To examine the psychometric properties of a Spanish version of the C-SSRS (Sp-CSSRS).
MethodData are from a naturalistic, cross-sectional, multicentre, validation study, including 467 psychiatric outpatients, 242 of whom had a history of suicide attempt. The study measures were: C-SSRS; the Hamilton Depression Rating Scale (HDRS); the Beck Suicide Intent Scale; the Medical Damage Scale.
ResultsConstruct validity: Pearson coefficient between the C-SSRS severity (C-Sev) and intensity (C-Int) of ideation subscale scores was 0.44 (p<0.000) for the total sample. Likewise, Pearson coefficient between C-Sev score and HDRS item 3 was 0.56 (p<0.000). For the sub-sample of patients with suicide attempt, significant Pearson correlations were found between the C-Sev and the Beck Suicide Intent Scale scores (r=0.22; p=0.001). Discriminant validity: Significant differences were found in C-Sev and C-Int scores between patients with and without suicide attempt (p<0.000). The C-Sev score discriminated between patients based on HDRS item 3 (p<0.009). Sensitivity to change: Linear regression showed that a one-unit decrease in HDRS item 3 corresponded to a decrease of 5.08 units in the C-Sev score (p=0.141). A one-unit change in HDRS item 3 corresponded to a change of 13.51 on the C-Int assessments (p=0.007). Cronbach's alpha was 0.53 for C-Int. The principal component analysis identified 2 components that explain 55.66% of the total variance (C-Int).
ConclusionThe data support that the Sp-C-SSRS is a reliable and valid instrument for assessing suicidal ideation and behaviour in daily clinical practice and research settings.
Examinar las propiedades psicométricas de la versión en español de la escala C-SSRS (Sp-CSSRS).
MétodoEstudio de validación naturalista, transversal y multicéntrico. Muestra: 467 pacientes psiquiátricos ambulatorios (242 con tentativa de suicidio previa). Instrumentos: C-SSRS; Escala de Hamilton para la Depresión (HDRS); Escala de Intencionalidad Suicida de Beck; Escala de Gravedad Médica de la Tentativa (MDS).
ResultadosValidez del constructo: el coeficiente de Pearson entre la subescala de gravedad (C-Grave) y la de intensidad (C-Int) de la Sp-C-SSRS fue 0,44 (p<0,000). El coeficiente de Pearson entre C-Grave y el ítem 3 de la HDRS fue 0,56 (p<0,000). Para la submuestra de pacientes con tentativa de suicidio previa, se encontró una correlación estadísticamente significativa entre C-Grave y la Escala de Intencionalidad Suicida de Beck (r=0,22; p=0,001). Validez discriminante: se encontraron diferencias estadísticamente significativas entre las puntuaciones de C-Grave y de C-Int entre pacientes con tentativa de suicidio y sin ella (p<0,000). La puntuación de la C-Grave clasificó adecuadamente a los pacientes en función de su puntuación en el ítem 3 de la HDRS (p<0,009). Sensibilidad al cambio: la regresión lineal mostró que la disminución de una unidad en el ítem 3 de la HDRS se correspondió con la disminución de 5,08 unidades en la C-Grave (p=0,141). El cambio de una unidad en el ítem 3 de la HDRS se correspondió con un cambio de 13,51 unidades en la C-Int (p=0,007). El alfa de Cronbach fue 0,53. El análisis factorial de la C-Int identificó 2 componentes que explicaron el 55,66% de la varianza total.
ConclusiónLa Sp-C-SSRS es un instrumento fiable y válido para evaluar la ideación y la conducta suicidas en la práctica clínica y en contextos de investigación.
Suicide is a major public health issue, and reduction or prevention depends on accurate identification of at-risk patients. Use of a precise evaluation method of lifetime suicidal behaviour and ideation may provide more accurate determination of risk in clinical and research settings.1 However, assessment of suicide risk is a complex task. One of the most common problems that arises in risk assessment is the failure to adequately document clinical judgments and observations.2 Furthermore, use of psychometric scales to assess suicidal behaviour has yet to be incorporated into everyday clinical practice.3 Indeed, a survey of 400 psychiatrists showed that only 10% habitually use structured scales and questionnaires to assess suicide risk.4
A comprehensive mental status examination targeting not only the present condition, but also including questions about past suicidal behaviour is valuable from a preventive point of view, especially since a history of suicidal behaviour is the most reliably replicated risk factor for future suicide attempt or completion.5
To address both the inconsistencies in nomenclature and their impact on accurate identification of suicide risk, as well as the need for a measure to assess the severity of suicidal ideation and behaviour and to track changes in them, a team of investigators developed the Columbia-Suicide Severity Rating Scale (C-SSRS).6 The scale was designed to6: “(i) provide definitions of suicidal ideation and behaviour and nonsuicidal self-injurious behaviour and corresponding probes; (ii) quantify the full spectrum of suicidal ideation and suicidal behaviour and gauge their severity over specified periods; (iii) distinguish suicidal behaviour from nonsuicidal self-injurious behaviour; and (iv) employ a user-friendly format that allows integration of information from multiple sources”. These criteria are considered essential for judging the utility of scales assessing suicide, and the C-SSRS meets all of these criteria.6,7
Additionally, the C-SSRS has been used to compare the full range of suicidal ideation and suicidal behaviour across clinical trial visits. It also has criteria for mental health referral that have shown adequate predictive power.6 The C-SSRS is the prospective counterpart to the FDA-commissioned Columbia Classification Algorithm for Suicide Assessment (C-CASA)8 adverse event system. C-CASA suicide attempt classification is required in central nervous system and obesity trials.6 The neuropharmacology division of the FDA has specifically requested that any instrument used prospectively to monitor trial visits should link to C-CASA suicide attempt definitions. The C-SSRS is the only scale currently deemed to do so7 and thus is approved by the FDA for prospective monitoring.9
The C-SSRS has not been validated in Spanish, the third most commonly spoken language in the world. The aim of this study was to translate and validate a Spanish version of the C-SSRS for broad use, and to examine its psychometric properties (reliability, construct and discriminant validity, and sensitivity to change) in a sample of psychiatric outpatients.
MethodsDesign and participantsThe data come from a naturalistic, 3-month follow-up validation study conducted at 3 sites (Oviedo, Barcelona and Granada). Baseline evaluation was conducted from January to December 2012. The 3-month follow-up assessment was extended to the end of March 2013. It was approved by the ethics committee for clinical research of one of the sites (University of Oviedo) and performed in accordance with the Declaration of Helsinki as revised in 1983. Written informed consent was obtained from all subjects prior to enrolment.
The baseline sample comprised a total of 467 psychiatric outpatients: 242 patients with a history of suicidal behaviour and 225 patients without a history of suicide attempt (SA). Only one site could perform the 3-month follow-up (n=61).
Eligibility criteria for inclusion in the suicidal group were: minimum age 18 years, either sex; written informed consent to participate in the study; currently in outpatient psychiatric treatment.
Study procedures and measurementsThe translation and cultural adaptation of the C-SSRS was compliant with the rules of the International Test Commission.10–12 The original instrument was first translated into Spanish by two Spanish psychiatrists fluent in English. The Spanish version was sent to the original authors of the C-SSRS who back-translated the instrument into English and approved the final Spanish version. Note that one of the original authors is bilingual. The final Spanish version was then sent to all psychiatrists participating in the study for their opinion about the wording of the different questions. Finally, the Spanish version was pilot tested, administered to ten patients who were then debriefed, to assess the understandability of the Spanish-C-SSRS (Sp-C-SSRS).
Demographic data and clinical information regarding diagnosis, medication and drug use was obtained for each patient. All assessments were conducted by the same clinician at baseline and the 3-month follow-up. Sixty-one (n=61) patients with history of SA at baseline were included in the 3-month follow-up study. Previous suicide attempts were identified in clinical records.
- -
The Columbia-Suicide Severity Rating Scale (C-SSRS) is a semi-structured interview that captures the occurrence, severity, and frequency of suicide-related thoughts and behaviour during the assessment period.6 Therefore, four constructs are measured. The first is severity of ideation, with a subscale rated on a 5-point ordinal scale that reflects five types of ideation of increasing severity (from 1=wish to be dead, to 5=suicidal intent with plan). The second is the intensity of ideation, with a subscale comprising 5 items, two of them (frequency and duration) rated on a 5-point (1–5) ordinal scale, and the other three (controllability, deterrents, and reason for ideation) rated on a 6-point (0–5) ordinal scale. The third is suicidal behaviour, including a subscale rated on a nominal scale that includes actual, interrupted and aborted attempts, preparatory acts, and nonsuicidal self-injurious behaviour. The fourth is suicidal behaviour lethality, with a subscale assessing the level of actual medical damage of the attempt rated on a 6-point ordinal scale (from 0=no physical damage, to 5=death); if actual lethality is zero, potential lethality of attempt is rated on a 3-point ordinal scale.6
- -
The Hamilton Depression Rating Scale (HDRS) is a 17-item clinician-rated questionnaire useful for determining the severity of depression, scored from 0 (no depression) to 52 (very severe depression). It probes depressive symptoms such as suicidal ideation and behaviour (item 3). The rater enters a number for each symptom construct that ranges from 0 (not present) to 4 (extreme symptoms).13 The HDRS has been validated in Spanish.14 The 6-, 17- and 21-item versions of the Spanish-HDRS showed appropriate discriminative validity according to Clinical Global Impression scores. Adequate internal consistency was found with Cronbach's alpha (α) values ranging between 0.6 and 0.7, and test–retest and inter-rater reliability were good.14
- -
The Beck Suicide Intent Scale (SIS) is a 15-item interviewer-rated questionnaire designed to assess the severity of suicidal intent associated with an episode of deliberate self-harm.15 Each item is scored 0–2, giving a total score range of 0–30. The questionnaire is divided into two sections: the first eight items assess the objective circumstances of the suicide attempt (Part 1), while the remaining seven items (Part 2) are based on the patient's own reconstruction of his/her feelings and thoughts at the time of the act. The SIS has been validated in Spanish.16 The internal consistency of the total Spanish SIS was α=0.8. Factor analysis of the Spanish SIS yielded a two-factor solution, expected lethality and planning subscales. Internal consistency of both subscales was excellent (α=0.8) to good (α=0.7), respectively.16
- -
The Medical Damage Scale (MDS) is a 1-item interviewer-rated questionnaire designed to score medical damage (lethality) of a suicide attempt from 0 (none) to 8 (death).17 An MDS score ≥4 was used to define severe medical damage, as it signifies that the medical consequences were such that inpatient treatment was required. There is no validated Spanish version.
The analyses were performed using SPSS 17.0 (SPSS Inc., Chicago, IL). The two-tailed level of significance used was 0.05. Demographic and clinical information were compared between suicide attempters and non-attempters using chi-square or Student's t-tests, as appropriate.
The internal consistency of the Sp-C-SSRS ideation subscale was assessed using Cronbach's alpha coefficients. As stated by the original authors of the C-SSRS, the severity and behaviour subscales use an ordinal scale and are therefore not subject to internal consistency analysis.6
Construct validity was assessed by calculating Pearson correlation coefficients for the Sp-C-SSRS severity of ideation subscale score and: (1) the Sp-C-SSRS intensity of ideation score and (2) the HDRS item 3 (suicide-related) score. For the sub-sample of patients with SA, Pearson correlations were calculated for Sp-C-SSRS severity of ideation score and SIS and MDS total scores.
For analysing the dimensional structure of the Sp-C-SSRS intensity of ideation subscale, we used the principal component analysis (PCA) with Varimax rotation. The criteria employed to determine the number of components to extract were the Kaiser rule, the screen plot, and the interpretation of the components.
Discriminant validity was calculated to test if the Sp-C-SSRS discriminated between patients with and without suicidal behaviour. For analysing discriminant validity, patients were classified into two groups based on the presence of SA, and Student's t-test was calculated for Sp-C-SRRS severity and intensity of ideation scores. Likewise, Student's t-test was calculated to identify statistically significant differences in the Sp-C-SSRS suicidal behaviour lethality scores (medical damage) according to the MDS cut-off point (<4 or ≥4). Regarding the severity of suicidal ideation, an ANOVA test with Duncan's post hoc test was calculated between Sp-C-SSRS severity of ideation subscale scores and item 3 of the HDRS.
In order to assess sensitivity to change, two linear regression models were performed with item 3 of the HDRS as the independent variable. The dependent variables for each model were the Sp-C-SSRS severity and intensity of ideation subscale scores, respectively. Also, two interaction plots tested how item 3 of the HDRS, and the Sp-C-SSRS severity of ideation and Sp-C-SSRS intensity of ideation subscales varied from baseline to the 3-month follow-up.
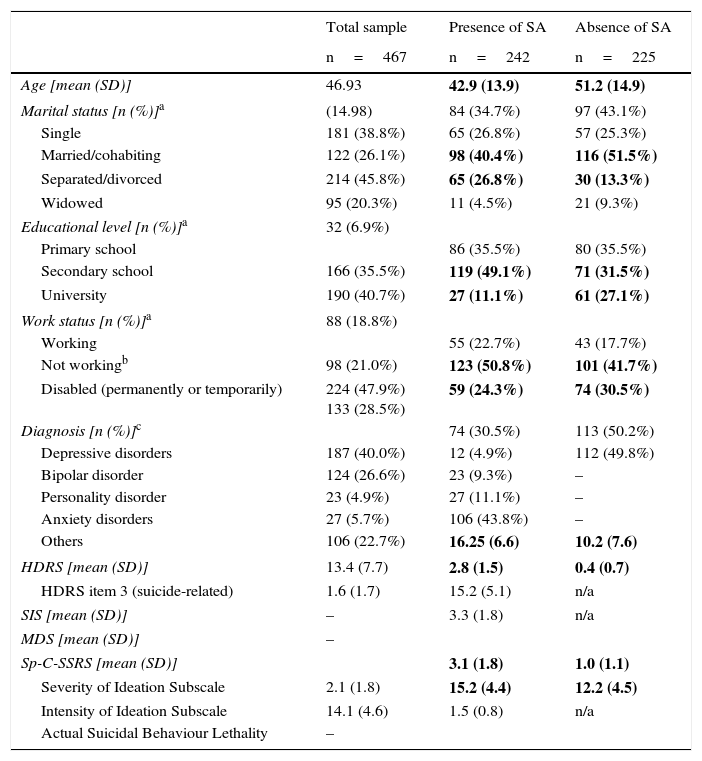
ResultsPatient demographic and clinical characteristicsTable 1 shows demographic and clinical characteristics of the total sample and by SA. Significant differences were found between patients with a history of SA and without a history of SA in the following variables: age (t=6.2; p<0.000), marital status (χ2=19.4; p=0.003), educational level (χ2=31.2; p<0.000), and work status (χ2=20.6; p=0.008). Significant differences were also found in both item 3 and HDRS total score (p<0.000), and in Sp-C-SSRS severity and intensity of ideation subscales (p<0.000).
Demographic and clinical characteristics of the sample and by presence or absence of suicide attempt (SA).
| Total sample | Presence of SA | Absence of SA | |
|---|---|---|---|
| n=467 | n=242 | n=225 | |
| Age [mean (SD)] | 46.93 | 42.9 (13.9) | 51.2 (14.9) |
| Marital status [n (%)]a | (14.98) | 84 (34.7%) | 97 (43.1%) |
| Single | 181 (38.8%) | 65 (26.8%) | 57 (25.3%) |
| Married/cohabiting | 122 (26.1%) | 98 (40.4%) | 116 (51.5%) |
| Separated/divorced | 214 (45.8%) | 65 (26.8%) | 30 (13.3%) |
| Widowed | 95 (20.3%) | 11 (4.5%) | 21 (9.3%) |
| Educational level [n (%)]a | 32 (6.9%) | ||
| Primary school | 86 (35.5%) | 80 (35.5%) | |
| Secondary school | 166 (35.5%) | 119 (49.1%) | 71 (31.5%) |
| University | 190 (40.7%) | 27 (11.1%) | 61 (27.1%) |
| Work status [n (%)]a | 88 (18.8%) | ||
| Working | 55 (22.7%) | 43 (17.7%) | |
| Not workingb | 98 (21.0%) | 123 (50.8%) | 101 (41.7%) |
| Disabled (permanently or temporarily) | 224 (47.9%) 133 (28.5%) | 59 (24.3%) | 74 (30.5%) |
| Diagnosis [n (%)]c | 74 (30.5%) | 113 (50.2%) | |
| Depressive disorders | 187 (40.0%) | 12 (4.9%) | 112 (49.8%) |
| Bipolar disorder | 124 (26.6%) | 23 (9.3%) | – |
| Personality disorder | 23 (4.9%) | 27 (11.1%) | – |
| Anxiety disorders | 27 (5.7%) | 106 (43.8%) | – |
| Others | 106 (22.7%) | 16.25 (6.6) | 10.2 (7.6) |
| HDRS [mean (SD)] | 13.4 (7.7) | 2.8 (1.5) | 0.4 (0.7) |
| HDRS item 3 (suicide-related) | 1.6 (1.7) | 15.2 (5.1) | n/a |
| SIS [mean (SD)] | – | 3.3 (1.8) | n/a |
| MDS [mean (SD)] | – | ||
| Sp-C-SSRS [mean (SD)] | 3.1 (1.8) | 1.0 (1.1) | |
| Severity of Ideation Subscale | 2.1 (1.8) | 15.2 (4.4) | 12.2 (4.5) |
| Intensity of Ideation Subscale | 14.1 (4.6) | 1.5 (0.8) | n/a |
| Actual Suicidal Behaviour Lethality | – | ||
Abbreviations: HDRS, Hamilton Depression Rating Scale; MDS, Medical Damage Scale; SA, suicide attempt; SD, standard deviation; SIS, Beck Suicide Intent Scale; Sp-C-SSRS, Spanish Columbia-Suicide Severity Rating Scale.
p<0.05.
Bold type shows significant differences.
Reliability judged by internal consistency (Cronbach's alpha) was 0.53 for the Sp-C-SSRS intensity of ideation subscale.
Construct validityPearson correlation coefficients between the Sp-C-SSRS severity and intensity of ideation subscale scores were 0.44 (p<0.000) for the total sample, 0.42 (p<0.000) for patients with SA, and 0.19 (p=0.026) for patients without SA. Regarding the C-SSRS severity of ideation subscale score and HDRS item 3, Pearson coefficients were 0.56 (p<0.000), 0.20 (p=0.002), and 0.57 (p<0.000), respectively, for the total sample, patients with SA, and patients without SA. For the sub-sample of patients with SA, significant Pearson correlations were found between the Sp-C-SSRS severity of ideation subscale and the SIS scores (r=0.22; p=0.001). For the MDS, the Pearson coefficient was not statistically significant (r=0.11, p=0.076). In this case, we employed data form 230 evaluation instead of 242.
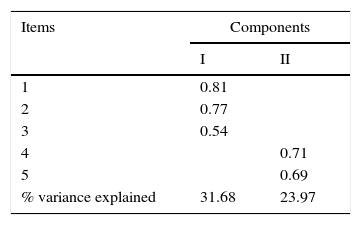
To identify the underlying internal structure of the Sp-C-SSRS intensity of ideation subscale, the principal component analysis with Varimax rotation was performed. The KMO's measure of sampling adequacy was 0.619 indicating that the data was suitable for factor analysis. Ther Bartlett's test of sphericity was significant (p<0.001), confirming that the variables were inert-correlated and therefore suitable for factoring. The principal component analysis identified two component that explain 55.66% of the total variance (factor loadings are shown in Table 3). The first component included the first three items and explained 31.66% of the total variance. The second component included items number 4 and 5 and explained 23.97% of the total variance.
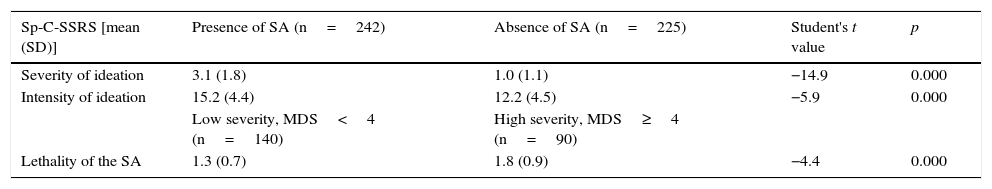
Discriminant validityTable 2 shows results of the analyses performed to calculate discriminant validity. The Sp-C-SSRS severity and intensity of ideation subscale scores discriminated between patients with and without SA. Moreover, significant differences were found in Sp-C-SSRS severity and intensity of ideation subscale scores between patients with and without SA (p<0.000). In addition, the Sp-C-SSRS suicidal behaviour lethality score discriminated among the different levels of medical lethality according to MDS scores (n=230). Likewise, those with high MDS scores based on the cut-off point had significant differences in the Sp-C-SSRS suicidal behaviour lethality score, compared with those who had low MDS scores. Similarly, the Sp-C-SSRS severity of ideation subscale score discriminated among patients of each severity group based on the HDRS (suicide-related item 3).
Discriminant validity of the Spanish Columbia-Suicide Severity Rating Scale (Sp-C-SSRS).
| Sp-C-SSRS [mean (SD)] | Presence of SA (n=242) | Absence of SA (n=225) | Student's t value | p |
|---|---|---|---|---|
| Severity of ideation | 3.1 (1.8) | 1.0 (1.1) | −14.9 | 0.000 |
| Intensity of ideation | 15.2 (4.4) | 12.2 (4.5) | −5.9 | 0.000 |
| Low severity, MDS<4 (n=140) | High severity, MDS≥4 (n=90) | |||
| Lethality of the SA | 1.3 (0.7) | 1.8 (0.9) | −4.4 | 0.000 |
| HDRS item 3 (suicide-related) | ANOVA F value | p | |||||
|---|---|---|---|---|---|---|---|
| Absent (n=25) | Life not worth living (n=44) | Wish to be dead (n=22) | Suicide ideas or gesture (n=10) | Attempts at suicide (n=141) | |||
| Severity of ideation | 1.9 (1.9) | 2.9 (1.6) | 3.0 (1.6) | 3.5 (1.6) | 3.3 (1.8) | 3.4 | 0.009a |
Abbreviations: HDRS, Hamilton Depression Rating Scale; MDS, Medical Damage Scale; SA, suicide attempt.
p<0.05.
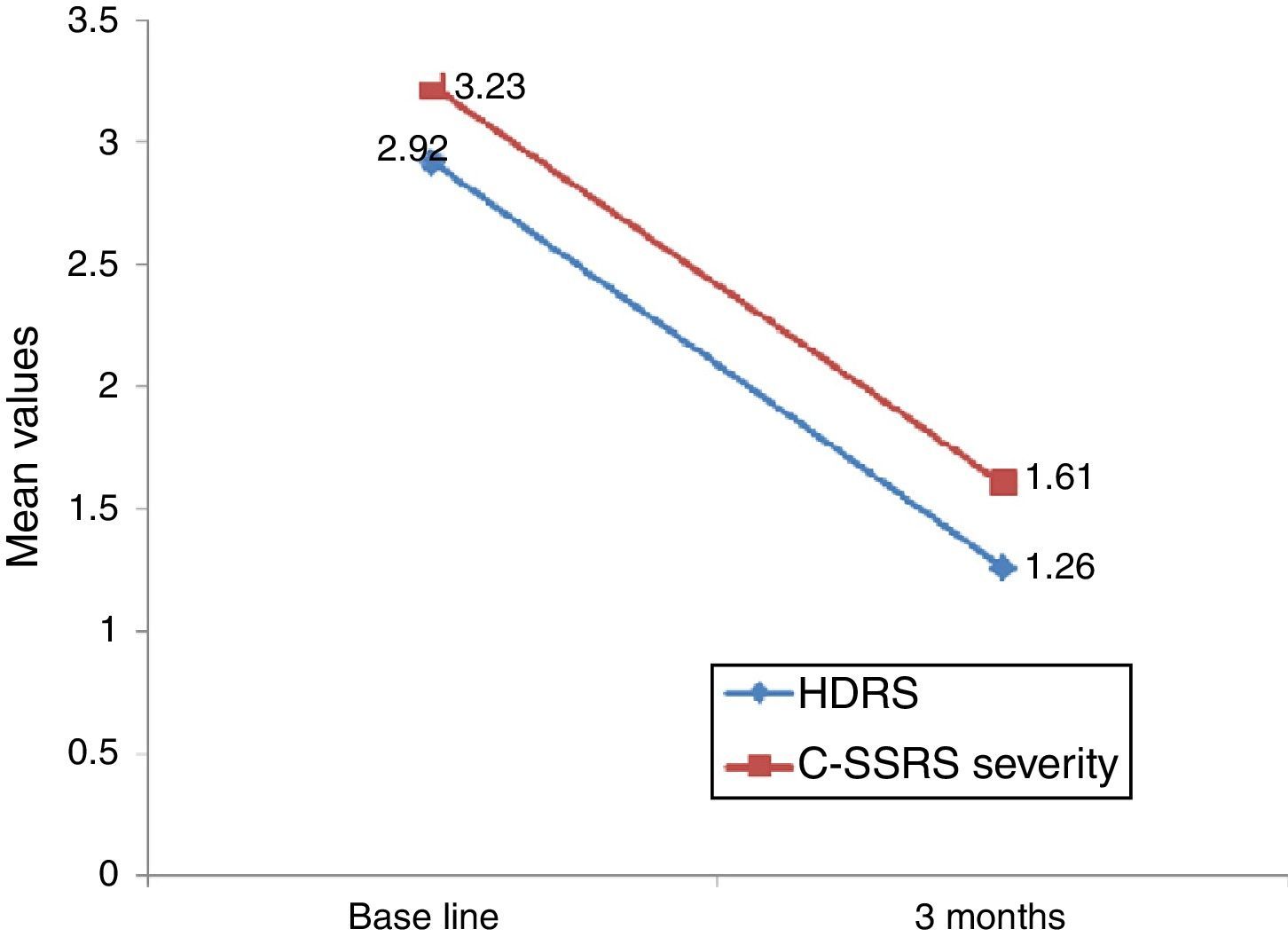
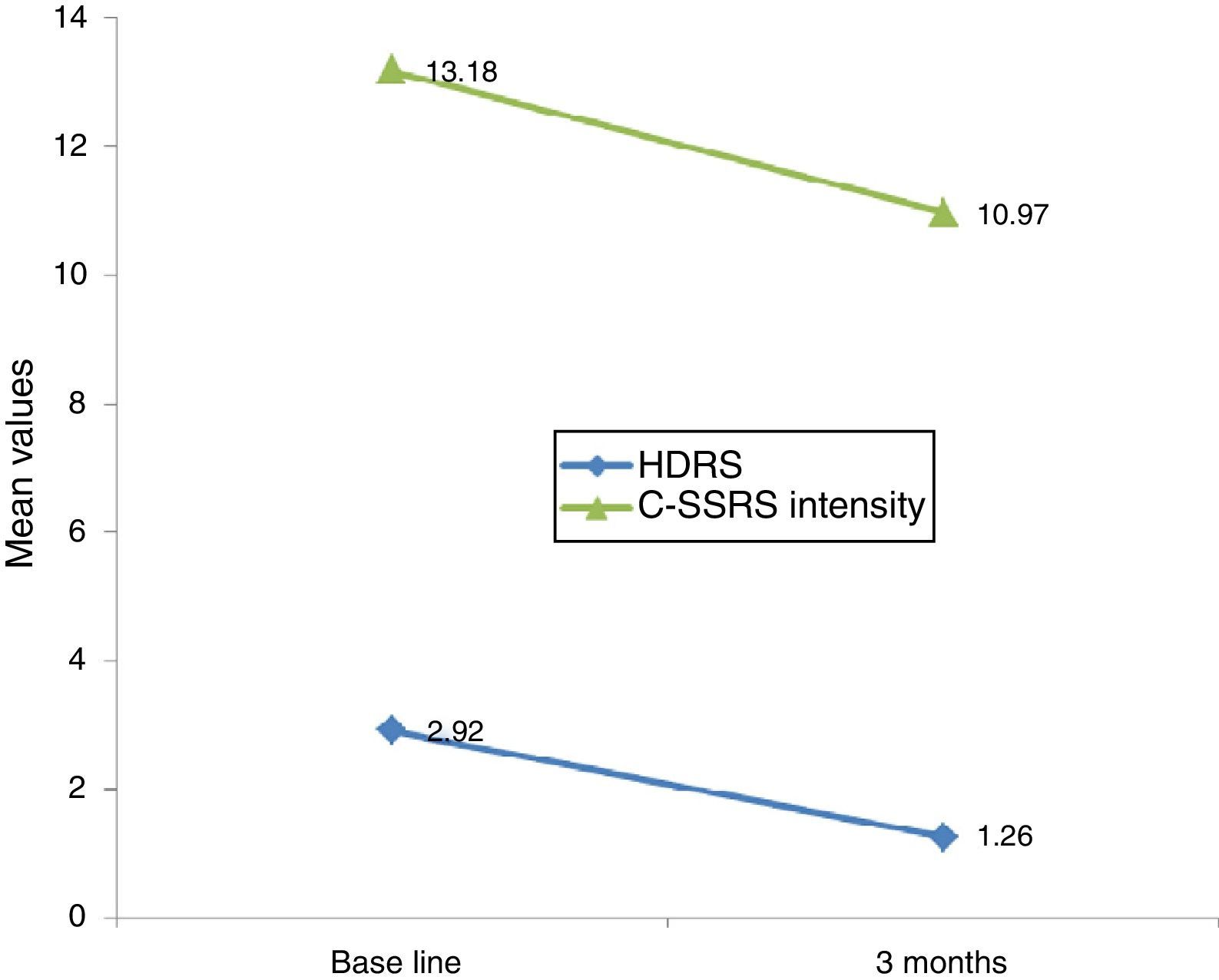
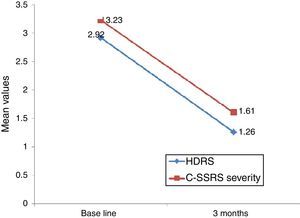
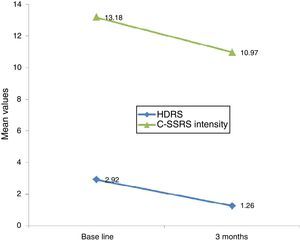
Linear regression showed that among suicide attempters, a one-unit decrease in HDRS item 3 corresponded to a decrease of 5.08 units in the Sp-C-SSRS severity of ideation subscale score (p=0.141). Similarly, a one-unit change in HDRS item 3 corresponded to a change of 13.51 on the Sp-C-SSRS intensity of ideation subscale assessments (p=0.007). Interaction plots showed that mean severity and mean intensity of ideation subscale scores responded similarly to the change in HDRS item 3: in the attempter subsample, an average decrease of 1.66 points in HDRS item 3 corresponded to a 1.62 point decrease in the Sp-C-SSRS severity of ideation subscale and a 2.21 point decrease in the Sp-C-SSRS intensity of ideation subscale (Figs. 1 and 2).
Suicidal behaviour is an important public health concern, and the development of valid methods for identifying patients at greater risk for engaging in suicidal behaviour has important implications for research and suicide prevention.1 However, the role of empathy is crucial when evaluating and treating suicidal individuals.18
We found adequate Sp-C-SSRS convergent and divergent validity, and sensitivity to change, as well as internal consistency of the severity and intensity of ideation subscales, which support the Sp-C-SSRS as a reliable and valid instrument for assessing suicidal ideation and behaviour in daily clinical practice and research settings.
There are many scales and measures that quantitatively and qualitatively measure the presence of suicidal ideation, intent, and motivation as well as the intensity, duration, frequency, and consequences of suicide attempts.19 Even when using the same measures across studies and across populations–as in this study–researchers often assume that all respondents are basing their responses on the same conceptualizations and definitions of suicide-related terms. The refinement of investigative tools and techniques can only improve their efficiency, effectiveness, sensitivity and specificity by clearly describing the studied populations and behaviours.19 In addition, a standardized nomenclature will lead to a standardized set of questions for determining the presence or absence of suicidal cognitions, motivations, emotions, and behaviours. Indeed, Silverman et al.20 provided a nomenclature built with an eye toward application in clinical research.
Posner et al.6 found good internal consistency for the C-SSRS intensity of ideation subscale with Cronbach's alpha values ranging between 0.73 and 0.93. However, in our sample the Cronbach coefficient value for the Sp-C-SSRS intensity of ideation subscale was 0.53, above what would be considered unacceptable. This rating reflects low internal reliability, showing that perhaps intensity of suicidal ideation is the most complex or heterogeneous aspect of suicidal behaviour. In this sense, it should be noted that three of the intensity subscale items assess “quantitative” aspects of the intensity of ideation such as frequency, duration, and controllability. Meanwhile, the other two items are more related to “qualitative” issues such as deterrents and reasons for ideation.
Regarding to this, the principal component analysis performed in the total sample confirms the existence of two components. The inconsistencies found in the internal reliability may be due to this new two-component solution. To the best of our knowledge, no previous studies have been done about this issue. We were able to obtain a one-component solution, even though the two-component showed a better fit, as shown in “Results” section. When examining the two-component solution, we observed that the first component, consisted of frequency, duration, and controllability, is more related to the characteristics of suicidal ideation, while the second component was represented by cognitive aspects (container vs content). These results suggest the need for further research on the structure of the C-CCSR intensity subscale.
We found lower internal validity for the intensity of ideation subscale. Because the samples in the original study6 and in this one are not similar or comparable, it was expected that the results would differ somewhat. The samples differed in at least 3 aspects: (i) Age: the original study6 was conducted with adolescents, while the mean age in our sample is nearly 47 years. It is known that scores tend to be less dispersed in adolescent samples than in others.21 Furthermore, educational level and personality characteristics of adolescents vs adults may also have led to differences. (ii) Cultural issues: the constructs of individualism and collectivism have been extensively researched.22 Empirical evidence supports the notion that Americans differ in terms of individualism and collectivism from those from Mediterranean countries (Spain). The US occupies the individualistic end of the spectrum while Spain would be in an intermediate position. However, prior data suggest a possible role of individualism in suicidality in Western societies.23 (iii) The same cultural influences may have been at work with respect to the clinicians conducting the evaluation, which may also have resulted in lower internal validity of the Sp-C-SSRS.
In fact, severity and intensity of ideation scores do not show correlation coefficients as high as expected (from 0.44 to 0.19). Nevertheless, in the initial study,6 C-SRRS severity and intensity of ideation subscales were moderately correlated (from 0.56 to 0.52). Pearson correlations for the Sp-C-SSRS scores were especially low in patients without any previous SA (0.19). Perhaps this reflects the complex relationship between suicidal ideation severity and intensity, which may generate different kinds of patient profiles (i.e. patients with a high score on the intensity of ideation subscale but no specific plan or intent to act, or patients with severe active suicidal ideation but with low frequency, duration, or controllability of those ideas).
In terms of construct validity, we found a moderate relationship between Sp-C-SSRS severity of ideation scores and the suicide-related item 3 of the HDRS, showing good convergent validity for the total sample. However, this correlation was lower for patients with SA (0.20). This finding is likely due to the fact that the C-SSRS severity of ideation subscale provides a retrospective lifetime assessment of the severity of suicidal behaviour, whereas the suicide-related item 3 of the HDRS rates only the past 2 weeks. Supporting this hypothesis, in our study, the best correlation was found in the subsample of patients without SA.
The Sp-C-SSRS severity of ideation subscale moderately but significantly correlated with the SIS (this analysis was conducted only for patients with SA). Posner et al.6 also found only moderate convergent validity between these two measures. Thus, this finding was expected and was likely due to non-overlap in items and to different operationalization of construct.6 Namely, the C-SSRS severity of ideation subscale was designed to reflect five different types of ideation of increasing severity whereas the SIS was designed to measure the seriousness of the intent to commit suicide among patients who have actually attempted suicide.
On the other hand, divergent validity with measures of other constructs is essential.6 We found that the Sp-C-SSRS severity of ideation subscale discriminates among the five levels of the HDRS suicide-related item 3 scores in the expected direction. Patients scoring higher on HDRS item 3 (“suicide ideas or gesture” and “attempts of suicide”) obtained significantly higher scores on the Sp-C-SSRS severity of ideation subscale, while patients with low scores on HDRS item 3 (“absent”, “life not worth living”, and “wish to be dead”) obtained significantly lower scores on this Sp-C-SSRS subscale than the other two groups. Of note, the Duncan post hoc test established a significant difference precisely between ideation (from “absent” to “wish to be dead”) and behaviour (“suicide gesture” and “attempts of suicide”) only. Posner et al.6 also demonstrated strong divergent validity of the C-SSRS with some items of scales for depression assessment (Beck Depression Inventory – BDI, and Montgomery-Asberg Depression Rating Scale – MADRS).
Likewise, the Sp-C-SSRS suicidal behaviour lethality subscale demonstrated good discriminant validity with the cut-off point of the MDS, similar to findings by Posner et al.,6 who found a robust correlation between these two measures.
Regarding sensitivity to change, Posner et al.6 found that a decrease in the severity of ideation (from “active suicidal thoughts” to “wish to die” or “no ideation”) was accompanied by a corresponding decrease in the Scale for Suicide Ideation (SSI) score, suggesting that the C-SSRS severity of ideation subscale is sensitive to clinical change. Our study found that decreases in Sp-C-SSRS severity and intensity of ideation subscale scores were associated with a decrease in HDRS suicide-related item 3. However, statistical significance was reached only with the intensity of ideation subscale. These data suggest that both Sp-C-SSRS suicide ideation subscales (i.e. severity and intensity subscales) are sensitive to clinical change. The lack of concordance between linear regression models and interaction plots may possibly be due to lack of statistical power associated with the small sample size used in the 3-month follow-up study.
Some limitations of this analysis should be noted. First of all, the study design precluded examination of other psychometric properties: (i) no independent evaluators assessed suicidal behaviour, so the sensitivity to change of the Sp-C-SSRS suicidal behaviour subscale could not be analyzed and (ii) interrater reliability was not evaluated (the data about the clinicians’ identity were not available), although previous data have demonstrated good interrater reliability of the original C-SSRS scale.24 Secondly, only patients with a current diagnosis of mood disorders were included as the non-suicidal control group, and only mid-term follow-up was conducted. For this 3-month follow-up, only Sp-C-SSRS and HDRS were used as assessment measures, limiting our ability to conduct a more robust validation study. Also, the small sample size used in the follow-up study may limit statistical power to detect concordance between regression analyses and interaction plots. Likewise, the comparison groups (patients with and without SA) are not completely similar in some socio-demographic and clinical features, and only mood disorders are included in the non-suicide attempter group. However, the demographic differences between suicide attempters and non-attempters reflect typical characteristics of populations displaying suicidal behaviour. Thirdly, the HDRS suicide item (item 3) may not be the best comparator for the suicidal ideation question, as the top anchor point is occurrence of a suicide attempt. The Montgomery-Asberg Depression Rating Scale (MADRS) or other single question suicide risk scales would have more data points to assess suicidal ideation. Fourthly, intent is not truly assessed by the C-SSRS scale, so using the SIS to determine construct validity for the SA sub-sample of patients may not be the best choice. Nonetheless, the medical lethality of a suicide attempt is commonly, but not always, associated with severity of ideation. Additionally, we used validated Spanish versions of all the measures, except the Medical Damage Scale, which has not been validated in Spanish.
In conclusion, our data indicate that the Sp-C-SSRS is a suitable instrument for assessing and monitoring suicidal ideation and behaviour in psychiatric patients, both in clinical and research settings. The impact of our findings is twofold if we take into account the FDA requirement for all central nervous system clinical drug trials to include a Columbia Classification Algorithm of Suicide Assessment (C-CASA)-compatible screening instrument for assessing and documenting the occurrence of treatment-emergent suicidal ideation and behaviour.7
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
FundingThis work was partly supported by the Spanish Ministry of Economy and Competitivity (grants PI10/01632, PI080247, and PI1200906). P Buron was funded by the Fundación para el Fomento en Asturias de la Investigación Científica Aplicada y la Tecnología, FICYT, through a Severo Ochoa predoctoral grant. E Jimenez is funded by the Institut d’investigacions Biomèdiques August Pi i Sunyer, IDIBAPS, through a predoctoral grant.
Conflict of interestDr Benabarre has received research grants and served as a speaker for the following companies: Bristol-Myers Squibb, Eli Lilly, Glaxo-Smith-Kline, Janssen-Cilag, Pfizer, and the Spanish Ministry of Economy and Competitivity.
Dr Oquendo receives royalties for use of the Columbia Suicide Severity Rating Scale and received financial compensation from Pfizer for the safety evaluation of a clinical facility, unrelated to this study. She has received unrestricted educational grants and/or lecture fees from Astra-Zeneca, Bristol-Myers Squibb, Eli Lilly, Janssen, Otsuka, Pfizer, Sanofi-Aventis, and Shire. Her family owns stock in Bristol-Myers Squibb.
Dr Posner has received salary support from the Research Foundation for Mental Hygiene (RFMH). She has served as director of the Center for Suicide Risk Assessment.
The rest of the authors declare no conflict of interest.
Please cite this article as: Al-Halabí S, Sáiz PA, Burón P, Garrido M, Benabarre A, Jiménez E, et al. Validación de la versión en español de la Columbia-Suicide Severity Rating Scale (Escala Columbia para Evaluar el Riesgo de Suicidio). Rev Psiquiatr Salud Ment (Barc). 2016;9:134–142.