Evidence-based clinical guidelines on Ulcerative colitis (UC) have been developed through a consensus, while GRADE methodology is the current standard for guideline development. This is the first one based on GRADE methodology on UC.
MethodsFollowing GRADE methodology, the Spanish Group of Ulcerative Colitis and Crohn's disease (GETECCU) have developed a guideline on UC treatment. After selection of relevant clinical scenarios, 32 clinical questions were chosen and recommendations were established.
ResultsIn 2 questions no recommendation was possible. Twenty-two actions were recommended for, 14 strongly and 8 weakly. However, in 8 questions a recommendation against doing something was obtained, weak in 5 and strong in 3. The majority of recommendations were based on moderate quality evidence, and only 5 on high-quality evidence.
ConclusionsWith GRADE methodology we find a clear recommendation on possible actions in most clinical decisions in UC treatment, but much more clinical high-quality research is needed.
Las guías clínicas para la colitis ulcerosa (CU), basadas en la evidencia, se elaboran a través del consenso; por otra parte, la metodología GRADE es el estándar actual para la elaboración de directrices. Esta es la primera guía para la CU que se basa en la metodología GRADE.
MétodosDe acuerdo con la metodología GRADE, el Grupo Español de Trabajo en Enfermedad de Crohn y Colitis Ulcerosa (GETECCU) ha elaborado una guía sobre el tratamiento de la CU. Después de seleccionar los escenarios clínicos pertinentes, se eligieron 32 preguntas clínicas y se establecieron recomendaciones.
ResultadosEn 2 preguntas no fue posible realizar recomendación alguna. Se recomendaron 22 acciones, 14 con un grado de recomendación sólido y 8 con grado débil. Sin embargo, para 8 preguntas se obtuvo una recomendación en contra de realizar una acción, y de ellas, 5 resultaron recomendaciones sólidas y 3 débiles. La mayoría de las recomendaciones se basaron en pruebas de una calidad moderada, y sólo 5 pruebas se pudieron considerar de alta calidad.
ConclusionesCon la metodología GRADE encontramos una clara recomendación acerca de acciones posibles durante la mayoría de las decisiones clínicas realizadas para el tratamiento de la CU, pero se necesita mucho más investigación clínica de alta calidad.
Ulcerative colitis (UC) is a chronic inflammatory bowel disease of complex etiology that mainly affects the colon. As there is no single pathognomonic criterion to define it, a combination of clinical, endoscopic, microbiologic and histological criteria is required to reach the final diagnosis.1 Its extension and severity vary from patient to patient, and from time to time in the same patient; hence, the definition of a clinical scenario demands to know both the severity and extension of the disease at a particular moment, according to the widely accepted “Montreal Classification”.2 The incidence and prevalence of UC have increased considerably over the last decades,3 Spain being no exception; in fact, recent data do show incidence rates that are very similar to those previously described in northern European countries.4
Currently, the different treatment methods include numerous nutritional, monitoring, follow-up, surgical, and especially pharmacological alternatives.5,6 Their application over the broad range of clinical scenarios, with such diverse individual, clinical and social circumstances, is not always easy, and this greatly justifies the need for guidelines. In fact, over the past years a number of guidelines have been published, the most notable being the ECCO guidelines,7 the BSG guidelines,8 the ACG guidelines,9 the WGO guidelines,10 the Asian-Pacific guidelines,11 and most recently, the Colombian guidelines.12 All of them are of high quality and evidence-based, but they are all based, without exception, on a relatively wide consensus process.
GETECCU (Grupo Español de Trabajo en Enfermedad de Crohn y Colitis Ulcerosa) is a 21-year-old Spanish working group on inflammatory bowel diseases, and its main goal is to improve the management of these diseases in Spain by promoting research, educational programs, and by improving care. After developing several consensus guidelines on the use of infliximab,13 TBC prevention,14 and apheresis,15 the GETECCU board decided to go a step further and start a program of evidence-based practice guidelines following the GRADE (Grades of Recommendation Assessment, Development and Evaluation) methodology16 and fulfilling AGREE (Appraisal of Guidelines, Research and Evaluation) collaboration requirements.17 This therapeutic UC guideline is the first result of this program. This report is the short form of the document; the complete document is fully available at http://dx.doi.org/10.1016/j.gastrohep.2012.11.002.
MethodsDefinitionsThe criteria for the primary diagnosis are those of Lennard–Jones, widely accepted,7,10 and for severity and extension, the Montreal Classification is used.2 To evaluate the results of the studies available in the literature, it is necessary to rely on several diverse and generally poorly validated indexes such as the Truelove-Witts, the Mayo Index, SEO's Index, Lichtiguer's Index, and Walmsley's Index (also known as SAI or simple activity index). The PUCAI index has the advantage of a correct methodological validation, but has been used only in pediatric studies. The details and references of these indexes are available in the full version of the guideline.
There are other definitions that are commonly used in UC literature, such as remission,18response, relapse, Steroid dependence, Steroide resistance, pouchitis and cuffitis that are somewhat arbitrary. A widely accepted consensus is needed, and for the purpose of the guideline, ECCO definitions are accepted19 (see the full version for details).
ObjectivesFirst, the Guideline Elaboration Committee defined the objectives, following the GRADE methodology. In sum, after choosing three broad clinical scenarios to which recommendations may be applied (induction of remission in severe colitis, induction of remission in mild-moderate colitis, and maintenance of remission), in each of them the possible outcome variables were defined in a scale of 1–9 (1–3 is not included, 4–6 is important but not critical, and 7–9 is considered critical for decision-making). After being scored by the nine members of the Committee, those outcome variables with an average score above 4 were included. In all the critical variables, the degree of internal agreement was excellent, with unanimity or with a maximum variability of one point. This process defined the following variables as Clinical Practice Guideline objectives:
- •
To establish recommendations for the induction treatment in severe UC flares, prioritizing the following assessment variables:
- •
To establish recommendations for the induction treatment in mild-moderate UC flares, prioritizing the following assessment variables:
- •
To establish recommendations for the maintenance of UC in remission, prioritizing the following assessment variables:
First, a Design Committee from the GETECCU board set out the general goals and a nine-member Elaboration Committee defined the clinical scenarios and assessment variables, as previously stated. Second, a five-member Working Committee drafted the first document. This document was submitted to be reviewed by an Internal Review Committee. After this process was carried out, the Working Committee prepared a second version of the document. This document was submitted to further review by an External Review Committee, which included gastroenterologists, surgeons, primary care physicians, nurses and patients. Finally, the Working Committee drafted the final document.
The Working Committee followed the GRADE methodology20,21 (see www.gradeworkinggroup.org) and classified the recommendations for the different clinical scenarios into four clear and easy-to-understand final categories:22strong recommendation for an intervention, implying for the clinician to do it; weak recommendation for an intervention, which implies to probably do it; weak against an intervention, implying to probably do not do it; and strong against an intervention, implying not to do it. Defined using the GRADE methodology, these recommendations were mainly, but not solely, based on the strict assessment of the quality of the evidence (high, moderate, low, or very low quality). The quality of the evidence may be downgraded as a result of limitations in the study design or in its implementation, imprecision of estimates (wide confidence intervals), variability in the results, indirectness of the evidence, or publication bias; or upgraded because of a very large magnitude of effects, a dose-response gradient, or if all the plausible biases would reduce an apparent treatment effect. Furthermore, the recommendations are also based on some other factors, such as desirable and undesirable consequences of alternative management strategies, variability in values and preferences, and the use of resources (costs).
Finally, we used the Agree instrument (see www.agreecollaboration.org) to ensure the high quality of our Clinical Practice Guideline, which was evaluated by the authors and the entire internal review Committee.
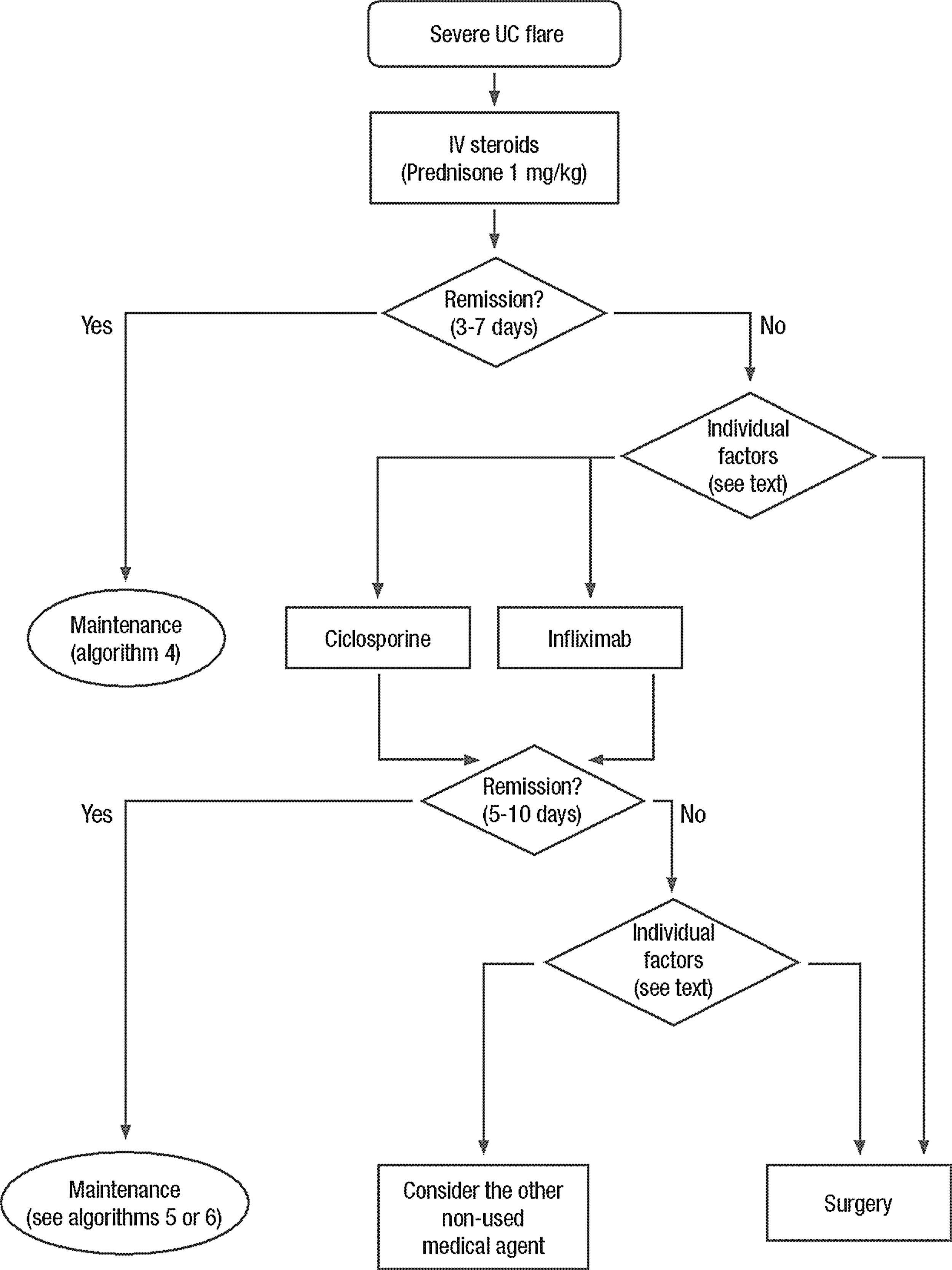
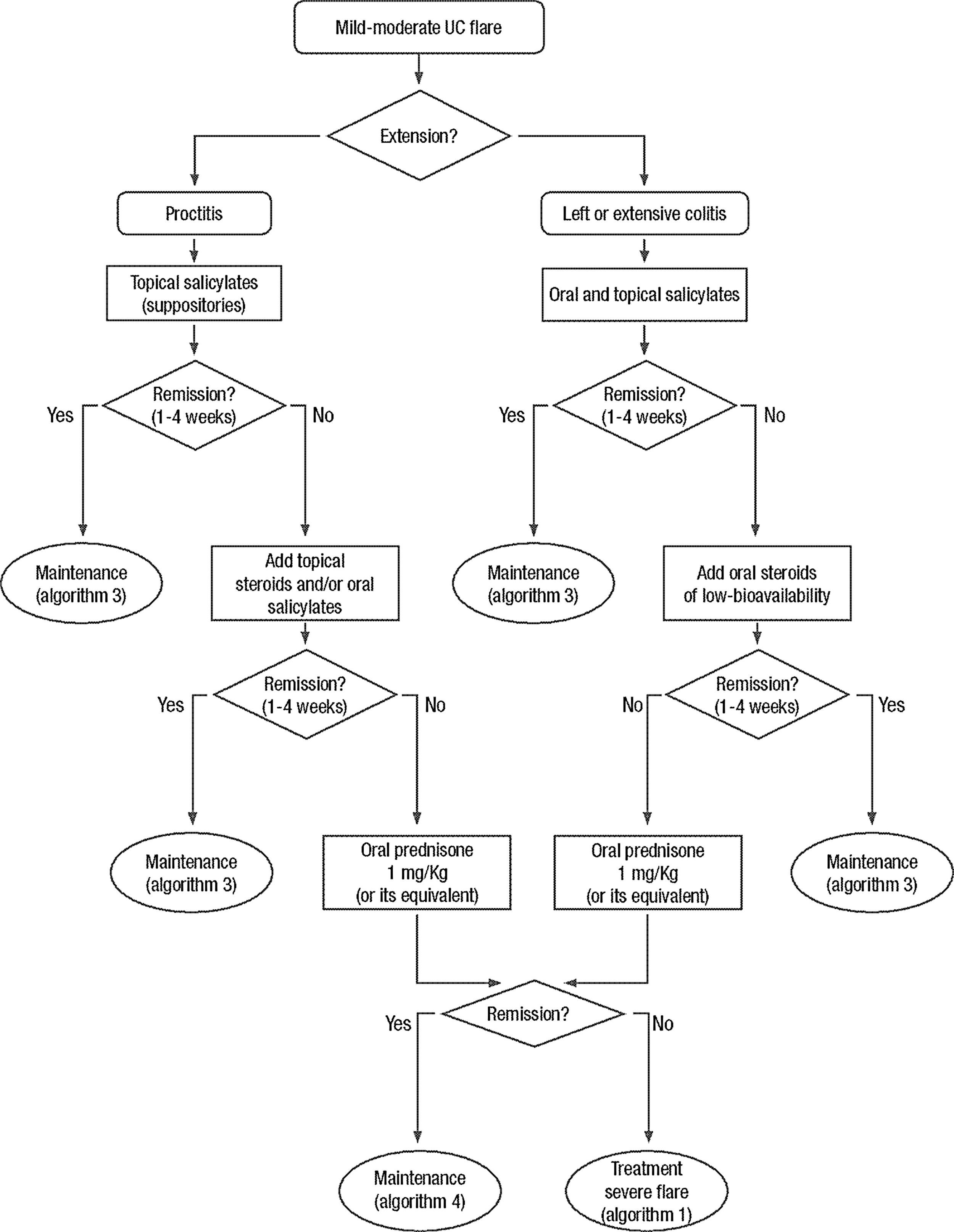
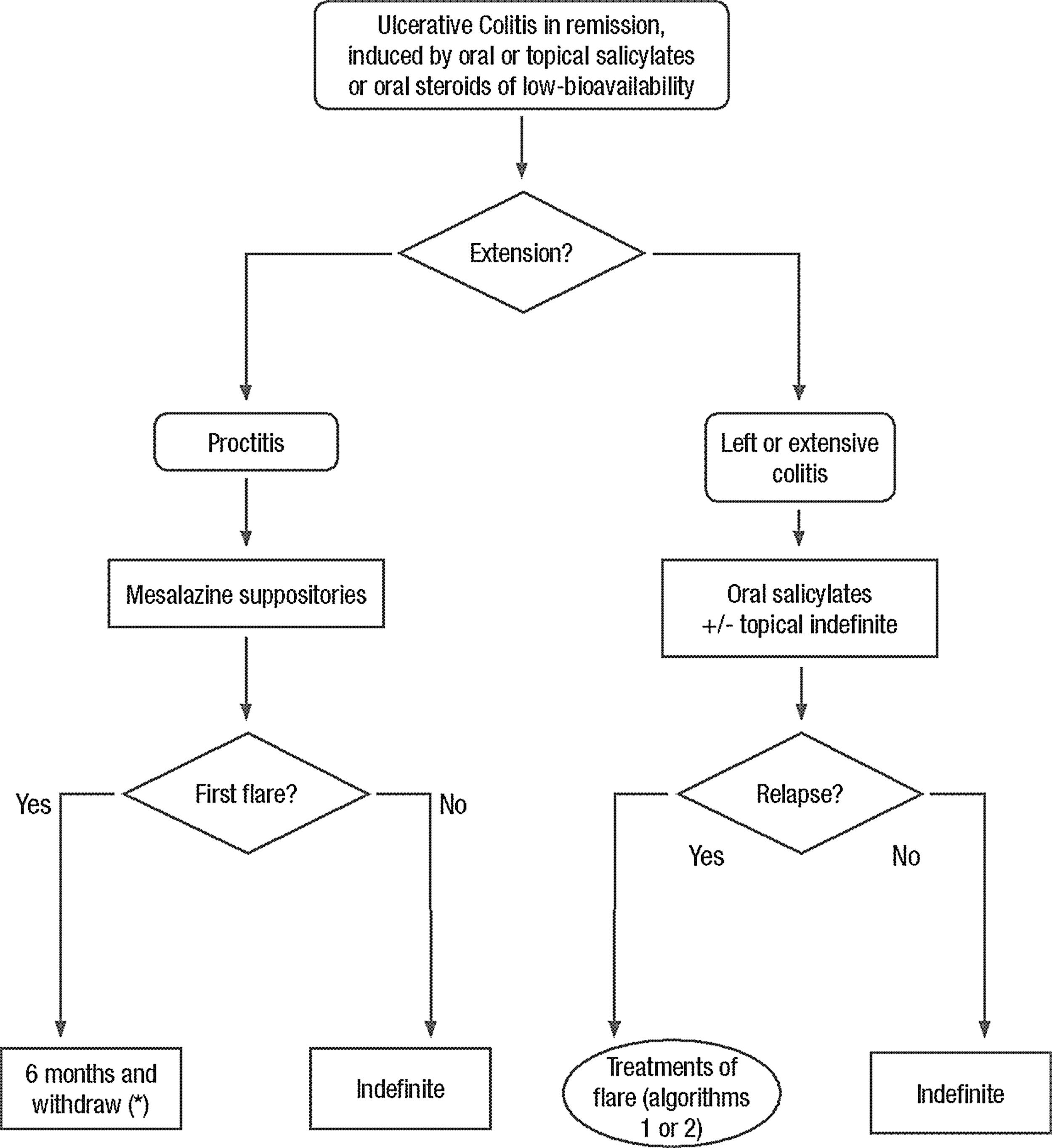
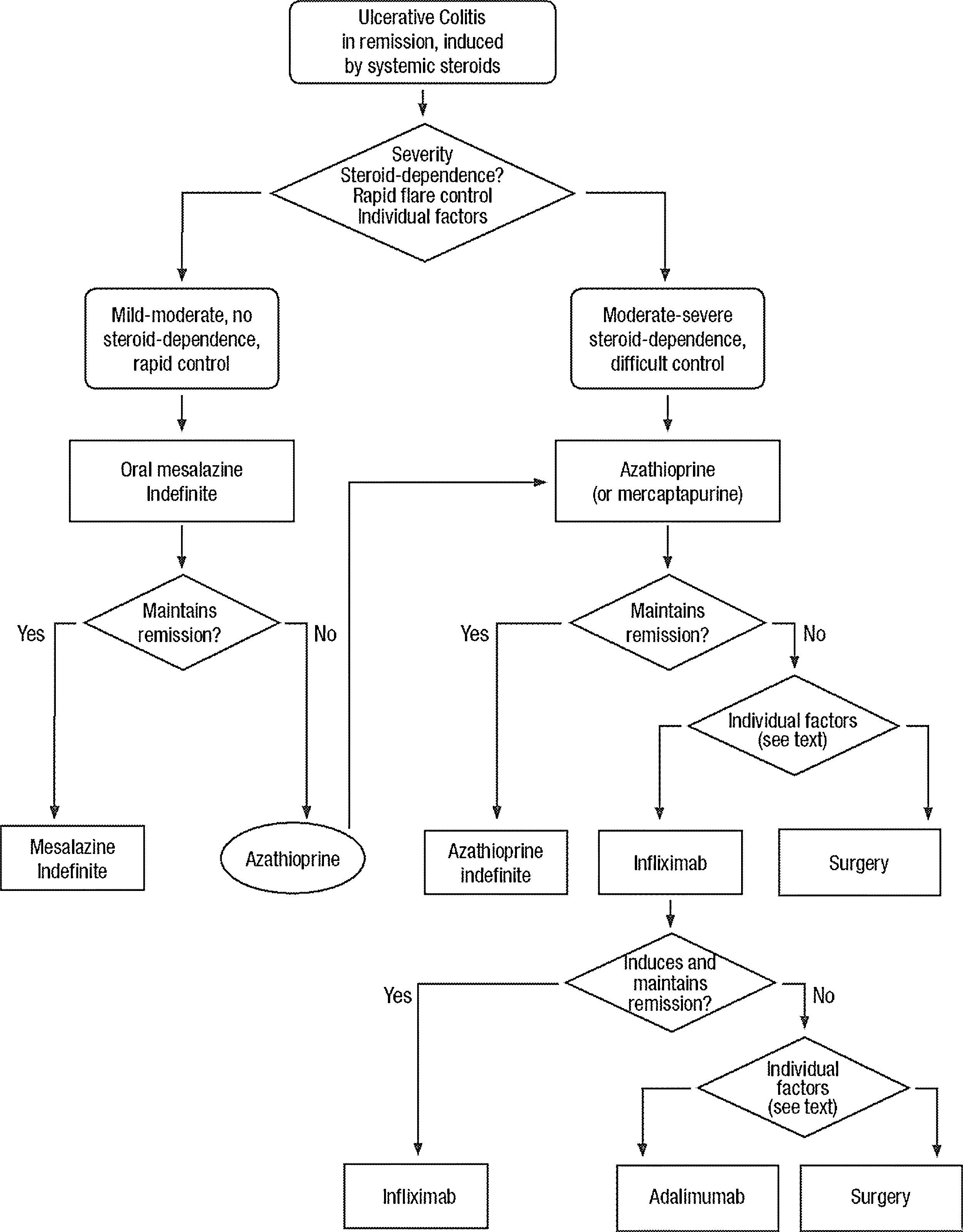
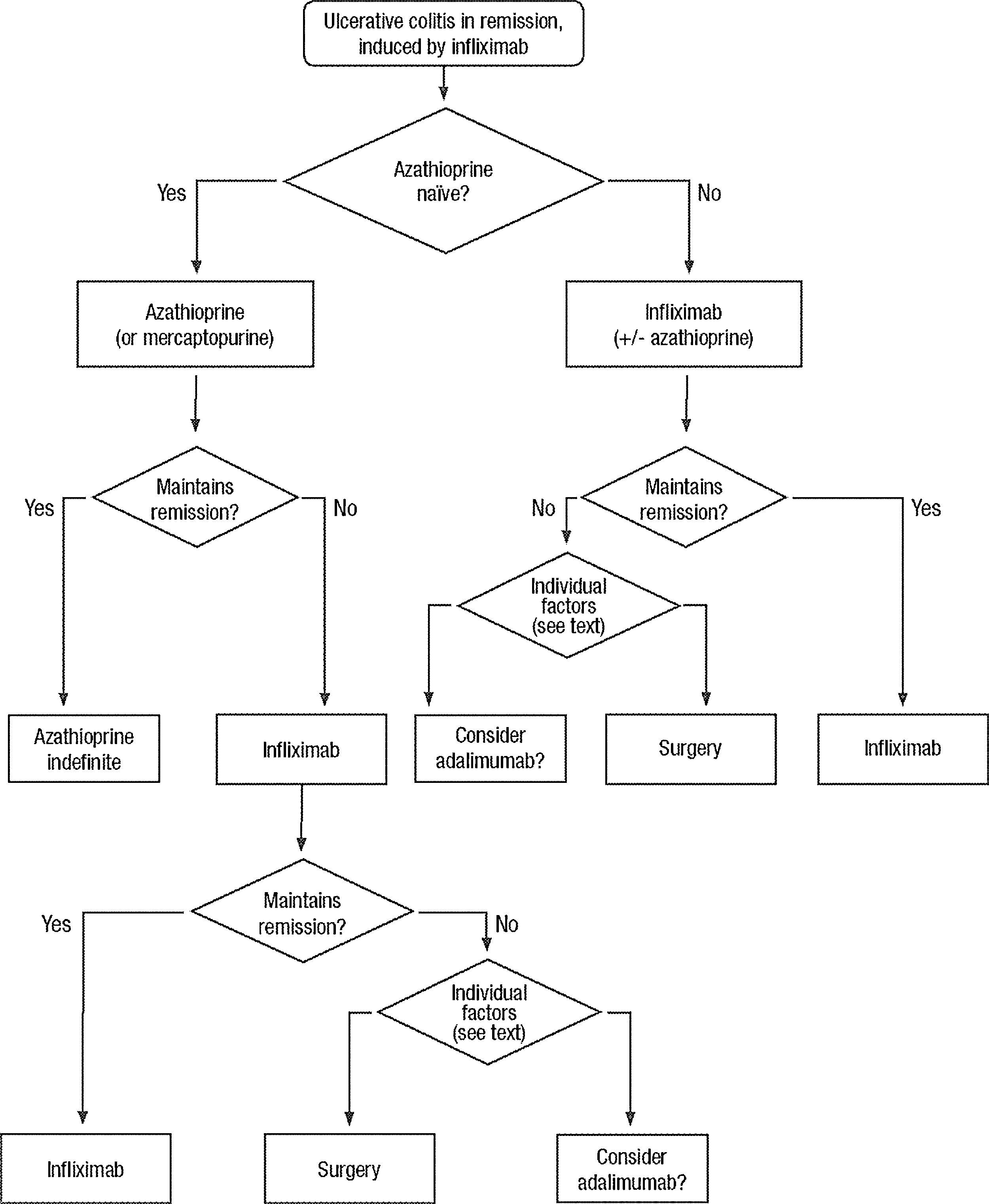
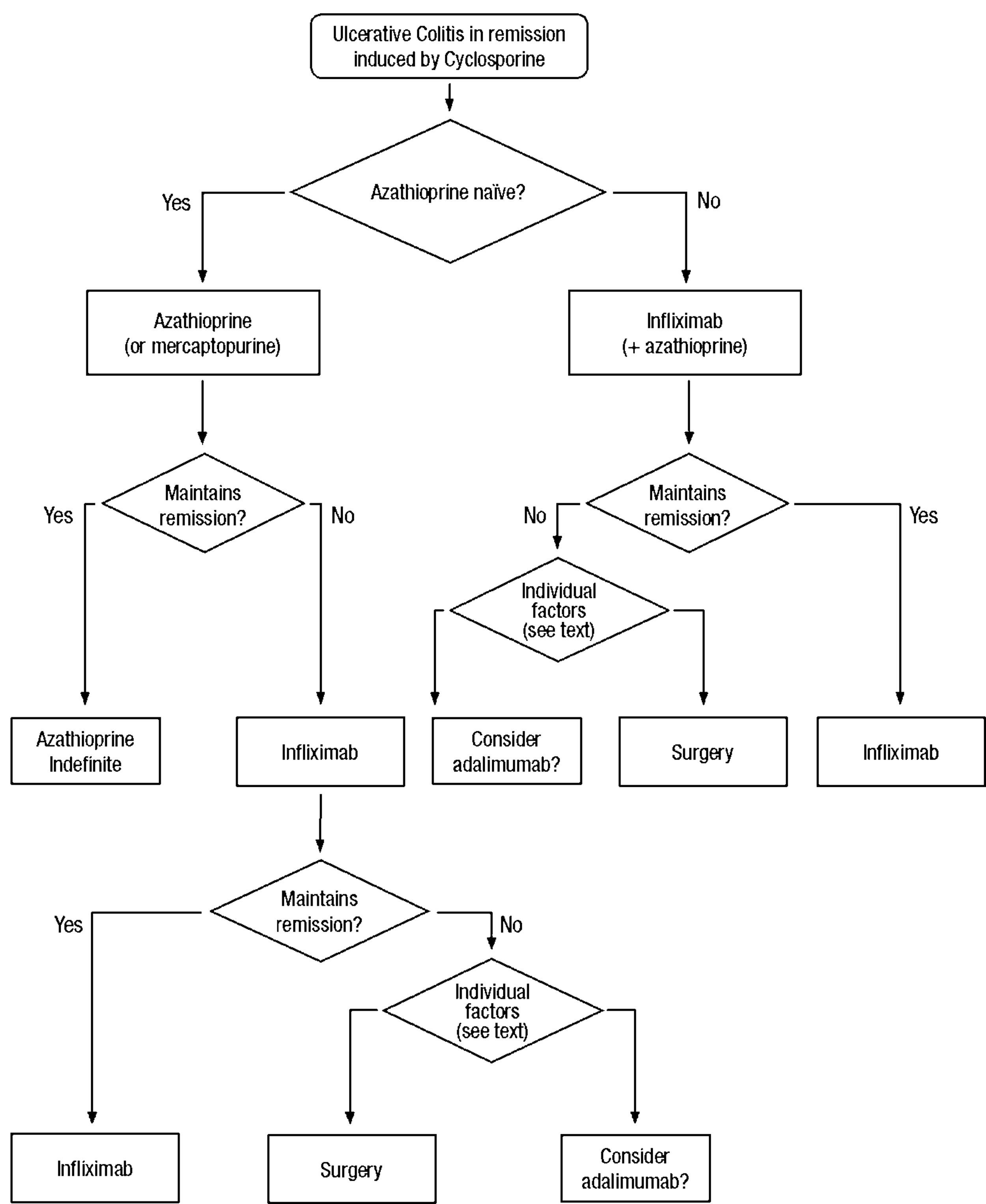
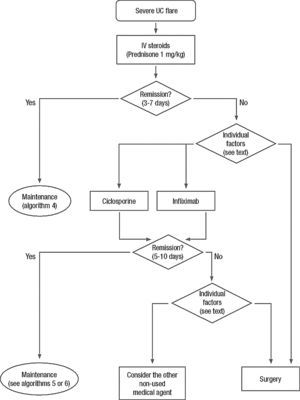
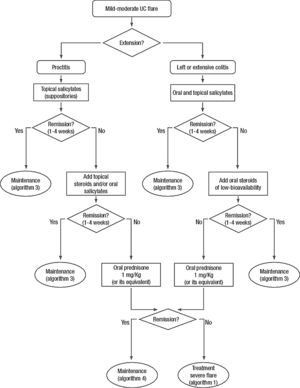
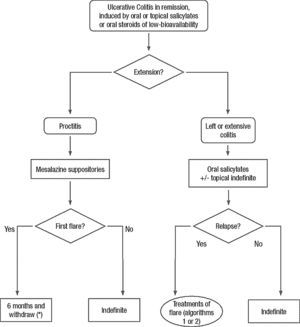
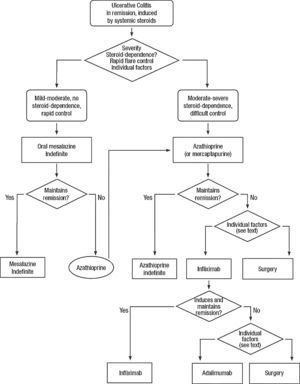
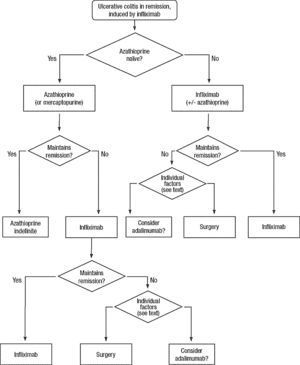
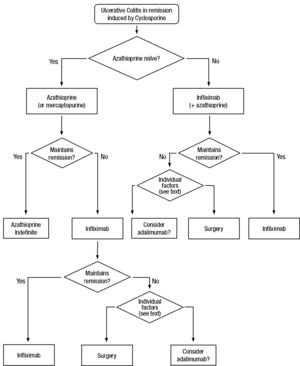
Clinical scenarios and clinical questions: Treatment algorithmsAfter defining those three broad clinical scenarios, the information available in the medical literature needs to be reviewed and classified. Taking into account the critical variables defined following the GRADE methodology, the possible clinical scenarios, the therapeutic alternatives available, and the available literature, a total of 32 relevant clinical questions were formulated (Table 1). A systematic review of the literature and an evaluation of the evidence were undertaken for each of the 32 questions, once again following the GRADE methodology, with the pertinent performance of specific tables when possible (available on request), finally making the appropriate recommendations (Table 2). The responses to these questions were the basis for six different algorithms for guiding clinical decisions (Figs. 1–6). The complete text of the guide contains a summary of the evidence for each particular question, recommendations, written comments for the algorithms, 17 tables, 3 “boxes” of general comments on methodology, 6 algorithms and several appendices (available at http://dx.doi.org/10.1016/j.gastrohep.2012.11.002).
The 32 clinically relevant questions formulated.
| Induction of remission in severe ulcerative colitis flares |
| Are steroids effective in the induction of remission in patients with a severe UC flare? |
| Is cyclosporine effective in the induction of remission in patients with a severe UC flare? And in steroid-resistance? |
| Is tacrolimus effective in the induction of remission in patients with a severe UC flare? And in steroid-resistance? |
| Is infliximab effective in the induction of remission in patients with a severe UC flare? And in steroid-resistance? |
| Is heparin (at anticoagulant dose) effective in the induction of remission in patients with a severe UC flare? |
| Are antibiotics effective in the induction of remission in patients with a severe UC flare? |
| Is surgery effective in the induction of remission in patients with a severe UC flare? |
| Is parenteral nutrition effective in the induction of remission in patients with a severe UC flare? |
| Induction of remission in mild to moderate ulcerative colitis flare |
| Are oral salicylates effective in the induction of remission in patients with a mild-moderate UC flare? |
| Is combined treatment with oral and rectal salicylates more effective than oral alone in the induction of remission in patients with a mild-moderate UC flare? |
| Are rectal salicylates alone effective in the induction of remission in mild-moderate flares of left-sided colitis? |
| Are rectal salicylates more effective than oral salicylates in the induction of remission in patients with a moderate flare of left-sided UC? |
| Are systemic steroids effective in the induction of remission in patients with mild-moderate UC flares? |
| Are oral steroids of low-bioavailability effective in the induction of remission in mild-moderate UC flares? |
| Are rectal steroids effective in the induction of remission in mild-moderate left-sided UC flares? |
| Are rectal steroids of low bioavailability more effective than those of high bioavailability in the induction of remission in patients with mild-moderate left-sided UC flares? |
| Are rectal salicylates more effective than rectal steroids in the induction of remission in patients with a mild-moderate left-sided UC flare? |
| Are thiopurines effective in the induction of remission in patients with a moderate flare of steroid-resistant or steroid-dependent UC? |
| Is methotrexate effective in the induction of remission in patients with a moderate flare of steroid-dependent UC? |
| Is infliximab effective in the induction of remission in patients with a moderate flare of steroid-dependent or steroid-resistant UC? |
| Is adalimumab effective in the induction of remission in patients with a moderate flare of steroid-dependent or steroid-resistant UC? |
| Is treatment with aphaeresis effective in the induction of remission in patients with a moderate flare of steroid-dependent UC? |
| Maintenance treatment of patients with ulcerative colitis in remission |
| Is treatment with oral salicylates effective in the maintenance of remission in patients after a mild-moderate UC flare treated with salicylates? |
| Is treatment with rectal salicylates effective in the maintenance of remission in patients after a mild-moderate flare of left-sided UC treated with salicylates? |
| Is treatment with thiopurines effective in the maintenance of remission in patients after a mild-moderate flare of steroid-dependent UC? |
| Is treatment with methotrexate effective in the maintenance of remission in patients after a mild-moderate flare of steroid-dependent UC? |
| Is treatment with infliximab effective in the maintenance of remission in patients after a mild-moderate flare of steroid-dependent or steroid-resistant UC? |
| Is maintenance treatment with thiopurines effective in patients with severe UC who have obtained remission with infliximab? |
| Is maintenance treatment with infliximab effective in patients with severe UC who have obtained remission with infliximab? |
| Is maintenance treatment with thiopurines effective in patients with severe UC who have obtained remission with cyclosporine? |
| Is maintenance treatment with cyclosporine effective in patients with severe UC who have obtained remission with cyclosporine? |
| Is maintenance treatment with tacrolimus effective in patients with severe UC who have obtained remission with tacrolimus? |
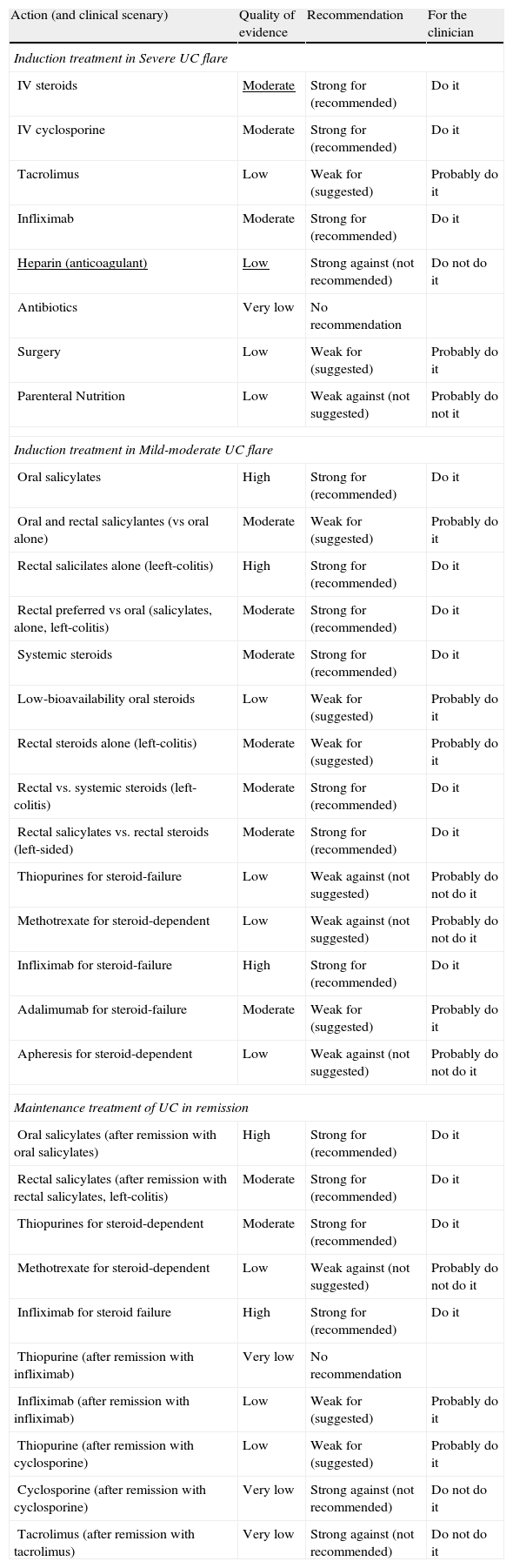
Summary of the quality of the evidence and grade of the recommendation for the 32 statements.
| Action (and clinical scenary) | Quality of evidence | Recommendation | For the clinician |
| Induction treatment in Severe UC flare | |||
| IV steroids | Moderate | Strong for (recommended) | Do it |
| IV cyclosporine | Moderate | Strong for (recommended) | Do it |
| Tacrolimus | Low | Weak for (suggested) | Probably do it |
| Infliximab | Moderate | Strong for (recommended) | Do it |
| Heparin (anticoagulant) | Low | Strong against (not recommended) | Do not do it |
| Antibiotics | Very low | No recommendation | |
| Surgery | Low | Weak for (suggested) | Probably do it |
| Parenteral Nutrition | Low | Weak against (not suggested) | Probably do not it |
| Induction treatment in Mild-moderate UC flare | |||
| Oral salicylates | High | Strong for (recommended) | Do it |
| Oral and rectal salicylantes (vs oral alone) | Moderate | Weak for (suggested) | Probably do it |
| Rectal salicilates alone (leeft-colitis) | High | Strong for (recommended) | Do it |
| Rectal preferred vs oral (salicylates, alone, left-colitis) | Moderate | Strong for (recommended) | Do it |
| Systemic steroids | Moderate | Strong for (recommended) | Do it |
| Low-bioavailability oral steroids | Low | Weak for (suggested) | Probably do it |
| Rectal steroids alone (left-colitis) | Moderate | Weak for (suggested) | Probably do it |
| Rectal vs. systemic steroids (left-colitis) | Moderate | Strong for (recommended) | Do it |
| Rectal salicylates vs. rectal steroids (left-sided) | Moderate | Strong for (recommended) | Do it |
| Thiopurines for steroid-failure | Low | Weak against (not suggested) | Probably do not do it |
| Methotrexate for steroid-dependent | Low | Weak against (not suggested) | Probably do not do it |
| Infliximab for steroid-failure | High | Strong for (recommended) | Do it |
| Adalimumab for steroid-failure | Moderate | Weak for (suggested) | Probably do it |
| Apheresis for steroid-dependent | Low | Weak against (not suggested) | Probably do not do it |
| Maintenance treatment of UC in remission | |||
| Oral salicylates (after remission with oral salicylates) | High | Strong for (recommended) | Do it |
| Rectal salicylates (after remission with rectal salicylates, left-colitis) | Moderate | Strong for (recommended) | Do it |
| Thiopurines for steroid-dependent | Moderate | Strong for (recommended) | Do it |
| Methotrexate for steroid-dependent | Low | Weak against (not suggested) | Probably do not do it |
| Infliximab for steroid failure | High | Strong for (recommended) | Do it |
| Thiopurine (after remission with infliximab) | Very low | No recommendation | |
| Infliximab (after remission with infliximab) | Low | Weak for (suggested) | Probably do it |
| Thiopurine (after remission with cyclosporine) | Low | Weak for (suggested) | Probably do it |
| Cyclosporine (after remission with cyclosporine) | Very low | Strong against (not recommended) | Do not do it |
| Tacrolimus (after remission with tacrolimus) | Very low | Strong against (not recommended) | Do not do it |
In two of the 32 clinical questions, it was not possible to reach a recommendation because the evidence available was considered of very low quality (Table 2).
The response was categorized as a strong recommendation against an intervention in three questions, with very low quality of evidence in two of them and low quality in one (Table 2).
In five questions, the response was a weak recommendation against an intervention, based all on low quality evidence.
In eight questions, there was a weak recommendation for an intervention, based three times on moderate quality evidence and five on low quality evidence.
In fourteen occasions, we found evidence to support a strong recommendation for an intervention, although high quality evidence was available only in five of them, the rest being only of moderate quality.
DiscussionUC is a chronic disease with very diverse clinical scenarios, in which several different treatments are available, and with a high variability in clinical practice.23 Therefore, the need of clinical guidelines seems evident. In fact, in the last 10 years, a number of consensus guidelines have appeared,7–12 in addition to a number of expert recommendations. Although we lack direct evidence of its use in real practice, the ECCO guidelines are probably widely known, as they are very frequently cited in the literature. In addition, ECCO has a specific program of workshops to disseminate the guidelines recommendations. In fact, at least in Spain we know that clinicians treating UC patients have a very good knowledge of the ECCO statements, and a very good agreement with its recommendations has been confirmed.24 All the guidelines available are evidence-based, but all of them rely on a consensus process without a specific systematic methodology for a true Clinical Practice Guideline.
As previously mentioned, the present guideline tries to follow the recommendations of the AGREE initiative,17 using the currently widely accepted GRADE methodology.16,22 A formal comparison with available guidelines is a difficult task that exceeds the scope of this report. In our view, to follow the GRADE methodology has the advantage of a systematic approach, taking into account not only the high-quality evidence available, but also observational data, social and cost-related considerations, and the experience of the authors and reviewers.20 The final 32 recommendations and 6 algorithms proposed could help the clinician in the rather complex process of making decisions in the daily practice, in a system that is very easy to grasp.
There are, however, some limitations to be considered. First, a final guideline can only be only as good as the evidence that is available.25 We could only base our recommendation on high-quality evidence in 5 out of 32 cases, and we had to classify the quality as moderate in 12 more questions; hence, in almost 50% of the scenarios defined, the quality of the evidence is low or very low. It is clear that we need more high-quality clinical research in this field. Furthermore, there is a clear limitation in the literature available: definitions of disease activity are very different among the studies reported. This makes it especially difficult to compare the results among the different reports and populations, and the elaboration of GRADE tables has been very difficult in some of the questions, and even impossible in others. Finally, the use of a systematic methodology cannot eliminate personal bias completely. Although we have undertaken a complete and elaborate process of internal and external review, with several rounds (with more than 40 people contributing, including surgeons, nurses and patients), we cannot completely exclude this bias.
This is the short version and its full version is available online.
Conflict of interestThis guide has been possible to some extent by an unrestricted grant of MSD. MSD was not involved in any part of the development or scientific aspects of the guide.
F. Gomollón has received grants for assistance to scientific meetings from Abbott and MSD. He has also received consultancy fees from FAES-FARMA, Abbott and MSD. He has also received research support from MSD.
S. García-López has received grants for assistance to scientific meetings from Abbott, MSD, FAES-FARMA, Ferring and Shire. He has also received consultancy fees from Abbott and MSD.
B. Sicilia has received grants for assistance to scientific meetings from Abbott, MSD, FAES-FARMA, Ferring y Shire.
Javier P Gisbert has received grants for assistance to scientific meetings from Abbott and MSD. He has also received consultancy fees from FAES-FARMA, Abbott and MSD.
Joaquin Hinojosa has received grants for assistance to scientific meetings from Abbott and MSD. He has also received consultancy fees from FAES-FARMA, Abbott and MSD.
On behalf of GETECCU (Grupo Español de Trabajo de Enfermedad de Crohn y Colitis Ulcerosa or Spanish Group for Working on Crohn's Disease and Ulcerative Colitis).