Coronavirus disease 2019 (COVID-19) is an infection caused by severe acute respiratory syndrome coronavirus 2 (SARS-COV-2), which has sparked growing interest and concern in the international community.
Although the disease's involvement is predominantly respiratory, with the lungs being the most damaged organs in severe cases, it has been suggested that this infection has a hematological impact as well.1
Decreased lymphocyte counts, thrombocytopenia, and increased ferritin and d-dimer levels are the most common abnormalities.1
We present a case of COVID-19 associated with Evans syndrome (hemolytic anemia plus thrombocytopenia, both with autoimmune causes) and antiphospholipid antibodies.
Case reportA 30-year-old woman presented with upper respiratory symptoms. At the time of her first visit to the outpatient clinic, she reported that on March 23rd, she began to experience nasal congestion, a sore throat, a cough, and the loss of her taste and smell. These symptoms were mild, lasted for a few days, and resolved spontaneously.
Her medical history was unremarkable except for a deep venous thrombosis of the right lower limb that she experienced when she was 11 years old. It was unprovoked and confirmed by ultrasonography. The patient denied a familiar history of thrombosis.
No underlying cause was found at that moment. Both systemic lupus erythematosus (SLE) and a secondary antiphospholipid syndrome (APS) were ruled out. She received anticoagulant treatment with coumarins for nine years.
Her annual medical follow-ups, including her history, physical examinations, and laboratory evaluations, were normal in recent years. The last complete blood cell count performed in February 2019 was normal without anemia or thrombocytopenia.
On April 1st, 2020, the woman had gingivorrhagia, which was self-limited. On April 5th, incoercible epistaxis appeared, so she went to a hospital close to her home in the Asunción metropolitan area and was treated with nasal tamponade. Petechiae were found on her skin all over her body. A lab analysis showed moderate anemia (8g/dL), leukopenia (2200/mm3), and severe thrombocytopenia (2000/mm3). Basic coagulation tests were normal with a prothrombin time (PT) of 13.3s (activity: 90%), an activated partial thromboplastin time (APTT) of 38s, and 335mg/dL of fibrinogen.
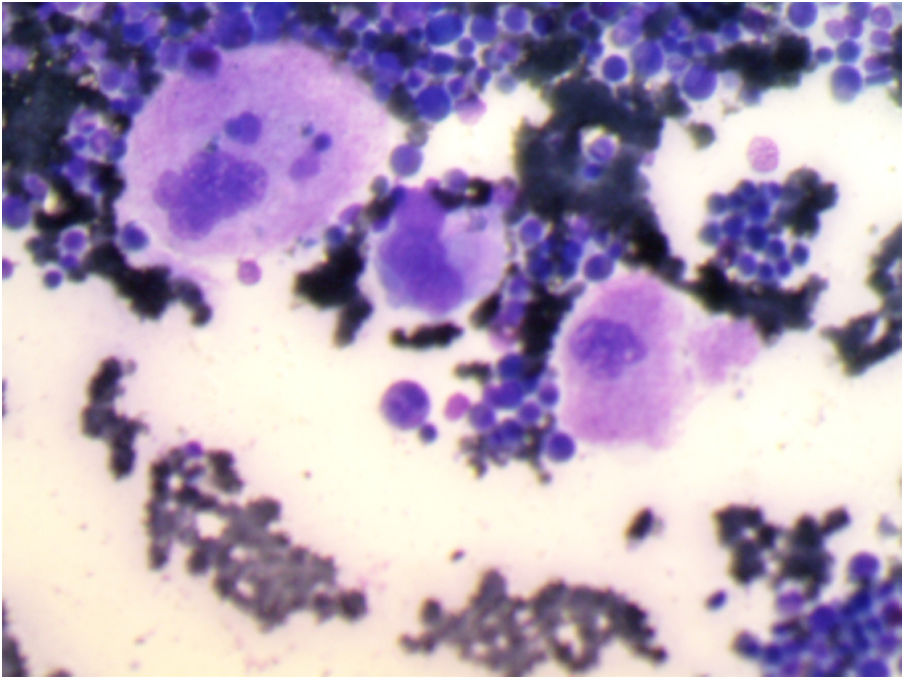
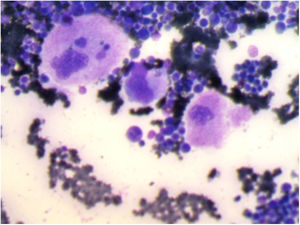
Bone marrow aspiration revealed increased cellularity, the relative hyperplasia of the erythroid precursors with dysplastic changes, myeloid precursors with normal maturation, and an increase in the number of megakaryocytes. Platelet detachment was not seen (Figure 1).
With the diagnosis of an immune thrombocytopenic purpura, treatment with 1g of methylprednisolone intravenously (IV) each day for three consecutive days was started, resulting in a decrease in bleeding and purpura.
On the third day of treatment, she reported that her father had tested positive for COVID-19. A Real-Time Polymerase Chain Reaction (RT-PCR) assay for SARS-COV-2 was performed and was found to be positive.
The patient was transferred to our hospital in the country's capital. Upon her admission, a physical examination showed a blood pressure of 100/70mmHg, a heart rate of 96beats/min, and a respiratory rate of 18breaths/min. Her skin and mucous membranes were pale. The pharynx was congestive. Her heart had a regular rhythm with no murmurs or gallops. Lung auscultation revealed bilateral rough vesicular murmurs. No adenomegalies or visceromegalies were palpable. In addition, petechiae were found on her skin all over her body.
Her laboratory results furthermore showed the following: 8.9g/dL of hemoglobin, a hematocrit of 25%, 10900/mm3 of white blood cells, a neutrophil count of 85%, a lymphocyte count of 15% (1635/mm3), 34000/mm3 of platelets, and a reticulocyte count of 7%. Increased neutrophils and platelets are likely due to methylprednisolone use a few days before.
The ferritin value was 194ng/mL, the PT was 81%, the APTT was 40s, the fibrinogen amount was 328mg/dL, and the d-dimer amount was 370ng/mL, and the liver and renal function tests were normal. The serology tests for the human immunodeficiency virus (HIV), hepatitis B and C, and VDRL were negative. The PCR amount was 6.16mg/L, and the procalcitonin amount was 0.02ng/mL.
The direct Coombs test was positive, and the following were also found: ANA+1: 320 with a homogeneous pattern, negative Anti DNA, a normal C3, a C4 amount of 8mg/dL (a normal value is 13–39mg/dL), an anti-cardiolipin IgG antibody>120U/mL (positive), an IgM anti-cardiolipin antibody>80U/mL (positive), a positive lupus anticoagulant, anti B2-glycoprotein 1, IgG antibody 90.3U/mL (positive), and an anti B2-glycoprotein 1 IgM antibody>120U/mL (positive). The anti Rho, Anti La, Anti Sm, Anca C and Anca P studies were negative.
Although she did not have respiratory symptoms during hospitalization, high-resolution lung tomography showed poorly delimited peripheral ground glass parenchymal infiltrates that involved the posterior segment of the left upper lobe, the apical segment of both lower lobes, and the lingula and the basal segment of the right lower lobe.
With these findings, her diagnoses upon admission were SARS-COV-2 infection, SLE with associated antiphospholipid antibodies, and Evans syndrome.
Empirical treatment was started with 500mg of azithromycin PO on day one followed by 250mg per day for four days, 400mg of hydroxychloroquine PO every 12h on day one followed by 200mg PO every 12h for the next four days, 100mg of prednisone PO once daily, and 1g of ceftriaxone IV every 24h.
As she had already experienced a significant decrease in bleeding and showed laboratory improvement, treatment with IV immunoglobulin was not performed. Despite the high risk of thromboembolic complications, it was decided not to start antithrombotic prophylaxis with low-molecular-weight heparin until the platelets reached a safety threshold, and it was recommended to avoid prolonged immobilization.
The clinical evolution and laboratory evolution were favorable. She showed good respiratory function with normal oxygen saturation without the need for any type of respiratory support.
On the third day of hospitalization, a significant increase in the number of platelets permitted the start of treatment with enoxaparin at prophylactic doses of 40mg every 24h.
The progressive improvement of the patient's health permitted her to be sent home. She was discharged with 50mg of prednisone daily, 200mg of hydroxychloroquine every 12h, and 40mg of enoxaparin daily. A close follow-up was indicated by all of the specialists involved in her care.
In early June 2020, a test for the detection of COVID-19 antibodies was performed, and she tested positive for IgG antibodies.
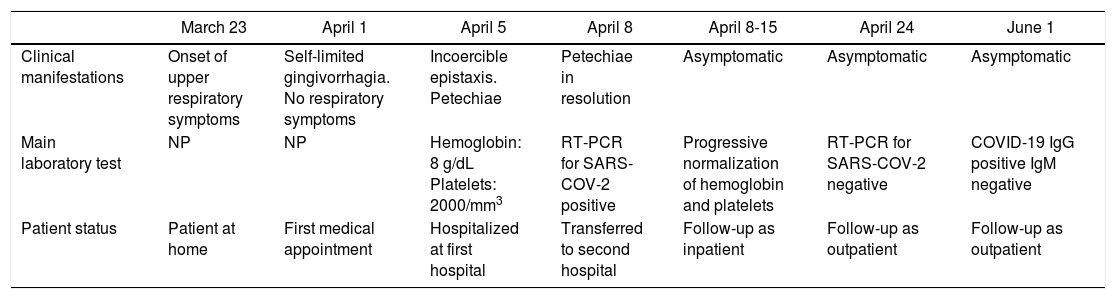
Table 1 shows a timetable of clinical manifestations and laboratory testing.
Timetable of clinical manifestations and laboratory testing.
| March 23 | April 1 | April 5 | April 8 | April 8-15 | April 24 | June 1 | |
|---|---|---|---|---|---|---|---|
| Clinical manifestations | Onset of upper respiratory symptoms | Self-limited gingivorrhagia. No respiratory symptoms | Incoercible epistaxis. Petechiae | Petechiae in resolution | Asymptomatic | Asymptomatic | Asymptomatic |
| Main laboratory test | NP | NP | Hemoglobin: 8 g/dL Platelets: 2000/mm3 | RT-PCR for SARS-COV-2 positive | Progressive normalization of hemoglobin and platelets | RT-PCR for SARS-COV-2 negative | COVID-19 IgG positive IgM negative |
| Patient status | Patient at home | First medical appointment | Hospitalized at first hospital | Transferred to second hospital | Follow-up as inpatient | Follow-up as outpatient | Follow-up as outpatient |
NP: not performed.
All dates refer to the year of 2020.
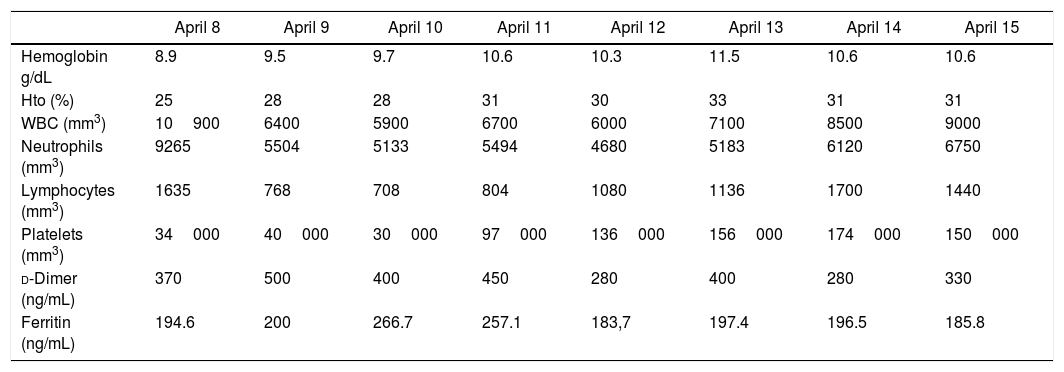
Table 2 shows the laboratory evolution during hospitalization.
Laboratory evolution during hospitalization.
| April 8 | April 9 | April 10 | April 11 | April 12 | April 13 | April 14 | April 15 | |
|---|---|---|---|---|---|---|---|---|
| Hemoglobin g/dL | 8.9 | 9.5 | 9.7 | 10.6 | 10.3 | 11.5 | 10.6 | 10.6 |
| Hto (%) | 25 | 28 | 28 | 31 | 30 | 33 | 31 | 31 |
| WBC (mm3) | 10900 | 6400 | 5900 | 6700 | 6000 | 7100 | 8500 | 9000 |
| Neutrophils (mm3) | 9265 | 5504 | 5133 | 5494 | 4680 | 5183 | 6120 | 6750 |
| Lymphocytes (mm3) | 1635 | 768 | 708 | 804 | 1080 | 1136 | 1700 | 1440 |
| Platelets (mm3) | 34000 | 40000 | 30000 | 97000 | 136000 | 156000 | 174000 | 150000 |
| d-Dimer (ng/mL) | 370 | 500 | 400 | 450 | 280 | 400 | 280 | 330 |
| Ferritin (ng/mL) | 194.6 | 200 | 266.7 | 257.1 | 183,7 | 197.4 | 196.5 | 185.8 |
All dates refer to the year of 2020.
We present the case of a young patient who was apparently in good health but had a history of venous thrombosis of unknown cause in her childhood, which started with Evans syndrome and a high titer of antiphospholipid antibodies, in coincidence with a SARS-COV-2 infection.
These hematological findings and the positivity of serological tests led to the diagnosis of SLE.
The concomitance of SLE and SARS-COV-2 does not necessarily mean that these conditions are related. The interesting aspect of this case is that in a situation where a new disease emerges as an epidemy, some symptoms related to a well-known disorder may be erroneously attributed to the emerging disease. Telling these diseases apart is not an easy task.
In addition to its widely known respiratory component, SARS-COV-2 infection affects the hematopoietic system.1
Regarding laboratory findings, lymphopenia and high ferritin levels have been related to poor evolution1,2 whereas high d-dimer has been associated with a marked clinical worsening.3 These hematological manifestations of COVID-19 were not present in our patient, although this could be explained because she presented a mild disease.
The pathophysiology of thrombocytopenia is unclear in patients with COVID-19, but Xu et al. suggested three possible mechanisms involved: first, the direct infection of the bone marrow cells by the virus and therefore the inhibition of platelet synthesis; second, the destruction of platelets by the immune system; and finally, platelet aggregation in the lungs, which results in the formation of microthrombi and the consumption of platelets.4 Although the decreased platelet count is usually mild in COVID-19, some cases of severe thrombocytopenia have been reported in the context of disseminated intravascular coagulation in these patients.5
Infected patients have a higher risk of suffering thromboembolic disease, so it is recommended that they receive antithrombotic prophylaxis with low-molecular-weight heparin.6
The marked positivity of the antiphospholipid antibodies was striking without evidence of arterial or venous thrombosis. We do not know the intimate mechanism, but one possible explanation could be the molecular mimicry and cross-reactivity between the virus and host B2-Glicoprotein-1. This would explain the induction of antiphospholipid antibodies in patients with COVID-19.7 Recently, Zhang et al. reported the cases of three severely ill patients with coronavirus who had antiphospholipid antibodies. All had had multiple strokes.8
The risk of developing antiphospholipid antibodies following viral infection has been demonstrated mainly in HIV and hepatitis C virus, but the mechanisms are unknown.9
It cannot be stated conclusively that the patient has APS because the increase in antiphospholipid antibodies could be only a transient alteration related to the current infection, or it could be related to SLE.
Obviously, our patient should be closely followed up to assess her evolution.
Finally, the history of venous thrombosis suggests that it could have an associated thrombophilia, which could never be demonstrated despite having been investigated.
Conflicts of interestThe authors declare no conflicts of interest.
Dr. Mario Ortega, Dr. Roberto López