Eslicarbazepine acetate (ESL), together with carbamazepine and oxcarbazepine, belongs to the dibenzazepine family. According to the latest clinical practice guidelines, tricyclic antidepressants, dual antidepressants (venlafaxine, duloxetine), and some antiepileptics (gabapentin, pregabalin) are first-line drugs for neuropathic pain; tramadol, lidocaine 5% patches, and capsaicin 8% patches are considered second-line drugs; and strong opioids constitute a third line of treatment. Such other antiepileptics as lamotrigine and lacosamide are not authorised as treatments for neuropathic pain by the regulatory agencies, but are nonetheless prescribed off-label in routine clinical practice. Carbamazepine, on the other hand, is indicated for trigeminal and glossopharyngeal neuralgia.
DevelopmentWe conducted a literature search to gather evidence on the use of ESL for neuropathic pain, headache, and cranial neuralgia.
ConclusionsEvidence is insufficient to recommend ESL for neuropathic pain, headache, and cranial neuralgia. Most of the available evidence comes from open and observational studies with small sample sizes and no control group; however, their favourable results call for further studies on the usefulness of ESL for neuropathic pain, headache, and cranial neuralgia.
El acetato de eslicarbazepina (ESL), junto con la carbamazepina y oxcarbazepina, pertenece a la familia de las dibenzazepinas. Las últimas guías de práctica clínica consultadas coinciden en señalar que los antidepresivos tricíclicos, duales (venlafaxina/duloxetina) y los antiepilépticos gabapentina/pregabalina, constituyen los fármacos de primera línea en el tratamiento del dolor neuropático. El tramadol, los apósitos de lidocaína al 5% y los parches de capsaicina al 8% son fármacos de segunda línea, mientras que los opioides potentes constituirían una tercera línea de tratamiento. Existen otros antiepilépticos que no tienen indicación en el dolor neuropático por las agencias reguladoras, como lamotrigina o lacosamida, pero se utilizan en la práctica clínica habitual fuera de indicación. Por otro lado, la carbamazepina está indicada en el tratamiento de la neuralgia esencial del trigémino y del glosofaríngeo.
DesarrolloTras una búsqueda bibliográfica, se realizó una revisión sobre el empleo del ESL en dolor neuropático, cefaleas y neuralgias craneales.
ConclusionesNo se dispone apenas de evidencia para recomendar el uso del ESL en dolor neuropático, cefaleas y neuralgias craneales. La mayor parte de la experiencia disponible corresponde a estudios abiertos y observacionales, sin grupo control y con bajo número de pacientes; pero los resultados favorables obtenidos invitan a seguir investigando la utilidad del ESL en dolor neuropático, cefaleas y neuralgias craneales.
Eslicarbazepine acetate (ESL) is a third-generation antiepileptic drug approved in 2009 by the European Medicines Agency and in 2013 by the US Food and Drug Administration. It has been available on the Spanish market since February 2011. The drug is currently indicated as an adjunctive therapy for adult patients with partial-onset seizures with or without secondary generalisation. The recommended initial dose is 400mg once daily, increasing to 800mg once daily after 1 to 2 weeks. The dose may be increased to 1200mg, depending on patient response.1
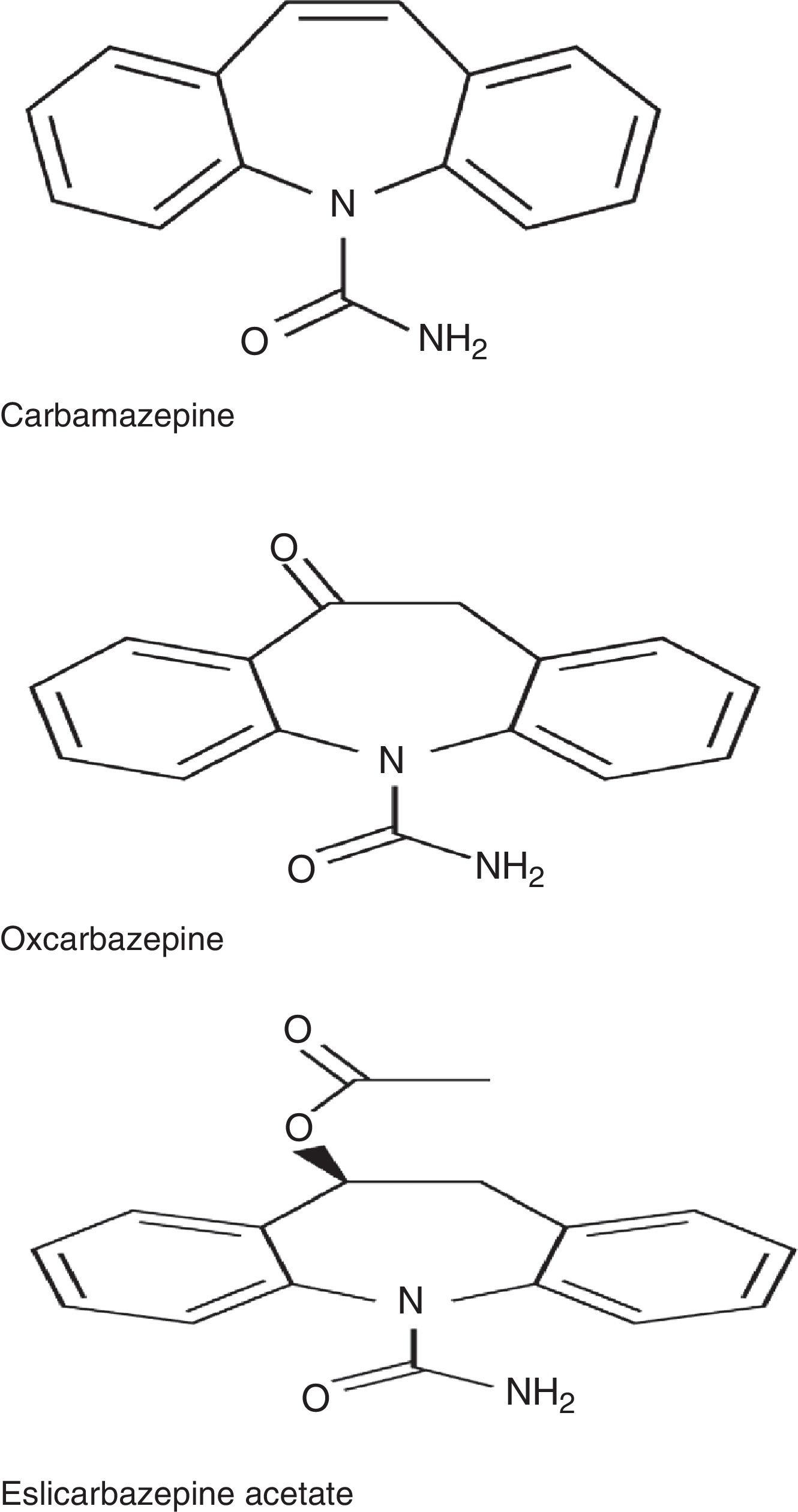
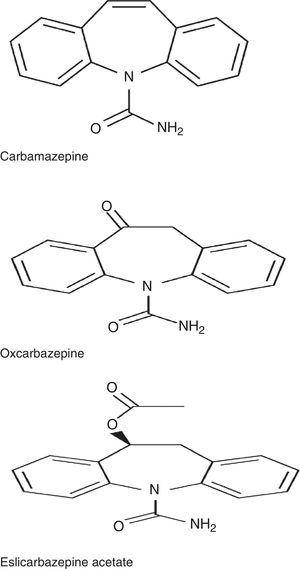
ESL belongs to the dibenzazepine family, as do carbamazepine (CBZ) and oxcarbazepine (OXC). These drugs are structurally different from one another at the 10,11 position. They also differ in terms of metabolism: CBZ is metabolised into CBZ-10,11-epoxide, unlike OXC, which is metabolised into the active metabolite S-licarbazepine (78.1%), and ESL, which is metabolised into the same metabolite but at a higher proportion (93.9%) (Fig. 1).2,3 The number of daily doses is also different: single doses in the case of ESL and extended-release OXC (not available in Spain), 2 doses of immediate-release OXC and extended-release CBZ (not available in Spain), and 2 to 3 doses of immediate-release CBZ. According to a recent study by Soares-da-Silva et al.,4 the mechanism of action of ESL has some distinctive features compared to those of other drugs in the same family: 1) the selectivity of interaction with the inactive state of the voltage-gated sodium channel (VGSC), 2) reduction in VGSC availability through enhancement of slow inactivation, rather than alteration of fast inactivation of VGSC, and 3) inhibition of high- and low-affinity hCaV3.2 inward currents with greater affinity than CBZ.
Similarly to OXC, ESL is moderately toxic, with the most common adverse effects being dizziness, nausea, vomiting, drowsiness, and diplopia. It has an elimination half-life of 13-24hours, and can therefore be administered as a single daily dose. ESL is a mild inducer of UDP-glucuronosyltransferase (UGT1A1) and the CYP3A4 isoform of cytochrome P450. This activity may cause some clinically relevant interactions with phenytoin, warfarin, and oestrogen-containing contraceptives.1
CBZ is indicated not only for epilepsy but also for manic episodes and for prophylaxis of bipolar affective disorder, essential trigeminal neuralgia, essential glossopharyngeal neuralgia, and alcohol withdrawal syndrome,5 whereas OXC is indicated for the treatment of partial seizures with or without secondary generalisation with tonic-clonic seizures.6
The latest clinical practice guidelines agree that tricyclic and dual-acting antidepressants (venlafaxine, duloxetine) and the antiepileptic drugs gabapentin and pregabalin constitute the treatment of choice for neuropathic pain. Tramadol, lidocaine 5% patches, and capsaicin 8% patches are second-line treatments, whereas strong opioids constitute the third line of treatment.7
Unlike CBZ, neither ESL nor OXC are indicated for neuropathic pain, headache, or cranial neuralgias. However, other antiepileptic drugs that are not indicated for these clinical scenarios, such as lamotrigine and lacosamide, are frequently used off-label in clinical practice.8–12
In this study, we review the available evidence on the use of ESL for neuropathic pain, headache, and cranial neuralgias to determine whether the drug may be a valid option in patients with poor response or intolerance to the traditional treatments.
DevelopmentWe conducted a literature search on PubMed and Google Scholar using the following search strategy: “acetate eslicarbazepine AND neuropathic pain” and “acetate eslicarbazepine AND headaches.” We only considered articles from indexed scientific journals and selected original articles and case reports published either in English or in Spanish. As this strategy yielded barely any results, we decided to broaden our search by including posters and oral communications on the same subject (“acetate eslicarbazepine and neuropathic pain” and “acetate eslicarbazepine and headaches”) presented at Annual Meetings of the Spanish Society of Neurology (SEN) between 2011 and 2015 and at other international congresses. For clarity, we structured the article with several different sections.
Experimental studies of eslicarbazepine acetate for neuropathic painIn experimental studies with mice, the analgesic effect of ESL has been studied extensively in a wide range of induced pain states, including trigeminal neuralgia, diabetic neuropathy, and visceral pain; results suggest that the drug may be useful for the treatment of inflammatory and neuropathic pain.13
Eslicarbazepine acetate for painful diabetic neuropathyWe found a poster on painful diabetic neuropathy (PDN) presented at the 2013 European Pain Federation EFIC congress. The poster presented the findings of a multicentre, randomised, double-blind, placebo-controlled, parallel-group, dose-finding phase II study. The trial comprised a 2-week wash-out period, during which previous treatments for neuropathic pain were discontinued, followed by a one-week titration period, a 12-week maintenance period, and a 2-week follow-up period. A total of 557 patients were randomly assigned to receive either placebo, 400mg ESL twice daily (BID), 800mg ESL once daily (QD), 600mg ESL BID, 1200mg ESL QD, or 800mg ESL BID. After 12 weeks of treatment, no statistically significant differences were observed between individuals receiving placebo and those receiving ESL for pain management (primary efficacy analysis) according to the Numeric Pain Rating Scale (NPRS). However, the percentage of individuals experiencing a ≥ 30% decrease in pain intensity was higher in the group receiving 400mg ESL BID than in the placebo group (62.5% vs 44.8%; P=.0230). The incidence of adverse reactions was 38.0% in the placebo group and 51.8% in the group treated with 1200mg ESL QD. The most frequent adverse reactions were vomiting, dizziness, and nausea.14
Our literature search also found a multicentre, randomised, double-blind, placebo-controlled, parallel-group phase III study of PDN; the study lasted 15 weeks (3 weeks’ dose adjustment+12 weeks’ follow-up). The primary efficacy variable was the change in pain intensity from study onset (baseline) to study completion (15 weeks), and was measured using the NPRS. A total of 332 patients with PDN were randomly assigned to receive either placebo, 1600mg ESL QD, 1200mg ESL QD, or 800mg ESL QD. After 15 weeks of treatment, no statistically significant differences in pain management were observed between the participants receiving placebo and those receiving ESL. The incidence of adverse reactions ranged from 12.20% in the placebo group to 63.10% in the group receiving 1600mg ESL QD. The most frequent adverse reactions were vertigo, nausea, and hyponatraemia (the latter was dose-dependent). The results from this trial were not published.15
García Escrivá et al.16 shared their experience with ESL as a second-line treatment for PDN refractory to traditional drugs. During 2013, the authors identified 8 patients with PDN whose previous treatments had failed to manage pain satisfactorily. In an initial visit, they substituted their previous pain treatment for ESL, gathered demographic data, and administered the following questionnaires: DN4 scale, Hospital Anxiety and Depression Scale, Visual Analogue Scale (VAS) for pain, Patient Global Impression of Improvement scale, and Clinical Global Impression of Improvement scale. In a follow-up consultation conducted 12 weeks after treatment, patients completed the same questionnaires and were asked about any adverse reactions. The data gathered were used for statistical analysis. Regarding previous treatment, 75% of the patients had received pregabalin, 25% amitriptyline, 12.5% clonazepam, and 12.5% fentanyl, either in monotherapy or in combination therapy. At onset of ESL treatment, patients were taking a mean of 4.6 drugs simultaneously (range, 1-11; median: 4.5). No changes were made to concomitant treatment during the follow-up period. Neuropathic pain, evaluated with the DN4 scale, decreased significantly at 3 months (P=.026, Wilcoxon test). The study found no significant changes in any of the pain characteristics measured by the scale. The authors observed a significant decrease in neuropathic pain as measured with the VAS (P=.018, Wilcoxon test). Patients’ scores improved on both the anxiety (P=.102, Wilcoxon test) and depression scales (P=.276, Wilcoxon test), although changes were not statistically significant. Subjectively, 65.5% of the patients reported feeling “much better” or “better” and 87.5% of physicians indicated that the patients were “better” or “much better”. ESL was withdrawn in one patient due to adverse reactions (dizziness). At 3 months, 7 patients (87.5%) continued taking ESL, 2 at 400mg/day and 5 at 800mg/day. Despite the small sample size, ESL was found to be an effective alternative for managing neuropathic pain in patients with PDN refractory to conventional treatment, and showed good tolerability and adherence.16,17
Eslicarbazepine acetate for postherpetic neuralgiaOur literature search found a poster on postherpetic neuralgia (PHN) presented at the 2013 European Pain Federation EFIC congress. The poster presented the findings of a multicentre, randomised, double-blind, placebo-controlled, parallel-group, dose-finding phase II study. The study comprised a 2-week wash-out period, during which previous treatments for PHN were discontinued, followed by a one-week titration period, an 8-week maintenance period, and a 2-week follow-up period. A total of 567 patients were randomly assigned to receive either placebo, 400mg ESL BID, 800mg ESL QD, 600mg ESL BID, 1200mg ESL QD, or 800mg ESL BID. The intention-to-treat analysis showed no statistically significant differences between the placebo and ESL groups in terms of pain intensity as measured with the NPRS (primary efficacy analysis). According to the per-protocol analysis, however, pain intensity decreased significantly more in the group receiving 800mg ESL BID than in the placebo group (P=.0277). The incidence of adverse reactions ranged from 31.2% in the placebo group to 54.4% in the group receiving 800mg ESL BID. The most frequent adverse reactions were dizziness, drowsiness, headache, nausea, and vertigo.18
Our literature search also found a multicentre, randomised, double-blind, placebo-controlled, parallel-group phase III study of PHN lasting 15 weeks. The primary efficacy variable was the change in pain intensity from study onset to study completion (15 weeks), and was measured using the NPRS. A total of 240 patients with PHN were randomly assigned to receive either placebo, 1600mg ESL QD, 1200mg ESL QD, or 800mg ESL QD. No significant differences in pain intensity were observed between the placebo and ESL groups after 15 weeks of treatment. The incidence of adverse reactions ranged from 10% in the placebo group to 76.67% in the group treated with 1600mg ESL QD. The most frequent adverse reactions were nausea, dizziness, and headache. The results of this study were not published.19
Eslicarbazepine acetate for cranial neuralgiasGaber et al.20 published the case of a 62-year-old woman with trigeminal neuralgia associated with multiple sclerosis adequately controlled with CBZ. However, treatment had to be discontinued due to recurrent symptomatic hyponatraemia. Several drugs, including topiramate, gabapentin, and amitriptyline, failed to improve the trigeminal neuralgia. ESL at low doses (400mg daily) achieved excellent pain control without altering the plasma sodium concentration.
Cuadrado et al.21 published the case of a 32-year-old man with epicrania fugax that was refractory to numerous treatments (occipital nerve block with triamcinolone and bupivacaine, gabapentin, lamotrigine, pregabalin, topiramate, zonisamide, sodium valproate, lacosamide, indometacin). CBZ dosed at 1200mg/day reduced pain intensity but had to be discontinued due to drowsiness. ESL dosed at 800mg/day significantly reduced pain frequency and intensity. The dose was increased to 1600mg/day; the patient showed good tolerance and remained asymptomatic. Symptoms reappeared when the dose was reduced.
Cação et al.22 studied a cohort of 15 patients with short-lasting unilateral neuralgiform headache with conjunctival injection and tearing (SUNCT), of whom a majority responded to lamotrigine, one responded to topiramate, and one to ESL.
The clinical case presented by Aledo Serrano et al.23 at the SEN's 66th Annual Meeting described the case of a 38-year-old man with a diagnosis of epicrania fugax refractory to lamotrigine, pregabalin, gabapentin, topiramate, zonisamide, indometacin, valproate, lacosamide, levetiracetam, anaesthetic block of the greater occipital nerve, and botulinum toxin injection at the site of pain. The patient responded partially to CBZ but experienced severe adverse reactions. The patient remained asymptomatic with ESL dosed at 1200mg daily.
Sánchez Larsen et al.24 presented an observational, retrospective study at the SEN's 66th Annual Meeting, analysing a group of patients with trigeminal neuralgia treated with ESL either in monotherapy or in combination therapy. The main variables were pain intensity before and after treatment (VAS), frequency of pain attacks before and after treatment, and adverse reactions. The study included 10 patients (4 in monotherapy); 7 were women, and mean age was 67.9 years (range, 28-92). Mean follow-up time was 24.7 months, with the exception of 2 drop-outs due to mild adverse reactions (dizziness). No patient experienced severe adverse reactions; one presented hyponatraemia. Pain intensity and frequency decreased in 9 of the 10 patients. In these patients, pain intensity decreased from 8.7 points to 2.6 and pain frequency decreased from 12.13 to 2.28 attacks per day (4 patients were asymptomatic, 3 experienced 1-2 attacks per week, 2 experienced mild improvements but attack frequency was still high [5-15 attacks/day]). In this series, ESL was found to be effective and safe for the treatment of trigeminal neuralgia, and superior to CBZ from the perspective of safety and pharmacokinetics, although the authors recommend close monitoring of sodium levels.
In a study presented at the SEN's 67th Annual Meeting (2015), Abril-Jaramillo et al.25 analysed response to treatment with ESL in patients with cranial neuralgias (56% had trigeminal neuralgia) who had responded well to CBZ or OXC but experienced adverse reactions to these drugs. Response to ESL was evaluated in 15 patients over a 6-month period; the initial evaluation included the data from the clinical history, physical examination, complementary tests, information about previous treatments, and the adverse reactions motivating drug discontinuation. Patients were subsequently evaluated at 3 and 6 months to assess treatment efficacy and any adverse reactions. Response was favourable in 13 of the 15 patients; ESL was better tolerated than CBZ and OXC.
Eslicarbazepine acetate in series of cases of different aetiologiesAt the SEN's 63rd Annual Meeting (2011, Barcelona), Tena Mora26 presented in poster format the results of a prospective, descriptive study of the efficacy and safety of ESL in monotherapy in 6 patients with different types of neuropathic pain. Analyses (complete blood count, coagulation test, sodium and potassium levels, kidney and liver function) were performed at 1 and 3 months after treatment onset; the VAS was completed during the first consultation (VAS 1), at 1 month (VAS 2), and at 3 months of follow-up (VAS 3). The study included 4 patients with primary trigeminal neuralgia (100% women; VAS 1: 8-10, VAS 2: 0-3, VAS 3: 0-3), one of whom had drowsiness which resolved when the maintenance dose was split into 2 daily doses; a patient with post-stroke pain (80-year-old man, VAS 1: 9; treatment was discontinued at 4 days due to drowsiness; CBZ was gradually discontinued in parallel); and a patient with persistent idiopathic facial pain (70-year-old woman; VAS 1: 9, VAS 2: 8, VAS 3: 8). None of the patients showed alterations in blood analysis results during the study period (3 months). The author stresses the analgesic effects of ESL for primary trigeminal neuralgia.
At the SEN's 66th Annual Meeting, García Arguedas et al.27 presented the results of a retrospective, descriptive study of 10 patients with neuropathic pain treated with ESL in monotherapy. They analysed the causes of pain, the dose used, the effectiveness of treatment (using baseline and 3-month VAS scores), and treatment tolerability. The causes of pain were trigeminal neuralgia in 5 patients, PDN in 3, and PHN in 2. The most frequently used dose was 800mg (maximum dose of 1200mg in one patient). Mean VAS score was 8.2 at the baseline consultation and 2.4 at the 3-month follow-up consultation, with a global decrease of over 50% in pain intensity. Regarding tolerability, the most frequent adverse reaction was dizziness; one patient developed mild hyponatraemia but did not discontinue treatment.
During the SEN's 67th Annual Meeting, Pagola Lorz et al.28 presented their experience with ESL as a second-line treatment for neuropathic pain refractory to traditional drugs. This descriptive, retrospective study analysed response to ESL in 10 patients with poorly-controlled neuropathic pain. The main variables were cause of pain, ESL dose, improvements in pain (rated as “much better,” “better,” “no change,” or “worse”), concomitant treatment for neuropathic pain, and adverse reactions. The causes of pain were trigeminal neuralgia in 6 patients, glossopharyngeal neuralgia in one, PHN in 2, and thalamic pain in one. Patients received a mean dose of 800mg ESL; 400mg was sufficient for 3 patients, whereas 2 required 1200mg. Seven of the 10 patients reported feeling “much better,” 2 felt “better,” and the remaining one reported “no change.” Regarding concomitant treatment, 3 patients received gabapentin, one received pregabalin, and another received amitriptyline. Adverse reactions (apathy, dizziness, blurred vision) were mild and transient, and did not require drug discontinuation in any case.
At the SEN's 67th Annual Meeting, Bermejo Velasco et al.29 presented the results of a retrospective study of the clinical histories of 10 patients with neuropathic pain and receiving ESL. The authors evaluated the efficacy of ESL using the VAS, the percentage of responders (> 50% decrease in pain intensity), percentage of pain-free patients, decrease in concomitant treatment, adverse reactions, dose used, titration schedule, and aetiology of neuropathic pain. Patients were followed up for a mean of 6.1 months. The study included a total of 32 patients. The mean dose of ESL was 920±220mg/day. Pain intensity decreased a mean of 4.4±1.6 points, with 46% of patients identified as responders and 13% pain-free. Around 78% of the patients continued with the treatment. The main reasons for treatment discontinuation were ineffectiveness, drowsiness, and dizziness.
Tables 1 and 2 summarise the results of the clinical trials, observational studies, and case reports included in this review.
Summary of the clinical studies included in the review.
| Type of study | Reference | Sample size | Disease | ESL dose | Duration | Main outcomes | Safety |
|---|---|---|---|---|---|---|---|
| Phase II trial | Kress et al.14 | 557 | PDN | Placebo, ESL 400mg BID, ESL 800mg QD, ESL 600mg BID, ESL 1200mg QD, and ESL 800mg BID | 15 weeks | No statistically significant differences were observed between individuals receiving placebo and those receiving ESL for pain management (primary efficacy analysis), according to NPRS scores.The percentage of individuals experiencing a≥30% decrease in pain intensity was higher in the group receiving 400mg ESL BID (62.5%) than in the placebo group (44.8%) (P = .0230). | The incidence of adverse reactions ranged from 38.0% in the placebo group to 51.8% in the group treated with 1200mg ESL QD. The most frequent adverse reactions were vomiting, dizziness, and nausea. |
| Phase III trial | https://clinicaltrials.gov/ct2/show/study/NCT01129960?term=eslicarbazepine&rank=1915 | 332 | PDN | Placebo, ESL 1600mg QD, ESL 1200mg QD, and ESL 800mg QD | 15 weeks | No statistically significant differences were observed between individuals receiving placebo and those receiving ESL for pain management, according to NPRS scores. | The incidence of adverse reactions ranged from 12.20% in the placebo group to 63.10% in the group treated with 1600mg ESL QD. The most frequent adverse reactions were vertigo, nausea, and hyponatraemia (the latter was dose-dependent). |
| Phase II trial | Kress et al.18 | 567 | PHN | Placebo, ESL 400mg BID, ESL 800mg QD, ESL 600mg BID, ESL 1200mg QD, and ESL 800mg BID | 11 weeks | The intention-to-treat analysis showed no statistically significant differences between the placebo and the ESL groups in terms of pain intensity as measured with the NPRS (primary efficacy analysis).According to the per-protocol analysis, pain intensity decreased significantly more in the group receiving 800mg ESL BID than in the placebo group (P = .0277). | The incidence of adverse reactions ranged from 31.2% in the placebo group to 54.4% in the group receiving 800mg ESL BID. The most frequent adverse reactions were dizziness, drowsiness, headache, nausea, and vertigo. |
| Phase III trial | https://clinicaltrials.gov/ct2/show/study/NCT01124097?term=eslicarbazepine&rank=18§=X7015619 | 240 | PHN | Placebo, ESL 1600mg QD, ESL 1200mg QD, and ESL 800mg QD | 15 weeks | No statistically significant differences were observed between individuals receiving placebo and those receiving ESL for pain management, according to NPRS scores. | The incidence of adverse reactions ranged from 10% in the placebo group to 76.67% in the group treated with 1600mg ESL QD. The most frequent adverse reactions were nausea, dizziness, and headache. |
BID: twice daily; ESL: eslicarbazepine acetate; NPRS: Numeric Pain Rating Scale; PDN: painful diabetic neuropathy; PHN: postherpetic neuralgia; QD: once daily.
Summary of the observational studies and clinical trials included in the review.
| Type of study | Reference | Sample size | Disease | ESL dose | Duration | Main outcomes | Safety |
|---|---|---|---|---|---|---|---|
| Prospective, descriptive, observational study | García Escrivá et al.16 | 8 | PDN with previous treatment failure | ESL 400mg/day (2 patients) and ESL 800mg/day (5 patients) | 12 weeks | Improvement in DN4, HADS, VAS, PGI-I, and CGI-I scale scores. | 1 drop-out due to dizziness |
| Clinical case | Gaber et al.20 | 1 | TN associated with multiple sclerosis was well managed with CBZ, but the drug was discontinued due to recurrent symptomatic hyponatraemia. | 400mg/day | – | Excellent pain management | Good tolerance with no alterations in plasma sodium levels |
| Clinical case | Cuadrado et al.21 | 1 | Refractory epicrania fugax with poor tolerance of CBZ | 1600mg/day | – | Significant improvement in pain frequency and intensity | Good tolerance |
| Case series | Cação et al.22 | 1 | SUNCT | 800mg/day | – | Management of pain and decrease in the number of attacks per day | The patient died due to causes unrelated to ESL a year later. |
| Clinical case | Aledo Serrano et al.23 | 1 | Refractory epicrania fugax with poor tolerance of CBZ | 1200mg/day | – | Asymptomatic | Good tolerance |
| Retrospective, descriptive, observational study | Sánchez Larsen et al.24 | 10 | TN (4 patients in monotherapy and 6 in polytherapy) | NA | Mean follow-up time: 24.7 months | Pain intensity and attack frequency decreased in 9 of the 10 patients. | Two patients dropped out due to mild adverse reactions (dizziness). No severe adverse effects were reported. One patient displayed hyponatraemia. |
| Prospective, descriptive, observational study | Abril-Jaramillo et al.25 | 15 | Cranial neuralgia (TN in 56% of patients), with good response to CBZ or OXC but associated with adverse drug reactions | NA | 6 months | Favourable response in 13 of the 15 patients | Tolerability of approximately 90% |
| Prospective, descriptive, observational study | Tena Mora26 | 6 | 4 TN, 1 post-stroke pain, 1 persistent idiopathic facial pain (all in monotherapy) | NA | 3 months | Significant analgesic effects for TN | One patient with TN displayed drowsiness, which resolved when the maintenance dose was split into 2 doses. The patient with post-stroke pain discontinued treatment at 4 days due to drowsiness. No patient showed alterations in analysis results during the study period. |
| Retrospective, descriptive, observational study | García Arguedas et al.27 | 10 | 5 TN, 3 PDN, and 2 PHN (monotherapy) | Mean dose: 800mg/day (maximum dose of 1200mg/day in one patient) | 3 months | Over 50% decrease in pain intensity (VAS) | Dizziness, mild hyponatraemia in one patient, no drop-outs |
| Retrospective, descriptive, observational study | Pagola Lorz et al.28 | 10 | 6 TN, 1 glossopharyngeal neuralgia, 2 PHN, and 1 thalamic pain (no response to traditional drugs) | Mean dose: 800mg/day; 3 patients required as little as 400mg/day whereas 2 required 1200mg/day. | NA | Seven patients reported feeling “much better,” 2 felt “better,” and the remaining one reported “no change.” | Mild, transient adverse reactions (apathy, dizziness, blurred vision) that did not result in drug discontinuation |
| Retrospective, descriptive, observational study | Bermejo Velasco et al.29 | 32 | Different aetiologies of neuropathic pain refractory to traditional drugs. | Mean dose: 920±220mg/day | Mean follow-up time: 6.1 months | Mean decrease in pain intensity of 4.4±1.6 points on the VAS; 46% responders (> 50% decrease in pain intensity) and 13% pain-free patients | 78% of the patients continued treatment. The main reasons for treatment discontinuation were ineffectiveness, drowsiness, and dizziness. |
CBZ: carbamazepine; CGI-I: Clinical Global Impression of Improvement scale; ESL: eslicarbazepine acetate; HADS: Hospital Anxiety and Depression Scale; NA: not available; OXC: oxcarbazepine; PDN: painful diabetic neuropathy; PGI-I: Patient Global Impression of Improvement scale; PHN: postherpetic neuralgia; SUNCT: short-lasting unilateral neuralgiform headache with conjunctival injection and tearing; TN: trigeminal neuralgia; VAS: Visual Analogue Scale.
Drugs from the dibenzazepine family may cause hyponatraemia (sodium level<135mmol/L); this is a common adverse reaction to CBZ and OXC. The incidence of hyponatraemia secondary to treatment with CBZ ranges between 4.8% and 40%.30,31 There is mounting evidence of the deleterious effects of hyponatraemia, even in mild cases, especially in elderly patients, among whom neuropathic pain is relatively frequent.32,33 Drug-induced hyponatraemia should be regarded as a reason to change treatment.
Physicians usually face a dilemma when CBZ is the only drug able to control a specific clinical problem, such as trigeminal neuralgia. ESL may constitute an alternative in patients experiencing adverse reactions to CBZ. Hyponatraemia has rarely been reported in clinical trials and observational studies of ESL in patients with epilepsy34–36; changing from CBZ or OXC to ESL in patients who have developed hyponatraemia therefore seems to be an efficacious and well-tolerated option. Sodium levels should still be monitored, however, as some patients continue to display hyponatraemia after drug switching.37
These differences in the profiles of antiepileptic drugs from the dibenzazepine family may result in different levels of effectiveness, tolerability, and adherence,38 which may lead physicians to consider switching from one drug to another in the same family to adapt treatment to each patient's characteristics. The EPICON project, in which a panel of epilepsy specialists adopted the Delphi method, evaluated specific situations and the methodology for switching from CBZ or OXC to ESL. The purpose of the study was to issue consensus recommendations for switching from CBZ or OXC to ESL in certain patients. The main recommendations are as follows: 1) Transition from CBZ to ESL should be carried out progressively over 1 to 3 weeks, employing a CBZ:ESL ratio of 1:1.3. 2) Switching from CBZ to ESL is recommended for patients who frequently forget to take their medication, those who work rotating shifts, polymedicated patients, patients with cognitive problems, patients with severe osteopaenia/osteoporosis, patients with dyslipidaemia or liver disease other than acute liver failure, and men with erectile dysfunction caused by CBZ. 3) OXC may be switched to ESL overnight at a dose ratio of 1:1; this is recommended for patients who frequently forget to take their medication, those who work rotating shifts, polymedicated patients, or patients with cognitive problems. 4) Drug switching is not recommended for patients displaying rash secondary to treatment with CBZ or OXC.39 Although these recommendations apply to patients with epilepsy, they may be helpful in other clinical situations (different types of neuropathic pain, trigeminal neuralgia and other types of headache) associated with poor adherence or tolerability of CBZ or OXC.
ConclusionsGiven the mechanism of action of ESL (selective interaction with the inactive state of VGSC through slow inactivation, in contrast with the fast inactivation of CBZ and OXC, and inhibition of hCaV3.2 currents with greater affinity than CBZ) it would initially appear to be a good option for the treatment of neuropathic pain, headache, and cranial neuralgia.
However, evidence is too scarce to recommend ESL for these indications. Most of the available evidence comes from open-label, observational studies with small samples and no control group. Furthermore, some of the studies included in this review address different clinical entities26–29 with different pathophysiological mechanisms. Therefore, the heterogeneity of the data prevents us from establishing any general conclusion on the efficacy of ESL. Interestingly, clinical trials on ESL as a treatment for neuropathic pain (PDN and PHN) have found no statistically significant decreases in pain intensity.
The scarcity of published studies on the subject may suggest a publication bias. Indeed, the phase III clinical trials included in our review were not published.
In conclusion, ESL may constitute a valid alternative for the treatment of neuropathic pain, headache, and cranial neuralgias in patients showing poor response or intolerance to traditional drugs, especially in the case of trigeminal neuralgia; further randomised studies with larger samples are necessary to confirm this hypothesis. The usefulness of ESL for trigeminal neuralgia may be associated with the condition's excellent response to CBZ and to the biochemical similarities between both drugs.
Conflicts of interestThe study complies with the ethical standards and authorship criteria of the journal. The authors have no conflicts of interest to declare.
Please cite this article as: Alcántara Montero A, Sánchez Carnerero C. Acetato de eslicarbazepina en dolor neuropático, cefaleas y neuralgias craneales: Evidencia y experiencia. Neurología. 2019;34:386–395.