Vitamin D (VD) is known to have multiple extra-skeletal health functions. There is emerging interest in exploring the relationship between vitamin D and chronic liver disease (CLD).
ObjectivesTo determine the prevalence of VD deficiency in patients with CLD in our setting and to assess whether VD supplementation influences plasma levels and is associated with improved liver function.
Material and methodsWe conducted a study in 2 phases. First, we analysed clinical and epidemiological characteristics in 94 patients with CLD; second, different doses of calcifediol (25-OH-VD) were administered to patients with VD deficiency (<20ng/mL) and insufficiency (20–30ng/mL). Plasma concentrations and liver function (Child–Pugh and MELD) at the end of treatment were compared with baseline data.
ResultsDeficient or insufficient VD levels were found in 87% of the patients, with an average concentration of 18.8ng/mL. Levels were lower in patients with cirrhosis (15.9ng/mL) (p=0.002) and in alcoholic liver disease. VD levels were inversely proportional to the degree of liver function: Child A (16.52ng/mL) vs Child C (7.75ng/mL). After VD supplementation, optimal serum levels were achieved in 94% of patients and significant improvements were observed in platelet count, albumin levels (p<0.05) and functional status assessed by the Child–Pugh scale (p<0.05).
ConclusionGiven the high prevalence of VD deficiency or insufficiency, the need for screening should be considered in the population with CLD. VD supplementation could be safe and effective.
La vitamina D (VD) participa en multitud de funciones extraesqueléticas en el organismo y cada vez es más importante su relación con las enfermedades hepáticas crónicas (EHC).
ObjetivosAnalizar la prevalencia de déficit o insuficiencia de VD en los pacientes con EHC de nuestra área. Evaluar si el aporte de VD influye en la concentración sérica y se asocia a mejoría de la función hepática.
Material y métodosRealizamos un estudio en 2 fases. En el primer tiempo se analizaron características clínico-epidemiológicas de 94 pacientes con EHC; en un segundo tiempo, se administraron diferentes dosis de calcifediol (25-OH-VD) a aquellos pacientes con déficit (<20ng/mL) e insuficiencia (20-30ng/mL) de VD. Se determinaron concentraciones plasmáticas, variables analíticas y de función hepática (Child-Pugh y MELD) al finalizar el tratamiento y se compararon con los datos basales.
ResultadosEl 87% de los pacientes tenían concentraciones deficitarias o insuficientes de VD, con una media de 18,8ng/mL, siendo menor en los cirróticos (15,9ng/mL) (p=0,002) y en la etiología por alcohol. Igualmente la concentración sérica de VD era inversamente proporcionales al grado de función hepática: Child A (16,52ng/mL) vs. C (7,75ng/mL). Tras el aporte con VD, se consiguió normalizar los niveles en el 94% de los pacientes, mejorar significativamente la cifra de plaquetas, de albúmina (p<0,05) y el grado funcional valorado por la escala de Child-Pugh (p<0,05).
ConclusiónDada la alta prevalencia de déficit o insuficiencia de VD debería plantearse la necesidad de cribado en la población con EHC. El aporte de VD podría ser seguro y eficaz.
Vitamin D (VD) is a lipid-soluble vitamin which, in addition to being an essential micronutrient, can also be considered a hormone involved in a complex system that regulates mineral homeostasis, protects skeletal integrity, and modulates cell growth and differentiation.1
We are currently witnessing a worldwide silent epidemic of VD deficiency. There is a widespread, though not undisputed, consensus that normal serum VD concentrations should be between 30–50ng/mL. VD insufficiency is considered to be levels of between 20 and 30ng/mL, and deficiency occurs when these fall below 20ng/mL.2 More than 1 billion people worldwide are estimated to have VD deficiency or insufficiency, with a higher risk in elderly patients or those with chronic diseases, such as chronic liver disease (CLD) or inflammatory bowel disease (IBD).3,4 Insufficient VD levels (20–30ng/mL) are almost universal in patients with CLD, and approximately two-thirds of this population have deficient levels (<20ng/mL). These levels drop further still in the case of advanced cirrhosis, and as hepatic dysfunction becomes more severe.5,6
There is growing interest in the non-classical or extra-skeletal functions of VD. Insufficient VD levels in CLD have been associated with an increase in bacterial infections,7 complications due to portal hypertension, severity of liver fibrosis and mortality.8 Given the increasingly important link between VD and liver disease, it seems appropriate to explore this relationship in patients with advanced CLD.
Our primary objective was to determine the prevalence of VD insufficiency or deficiency in patients with CLD in our health area, and to examine whether VD differed according to the aetiology of the CLD, presence of liver cirrhosis or grade of liver failure. In a second phase, we also examined whether VD supplementation could correct deficient levels, and if this led to an improvement in the CLD functional class.
Materials and methodsAll patients with CLD seen consecutively in the specialised liver clinic of our tertiary hospital (Complejo Asistencial Universitario de León, Spain) during the months of March and April 2014 were included in the study.
In the first phase, all patients with CLD seen in our clinic during the study period were registered. Patients receiving calcium or VD supplements were excluded. Plasma liver enzymes, albumin, creatinine and platelet levels were determined, as well as baseline serum VD concentrations (25-hydroxy-vitamin D [25-OH-VD]) and coagulation parameters. Various epidemiological variables, aetiology of the CLD, stage of the disease, presence or absence of cirrhosis, associated episodes of decompensation and need for treatment were recorded.
Normal (30–50ng/mL), insufficient (20–30ng/mL) or deficient (<20ng/mL) serum VD levels were established according to the consensus of the endocrinology societies.2
In the second phase, different doses of vitamin D3, calcifediol (25-OH-VD) were administered to patients with VD deficiency and insufficiency for approximately 3 months. Subjects with VD insufficiency were given 2285IU/day, while those with deficiency were given 3200IU/day. Plasma VD concentrations, and analytical and liver function variables (Child–Pugh, used since 1973, and model for end-stage liver disease [MELD] score from 2000) were determined at the end of treatment and compared with baseline data. Patients were interviewed by telephone 1 month after starting treatment to check dose adherence and compliance, the possible presence of adverse effects and to ensure that they had their follow-up blood test on the date indicated.9,10
Before receiving the VD supplements, all patients were informed of the foreseen beneficial effects, either during the consultation itself or when they came to our department to receive their assigned treatment regimen and instructions, and to have the follow-up blood test at the end of treatment.
Statistical analysis was performed using PASW Statistics 18 (SPSS). Qualitative variables were expressed as absolute number and percentage. Quantitative variables were expressed in terms of mean and standard deviation. The Chi square test was used to compare proportions relating to quantitative variables, and the Student t-test to assess differences between the means of the qualitative variables. The Mann–Whitney U test for related samples was used for intra-subject comparisons. Spearman correlation analysis was performed to assess the association between 2 quantitative variables. A p value<0.05 was considered statistically significant.
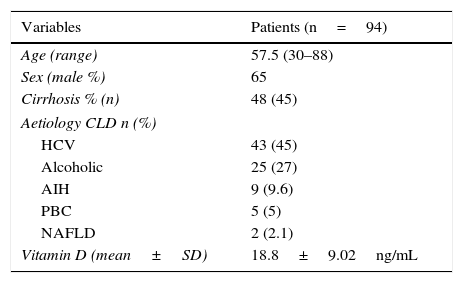
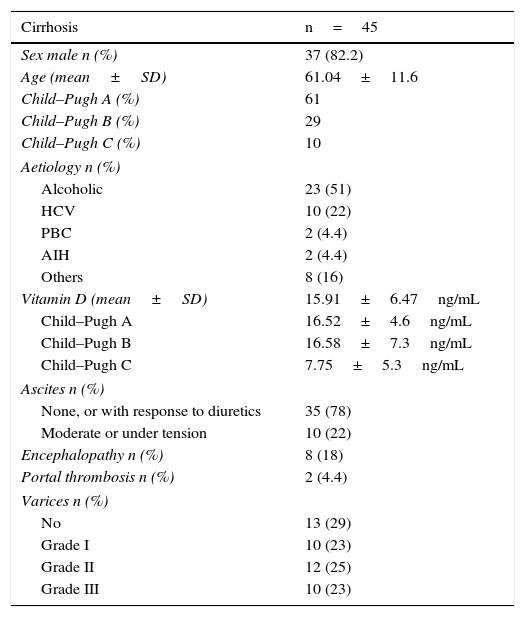
ResultsPopulation and baseline characteristicsA total of 94 patients with CLD were recruited during the study period; 65% were men, with a mean age of 57.5±13.7 years. Patient baseline characteristics are shown in Table 1. Almost half the patients in the series (48%, 45/94) had liver cirrhosis; 82% of these were men, all with compatible fibroscans (Table 2). Sixty-one percent of patients were Child–Pugh class A, 29% class B and 10% class C; 8.5% had a MELD score of over 20 and 55% had a score of less than 10. Of the subjects with liver cirrhosis, 29% had no varices. Thirty-five patients had ascites grade I, 2 patients had portal vein thrombosis and 4 had hepatocarcinoma.
Baseline characteristics of the study population.
| Variables | Patients (n=94) |
|---|---|
| Age (range) | 57.5 (30–88) |
| Sex (male %) | 65 |
| Cirrhosis % (n) | 48 (45) |
| Aetiology CLD n (%) | |
| HCV | 43 (45) |
| Alcoholic | 25 (27) |
| AIH | 9 (9.6) |
| PBC | 5 (5) |
| NAFLD | 2 (2.1) |
| Vitamin D (mean±SD) | 18.8±9.02ng/mL |
AIH, autoimmune hepatitis; HCV, hepatitis C virus; NAFLD, non-alcoholic fatty liver disease; PBC, primary biliary cirrhosis; SD, standard deviation.
Baseline characteristics of the cirrhotic population.
| Cirrhosis | n=45 |
|---|---|
| Sex male n (%) | 37 (82.2) |
| Age (mean±SD) | 61.04±11.6 |
| Child–Pugh A (%) | 61 |
| Child–Pugh B (%) | 29 |
| Child–Pugh C (%) | 10 |
| Aetiology n (%) | |
| Alcoholic | 23 (51) |
| HCV | 10 (22) |
| PBC | 2 (4.4) |
| AIH | 2 (4.4) |
| Others | 8 (16) |
| Vitamin D (mean±SD) | 15.91±6.47ng/mL |
| Child–Pugh A | 16.52±4.6ng/mL |
| Child–Pugh B | 16.58±7.3ng/mL |
| Child–Pugh C | 7.75±5.3ng/mL |
| Ascites n (%) | |
| None, or with response to diuretics | 35 (78) |
| Moderate or under tension | 10 (22) |
| Encephalopathy n (%) | 8 (18) |
| Portal thrombosis n (%) | 2 (4.4) |
| Varices n (%) | |
| No | 13 (29) |
| Grade I | 10 (23) |
| Grade II | 12 (25) |
| Grade III | 10 (23) |
AIH, autoimmune hepatitis; HCV, hepatitis C virus; PBC, primary biliary cirrhosis; SD, standard deviation.
With regard to the origin of the CLD, 46.2% was due to hepatitis C virus (HCV), 27% to alcohol consumption, 9.6% to autoimmune hepatitis and 5.4% to primary biliary cirrhosis; the remainder were caused by hepatitis B virus and non-alcoholic fatty liver disease. In patients with liver cirrhosis, 52% was secondary to alcohol and 22% was due to HCV.
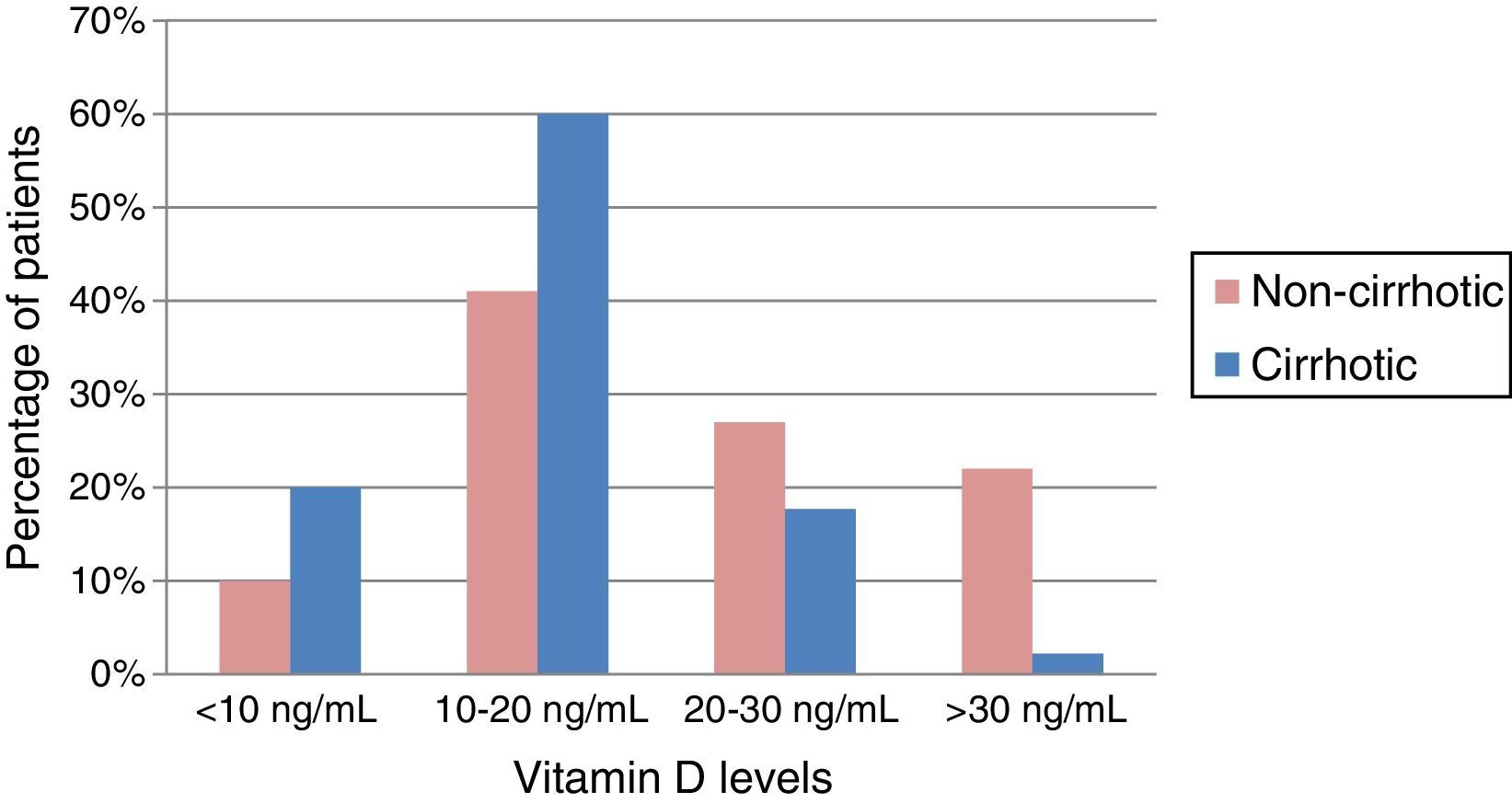
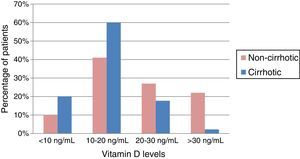
Baseline serum vitamin D concentrationOf the patients in our cohort with CLD, 87% had insufficient or deficient VD levels. The mean VD concentration in the series was 18.8±9.02ng/mL. In cirrhotic patients, the mean was 15.9ng/mL, lower than in non-cirrhotic patients, who had mean values of 21.6ng/mL; the difference was statistically significant (p=0.002). Among the cirrhotic patients, greater deficiency was observed when the CHD was due to alcohol consumption, with a mean VD concentration of 14.1ng/mL (p=0.002). Almost 80% of cirrhotic patients had VD deficiency, which was extreme (<10ng/mL) in 20% of cases, as shown in Fig. 1.
Relationship between vitamin D and liver functionVD values were found to be directly correlated with liver function grade, as shown in Table 2, since quantitative VD concentration was lower in patients with Child–Pugh class C (p=0.019). A direct correlation was also observed between plasma VD concentration and albumin and platelet values, and an inverse relationship with the international normalised ratio (INR) and bilirubin (p<0.005). Thus, patients with <50×109platelets/L had a serum VD concentration of 9.5±5.9ng/mL, while those with >100×109platelets/L had a mean concentration of 21.21±9.37ng/mL.
Response to vitamin D supplementsWe also determined the degree of response or normalisation of VD levels after administering the supplement in individuals with VD insufficiency or deficiency. Forty-four patients were treated with VD supplements at the previously indicated doses for 90 days. The mean dose used was 2914IU/day. After treatment, mean VD values were 81.89ng/mL, achieving normal values of >30ng/mL in 95.45% of patients. Values returned to normal in 93.8% of non-cirrhotic patients compared to 89.3% of cirrhotic patients, despite receiving lower doses; VD levels in non-cirrhotic patients were higher (p<0.05). The mean post-treatment serum concentration in non-cirrhotic patients was 109ng/mL, and 77.8ng/mL in cirrhotic patients; although doses considered toxic were exceeded, no adverse effects were recorded in any case. Patients with CLD secondary to alcohol consumption and as a result of HCV had the lowest VD levels. The grade of liver failure measured by the Child–Pugh score improved significantly in cirrhotic patients in whom the plasma VD concentration reached normal values (p=0.05), unlike the MELD scores, which fell from 11.02 to 8.96 (p=0.09); this did not reach statistical significance, probably due to the small sample size. Serum albumin levels >3.5g/dL were found in 92.6% of patients at the end of treatment (p<0.001); this was the only laboratory test parameter to significantly improve after VD supplementation. Treatment was discontinued in 3 patients: 1 for suspected hypercalcaemia and 2 due to tremors. However, no differences in blood calcium levels before and after treatment were observed. There were 3 deaths during the follow-up period as a result of cirrhosis-related complications.
DiscussionThe importance of VD lies in its many different functions, as it has been reported to control the expression of at least 200 genes involved in cell proliferation, differentiation, angiogenesis and immunomodulation.11,12 Thus, VD deficiency has been associated with an increased risk of autoimmune diseases, progression of certain types of cancer (colon, prostate and breast), an increase in cardiovascular phenomena, diabetes mellitus and even infections.13–16 Its anti-inflammatory and immunomodulatory functions and antifibrotic properties play an important role, particularly in the pathogenesis and treatment of a multitude of diseases, among them liver diseases.11,12
VD metabolism is well known. The main source of VD comes from exposure to sunlight. Through ultraviolet radiation, it is converted to vitamin D3 (cholecalciferol) in the skin. Another small proportion comes from the diet, in the form of vitamin D2 (ergocalciferol) or D3, which is absorbed in the small intestine due to the action of bile acids. VD can be stored in fat cells or can undergo primary hydroxylation in the liver to form the metabolite 25-OH-VD, its most abundant form. 25-OH-VD is biologically inactive and is used to determine the serum concentration of VD in peripheral blood.17 This metabolite undergoes a second hydroxylation in the proximal renal tubules, converting to 1,25 dihydroxy-vitamin D or calcitriol, which is biologically active.
As already mentioned, VD deficiency is a worldwide epidemic, and is even common among the young, healthy population. In the United States, between 25% and 50% of the adult population is estimated to have deficient VD levels.18 In liver disease, low VD values have been associated with a greater degree of liver fibrosis, higher risk of hepatic dysfunction, non-alcoholic fatty liver disease, osteodystrophy and hepatocarcinoma.7 In HCV infection, sub-optimal VD levels have been related with poor response to interferon- and ribavirin-based antiviral treatments.8,19,20 This is a controversial finding, as shown by the study by Melo-Villar et al.,21 in which the authors found that optimal VD levels are not an independent factor for achieving sustained viral response to antiviral treatment. However, according to Ladero et al.22 in 2012, VD levels do not appear to have any relationship with liver function tests, grade of fibrosis or IL28B genotype. Even in fatty liver disease, which is the main cause of CLD in developed countries, VD has been shown to reduce inflammation at systemic level, which could have an important role within the therapeutic options.23
Several studies have suggested a multifactorial origin for VD deficiency.24 This is not only due to hepatocyte dysfunction and inability to hydroxylate VD, but may also be due to intestinal malabsorption, excess urinary secretion, altered enterohepatic circulation, increased catabolism, decreased intake and lack of sunlight.23–25 Low serum VD concentrations have recently been related with high LDL and HDL cholesterol levels, and low alkaline phosphatase and haemoglobin levels.21 Some authors recommend treatment with VD supplements in patients with liver cirrhosis to prevent loss of bone mass.26
As we found in our cohort, most patients with CLD have insufficient serum VD concentrations. This was initially believed only to affect patients with cholestatic diseases, but there is evidence that patients with other CLDs, such as those caused by alcohol, HCV or steatohepatitis also have sub-optimal VD levels.27 In our study, which included patients with CLD of different aetiologies, 87% had insufficient VD levels (<30ng/mL), with this percentage being higher in cirrhotic patients. This finding is similar to that published by Arteh et al.,28 where prevalence of VD insufficiency was 92% overall, and higher in cirrhotic patients. These findings were replicated in another study by Miroliaee et al.,29 in which 68% of subjects had serum VD levels below the limit of normal; lower levels were also found in patients with more severe liver dysfunction. In the study by Melo-Villar et al.,21 66% of patients with HCV had VD levels <30ng/mL.
Similar to the findings presented previously by Malham et al.,7 in our cohort, the group of patients with CLD due to alcohol was the group with the greatest VD deficiency, with mean levels of 14ng/mL. This is probably due to the higher proportion of alcoholic cirrhotic patients in our setting, and to the known association between alcoholic cirrhosis and malnutrition, which may act as a coadjuvant factor to VD deficiency.30 Previous studies have also shown that low VD levels are directly related with the level of malnutrition in patients with CLD.26
The findings of most studies published to date, including ours, show that the functional grade of CLD is directly related with serum VD concentration. This occurs with laboratory parameters such as albumin and platelets. The opposite is true of INR and bilirubin levels, which are inversely related to VD levels.
All CLD patients presenting VD deficiency need to be treated. However, it should be noted that VD therapy is not without its risks, since excessive intake can trigger mild toxic effects such as nausea, weakness, constipation and irritability, and even serious effects such as symptomatic hypercalcaemia, bone loss or renal calculi.31 In our series, 95% of patients achieved normal serum concentrations after oral VD supplementation, while Ladero et al.,22 in a study of 108 patients with HCV, found that all achieved normal VD levels after vitamin supplementation, although normalisation of VD levels did not change RNA-HCV concentration or improve biochemistry tests. Our findings suggest a possible relationship between normalisation of VD levels and improvement in liver function measured by the Child–Pugh score. We observed an improvement in the Child–Pugh class as well as an increase in the plasma albumin concentration to a mean of 1.30g/dL. The therapy also seems to be safe, since even though the maximum level (100ng/mL) was exceeded in some cases, no toxicity symptoms were recorded. VD supplements were discontinued in only 3 cases due to suspected hypercalcaemia, although this was not confirmed analytically in any of the patients. It is important to note that in patients in whom a VD level of 100ng/mL was exceeded, laboratory tests following treatment discontinuation showed that the VD returned to optimal values around 50ng/mL.
Our study has certain limitations. Serum VD levels are known to vary greatly, and depend on exposure to sunlight. Data was collected and treatment administered at the end of winter and beginning of spring (mainly March and April), months that are characterised by few hours of sunlight due to the adverse weather conditions in the Madrid region, thus reducing the effect of exposure to sunlight. Patients were only followed up for a few months, so we had no access to long-term data. As a result, we do not know if VD supplementation should be given occasionally or periodically to maintain blood levels within an optimal range. We do not have fibrosis data measured by elastometry, which would have been useful to show a potential association between the grade of fibrosis and blood VD levels. Similarly, further studies are needed to determine whether it is the optimal VD levels that affect the liver function, or the improvement in CLD that increases serum VD levels. We were unable to confirm this due to the lack of a control group and randomisation, since the treatment was given within routine clinical practice.
In any case, we believe that our series represents the type of patients with CLD usually seen in our specialised clinics, so our findings could be applicable in other centres with similar population characteristics. Although we collected data from nearly 100 patients, fewer than half received treatment; therefore, a larger sample would have been more appropriate. Likewise, a number of factors are still unclear: the optimal dose of VD supplements in these patients, the baseline threshold to consider initiating treatment, its duration, and whether treatment should differ according to the aetiology of CLD.
In conclusion, given the high prevalence of VD sufficiency and/or deficiency in patients with CLD and the potential benefit of treatment, periodic screening to determine VD levels in this population seems advisable. Treatment with VD when required could be both safe and effective.
Conflict of interestsThe authors declare that they have no conflict of interests.
Please cite this article as: Fernández Fernández N, Linares Torres P, Joáo Matias D, Jorquera Plaza F, Olcoz Goñi JL. Déficit de vitamina D en la enfermedad hepática crónica, análisis clínico epidemiológico y tras aporte vitamínico. Gastroenterol Hepatol. 2016;39:305–310.