombocytopenic, Idiopathic OR Werlhof Disease OR Autoimmune Thrombocytopenic Purpura OR Purpura, Thrombocytopenic) AND (CD20 antibody OR rituximab OR Mabthera) AND Random*=33.
Estratégia 1=(Purpura, Thrombocytopenic, Idiopathic OR Werlhof Disease OR Autoimmune Thrombocytopenic Purpura OR Purpura, Thrombocytopenic) AND (Sulfonyldianiline OR Diaminodiphenylsulfone OR Diaphenylsulfone OR 4,4′-Diaminophenyl Sulfone OR DADPS OR Sulfona OR Orsade Brand of Dapsone OR Dapson-Fatol OR Disulone OR Avlosulfone OR Dapsoderm-X OR Dapsone) AND (Therapy/Broad [filter] OR Random* OR Comparative Study OR Comparative Studies)=36 Selecionados: 7.
Estratégia 2=(Purpura, Thrombocytopenic, Idiopathic OR Werlhof Disease OR Autoimmune Thrombocytopenic Purpura OR Purpura, Thrombocytopenic) AND (Rho(D) Immune Globulin OR Immune Globulin, Rh OR Anti-D-Immunoglobulin OR Anti D Immunoglobulin OR Rh Immune Globulin OR Rhophylac OR ZLB Bioplasma AG Brand of Rho(D) OR Immune Globulin OR Rho(D) Immune Globulin Intravenous (Human) OR RhoGAM OR Gamulin Rh OR MICRhoGAM) AND (Random*)=23 Selecionados=8.
Estratégia 3=(Purpura, Thrombocytopenic, Idiopathic OR Werlhof Disease OR Autoimmune Thrombocytopenic Purpura OR Purpura, Thrombocytopenic) AND (Azathioprine OR Azothioprine OR Azathioprine Sulfate OR Azathioprine Sodium Salt OR Imuran OR Immuran OR Imurel) AND (Therapy/Broad [filter] OR Random* OR Comparative Study OR Comparative Studies)=156 – Selecionados=7.
Estratégia 4=(Purpura, Thrombocytopenic, Idiopathic OR Werlhof Disease OR Autoimmune Thrombocytopenic Purpura OR Purpura, Thrombocytopenic) AND (Cyclosporine OR Cyclosporin OR Cyclosporine A OR Neoral OR Sandimmune OR Sandimmun OR Restasis OR Sandimmun Neoral OR CyA-NOF OR CyA NOF OR CsA-Neoral OR OL 27–400) AND (Therapy/Broad [filter] OR Random* OR Comparative Study OR Comparative Studies)=175 – Selecionados=12.
Estratégia 5=(Purpura, Thrombocytopenic, Idiopathic OR Werlhof Disease OR Autoimmune Thrombocytopenic Purpura OR Purpura, Thrombocytopenic) AND (Cyclophosphamide OR Cyclophosphane OR Procytox OR Cyclophosphamide Monohydrate OR Sendoxan OR Cyclophosphamide, (S)-Isomer OR Cytoxan OR Endoxan OR Neosar OR NSC-26271 OR B-518 OR Cyclophosphamide, (R)-Isomer)=0.
Estratégia 6=(Purpura, Thrombocytopenic, Idiopathic OR Werlhof Disease OR Autoimmune Thrombocytopenic Purpura OR Purpura, Thrombocytopenic) AND (Danazol OR Danazant OR Azol OR Norciden OR Danoval OR Panacrine OR Danocrine OR Cyclomen OR Danatrol OR Danol OR Ladogal) AND (Therapy/Broad [filter] OR Random* OR Comparative Study OR Comparative Studies)=147 Selecionados=11.
Estratégia 7=(Purpura, Thrombocytopenic, Idiopathic OR Werlhof Disease OR Autoimmune Thrombocytopenic Purpura OR Purpura, Thrombocytopenic) AND (Vincristine OR Leurocristine OR Citomid OR Oncovin OR Oncovine OR Onkocristin OR Vintec OR Vincasar PFS OR Vincristin Bristol OR Vincristine Sulfate OR Vincrisul OR cellcristin OR Vincasar OR Farmistin) AND (Therapy/Broad [filter] OR Random* OR Comparative Study OR Comparative Studies)=263- Selecionados=11.
Estratégia 8=(Purpura, Thrombocytopenic, Idiopathic OR Werlhof Disease OR Autoimmune Thrombocytopenic Purpura OR Purpura, Thrombocytopenic) AND (mycophenolate mofetil OR mycophenolic acid morpholinoethyl ester OR RS 61443 OR Mycophenolate Sodium OR Myfortic OR Cellcept OR mycophenolate mofetil hydrochloride OR Mycophenolic Acid) AND (Therapy/Broad [filter] OR Random* OR Comparative Study OR Comparative Studies)=30 – Selecionados=8.
((drug resistance OR refractory OR recurrence)) AND (((Purpura, Thrombocytopenic, Idiopathic OR Werlhof Disease OR Autoimmune Thrombocytopenic Purpura OR Purpura, Thrombocytopenic)) AND (platelet OR platelets)) AND (Prognosis/broad[filter] OR Therapy/broad[filter])=657 – Selecionados=49.
(((Purpura, Thrombocytopenic, Idiopathic OR Werlhof Disease OR Autoimmune Thrombocytopenic Purpura OR Purpura, Thrombocytopenic)) AND (eltrombopag OR romiplostim)) AND Random*=61.
((Purpura, Thrombocytopenic, Idiopathic OR Werlhof Disease OR Autoimmune Thrombocytopenic Purpura OR Purpura, Thrombocytopenic) AND (((azobisformamide OR tetrahydroxybutylimidazole OR Mercaptopurine OR aminoguanosine OR abatacept OR acteoside OR alicaforsen OR Azaserine OR Azathioprine OR basiliximab OR benzonidazole OR bestrabucil OR bredinin OR brequinar OR Busulfan OR butenolide OR Cladribine OR Coformycin OR Complement OR Cyclophosphamide OR Cyclosporine OR Cytarabine OR daclizumab OR daltroban OR deflazacort OR enisoprost OR everolimus OR fingolimod OR Fluorouracil OR gemcitabine OR glimepiride OR leflunomide OR Methotrexate OR Muromonab OR mycophenolate mofetil OR pimecrolimus OR Sirolimus OR Tacrolimus OR Thalidomide OR Triamcinolone Immunosuppressive Agents OR Immunosuppressant* OR Immunosuppression* OR alemtuzumab) OR (Antineoplastic Combined Chemotherapy Protocols OR Antineoplastic Agents, Combined OR Agent, Combined Antineoplastic OR Agents, Combined Antineoplastic OR Antineoplastic Agent, Combined OR Combined Antineoplastic Agent OR Drug Combinations, Antineoplastic OR Antineoplastic Drug Combinations OR Antineoplastic Drug Combination OR Combinations, Antineoplastic Drug OR Drug Combination, Antineoplastic OR Combined Antineoplastic Agents OR Anticancer Drug Combinations OR Anticancer Drug Combination OR Drug Combination, Anticancer OR Drug Combinations, Anticancer OR Antineoplastic Combined Chemotherapy Regimens OR Antineoplastic Chemotherapy Protocols OR Antineoplastic Chemotherapy Protocol OR Chemotherapy Protocol, Antineoplastic OR Protocol, Antineoplastic Chemotherapy OR Protocols, Antineoplastic Chemotherapy OR Chemotherapy Protocols, Antineoplastic OR Cancer Chemotherapy Protocols OR Cancer Chemotherapy Protocol OR Chemotherapy Protocol, Cancer OR Chemotherapy Protocols, Cancer OR Protocol, Cancer Chemotherapy OR Protocols, Cancer Chemotherapy OR Combination Chemotherapy OR Drug Polytherapy OR Drug Polytherapies OR Polytherapies, Drug OR Polytherapy, Drug OR Therapy, Combination Drug OR Chemotherapy, Combination OR Chemotherapies, Combination OR Combination Chemotherapies OR Combination Drug Therapy OR Combination Drug Therapies OR Drug Therapies, Combination OR Therapies, Combination Drug OR Polychemotherapy OR Polychemotherapies OR Drug Therapy, Combination OR Combined Modality Therapy OR Multimodal Treatment)) AND (Therapy/broad [filter] OR Comparative study OR Comparative studies)))=1387 Selecionados=13.
(Pregnancy OR Prenatal Care OR Gestation OR Postnatal Care OR Infant, Newborn) AND (Purpura, Thrombocytopenic, Idiopathic OR Werlhof Disease OR Autoimmune Thrombocytopenic Purpura OR Purpura, Thrombocytopenic)=1670.
(((((Purpura, Thrombocytopenic, Idiopathic OR Werlhof Disease OR Autoimmune Thrombocytopenic Purpura OR Purpura, Thrombocytopenic))) AND (splenectomy))) AND (Survival Analysis OR Prognosis OR Risk)=694.
(((((Purpura, Thrombocytopenic, Idiopathic OR Werlhof Disease OR Autoimmune Thrombocytopenic Purpura OR Purpura, Thrombocytopenic))) OR (splenectomy))) AND (vaccinations OR vaccination OR immunization, active OR active immunization OR active immunizations OR immunizations, active OR vaccination OR vaccine)=906.
(Purpura, Thrombocytopenic, Idiopathic OR Werlhof Disease OR Autoimmune Thrombocytopenic Purpura OR Purpura, Thrombocytopenic) AND (bone marrow transplantation OR transplant OR transplantation)=631 – Selecionados=12.
(Pregnancy OR Prenatal Care OR Gestation OR Postnatal Care OR Infant, Newborn) AND (Purpura, Thrombocytopenic, Idiopathic OR Werlhof Disease OR Autoimmune Thrombocytopenic Purpura OR Purpura, Thrombocytopenic)=1670.
(Pregnancy OR Prenatal Care OR Gestation OR Postnatal Care OR Infant, Newborn) AND (Purpura, Thrombocytopenic, Idiopathic OR Werlhof Disease OR Autoimmune Thrombocytopenic Purpura OR Purpura, Thrombocytopenic)=1670.