Clostridium difficile ribotype 027 (Cd027) has caused outbreaks in the United States, Canada, and Europe since 2001. In Spain, the importance of Cd027 is still unknown. In 2007, we began active surveillance of Cd027 to determine its incidence in our hospital.
MethodsFrom January 2007 to April 2012, isolates of C. difficile by multiplex PCR were studied to detect toxin genes. Binary toxin-positive isolates were characterized using PCR-ribotyping. Cd027 were further characterized by toxino-typing, sequencing of tcdC gene, and MLVA (multilocus-variable-number-tandem-repeat-analysis).
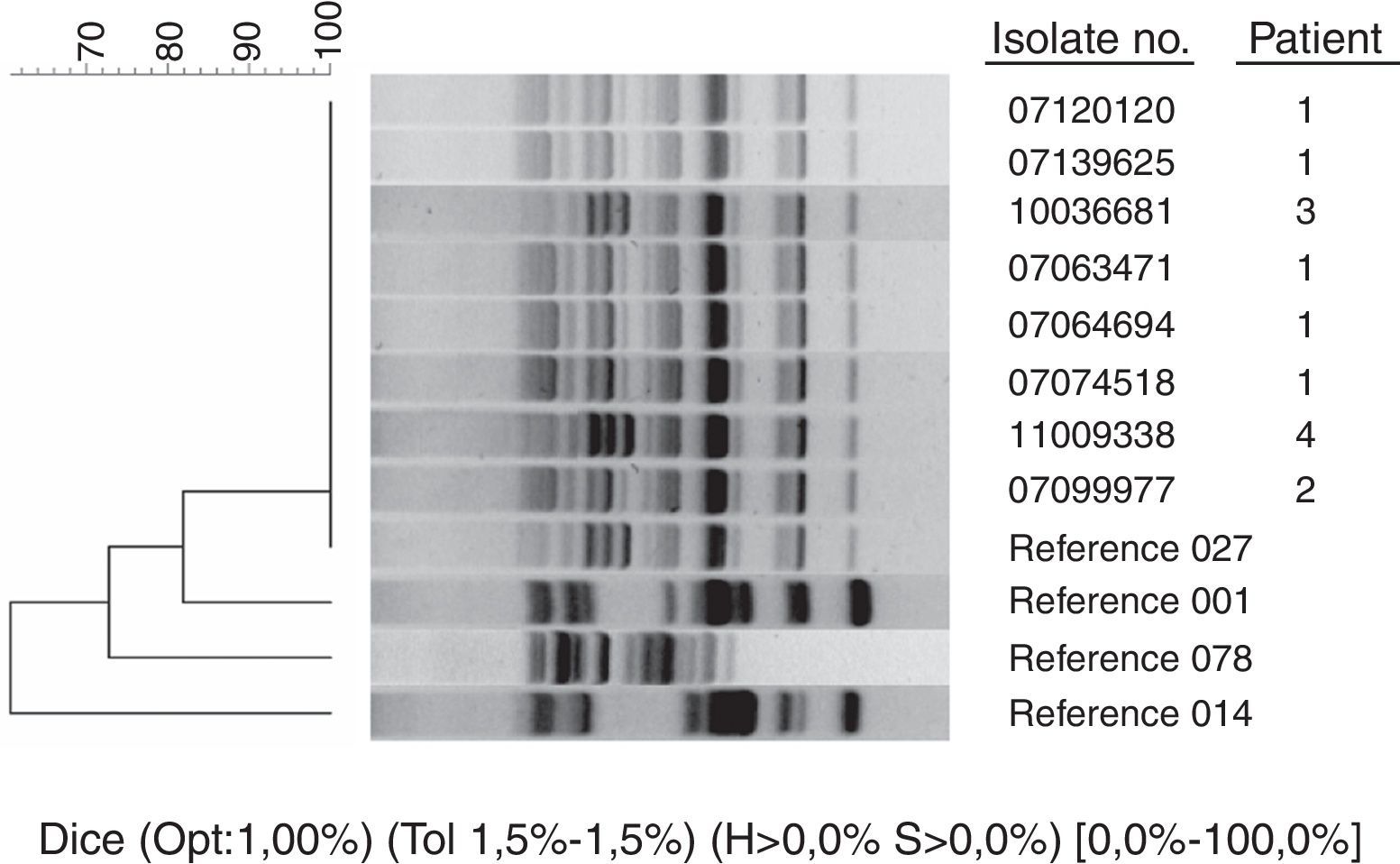
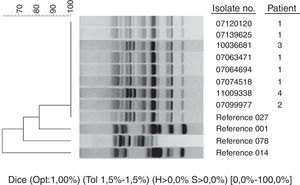
ResultsOnly 8 strains were Cd027 from 3666 isolates of C. difficile analyzed during the study period. These strains were isolated from 4 patients: a Spanish patient previously hospitalized in the UK, a pregnant laboratory technician, a British tourist, and a Spanish patient without epidemiological antecedents for acquiring Cd027. MLVA typing of Cd027 isolates revealed 4 different patterns. The first patient had 2 episodes of diarrhea caused by different Cd027. The strains from the first episode of patient 1 and the strain from patient 2 were grouped in the same clonal cluster (these cases were previously published as laboratory transmission), while strains from patients 3 and 4 were genetically unrelated to each other, and to the strains from patients 1 and 2.
ConclusionWe report the first finding of an autochthonous case of non-severe Cd027 infection. Our results indicate that Cd027 diarrhea is uncommon in our area, and it appears mainly as imported cases. MLVA typing enables us to distinguish different genotypes among our Cd027 isolates.
Clostridium difficile del ribotipo 027 (Cd027) ha causado importantes brotes en EE. UU, Canadá y Europa desde 2001. Actualmente su importancia en España es poco conocida. En 2007, nuestro grupo inició la búsqueda de Cd027 para determinar su incidencia en nuestro hospital.
MétodosDesde enero de 2007 hasta abril de 2012 se estudiaron todos los aislados de C. difficile mediante PCR multiplex de los genes de las toxinas. Las cepas toxina binaria positivas se caracterizaron por PCR-ribotipado. Las cepas de Cd027 encontradas se genotiparon por toxinotipo, secuenciación del gen tcdC y MLVA.
ResultadosDurante el periodo de estudio se analizaron 3.666 cepas de C. difficile de las que solo 8 fueron Cd027. Estas cepas se aislaron de 4 pacientes: una paciente española previamente hospitalizada en el Reino Unido, una técnico de nuestro laboratorio, una turista británica y un paciente español sin antecedentes de riesgo para haber adquirido Cd027. Mediante MLVA obtuvimos 4 patrones de tipado diferentes. La primera paciente tuvo 2 episodios de diarrea causados por cepas diferentes de Cd027. Una de estas cepas fue la misma que la de nuestra técnico de laboratorio (este caso está publicado como una transmisión de laboratorio). Las cepas de los pacientes 3 y 4 tuvieron MLVA únicos.
ConclusiónEn este trabajo describimos el primer autóctono de diarrea causada por Cd027. Nuestros resultados indican que es infrecuente en nuestro medio y que aparece principalmente como casos importados. El tipado por MLVA nos ha permitido diferenciar genotipos diferentes entre los aislados de Cd027 de nuestro hospital.
Hypervirulent epidemic strains of Clostridium difficile belonging to ribotype 027 (toxinotype III, toxin A+B+binary+) have been reported to cause important outbreaks of severe infection in the United States and Canada since 2001.1,2 Since 2003, C. difficile ribotype 027 (Cd027) has been detected in major outbreaks or sporadic cases in several European countries.3 In Spain, data describing the molecular epidemiology of C. difficile are scarce, and the importance of Cd027 is still unknown.4,5
In this report, we describe the first Spanish autochthonous case of Cd027 infection and present an additional 3 cases of diarrhea caused by Cd027 strains isolated at our institution.
Materials and methodsSince January 2007, our laboratory has performed active surveillance of Cd027 based on multiplex polymerase chain reaction (PCR) to detect C. difficile toxin genes in all strains of C. difficile isolated in our hospital. tdcA conserved fragment, tcdA deleted fragment, tcdB, cdtA, cdtB, and 16SrRNA (internal control) were amplified by PCR following a method adapted from other authors.6,7
As Cd027 is characterized by binary toxin production, all binary toxin-positive strains were ribotyped.8 Strains belonging to ribotype 027 were further characterized by toxinotyping,9 sequencing of tcdC,10 and multilocus-variable-number of tandem repeat analysis (MLVA).11 MLVA patterns were interpreted using summed tandem repeat differences (STRD).11 Susceptibility to erythromycin, clindamycin, moxifloxacin, rifampin, imipenem, metronidazole, and vancomycin was assessed using the E-test in Brucella agar.
By April 1st, 2012 we had studied 3666 strains of C. difficile of which 444 (12%) had binary toxin genes. A total of 360 strains (10%) belonged to toxinotype V ribotype 078/126, and only 8 (0.21%) belonged to toxinotype III ribotype 027 (Fig. 1, ribotyping pattern compared to epidemic strains kindly provided by E.J. Kuijper). All Cd027 strains had a single 1-bp deletion at position 117 and an 18-bp deletion in the tcdC gene that is characteristic of the epidemic C. difficile strain ribotype 027. These strains were isolated from 4 patients (Table 1).
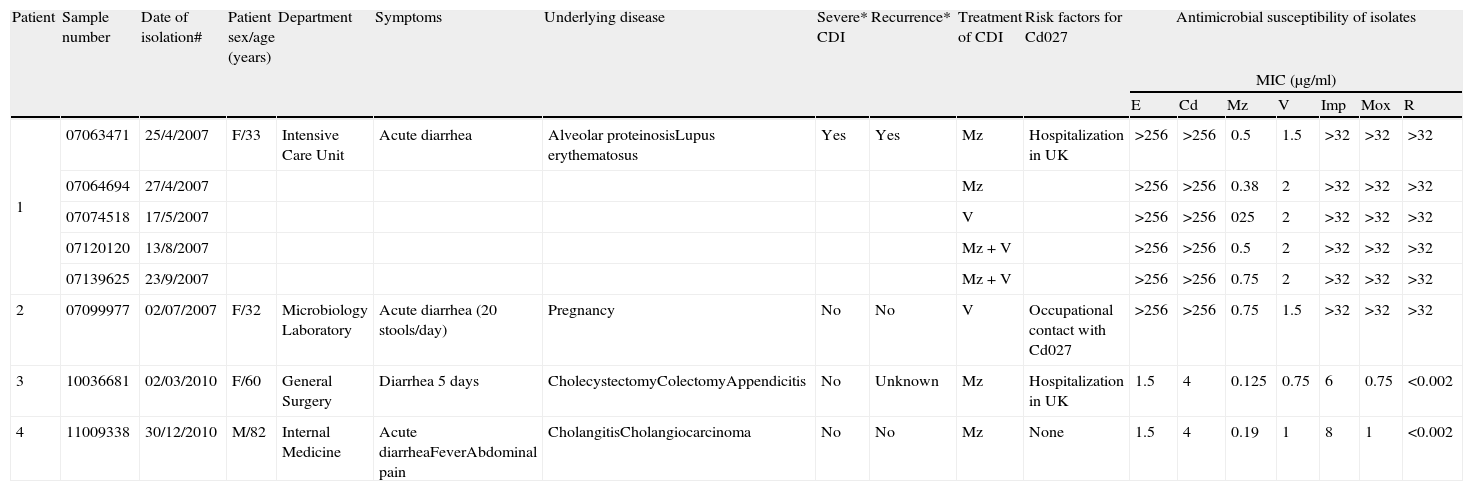
Characteristics of patients with Cd027 infection and susceptibility of isolates.
| Patient | Sample number | Date of isolation# | Patient sex/age (years) | Department | Symptoms | Underlying disease | Severe* CDI | Recurrence* | Treatment of CDI | Risk factors for Cd027 | Antimicrobial susceptibility of isolates | ||||||
| MIC (μg/ml) | |||||||||||||||||
| E | Cd | Mz | V | Imp | Mox | R | |||||||||||
| 1 | 07063471 | 25/4/2007 | F/33 | Intensive Care Unit | Acute diarrhea | Alveolar proteinosisLupus erythematosus | Yes | Yes | Mz | Hospitalization in UK | >256 | >256 | 0.5 | 1.5 | >32 | >32 | >32 |
| 07064694 | 27/4/2007 | Mz | >256 | >256 | 0.38 | 2 | >32 | >32 | >32 | ||||||||
| 07074518 | 17/5/2007 | V | >256 | >256 | 025 | 2 | >32 | >32 | >32 | ||||||||
| 07120120 | 13/8/2007 | Mz+V | >256 | >256 | 0.5 | 2 | >32 | >32 | >32 | ||||||||
| 07139625 | 23/9/2007 | Mz+V | >256 | >256 | 0.75 | 2 | >32 | >32 | >32 | ||||||||
| 2 | 07099977 | 02/07/2007 | F/32 | Microbiology Laboratory | Acute diarrhea (20 stools/day) | Pregnancy | No | No | V | Occupational contact with Cd027 | >256 | >256 | 0.75 | 1.5 | >32 | >32 | >32 |
| 3 | 10036681 | 02/03/2010 | F/60 | General Surgery | Diarrhea 5 days | CholecystectomyColectomyAppendicitis | No | Unknown | Mz | Hospitalization in UK | 1.5 | 4 | 0.125 | 0.75 | 6 | 0.75 | <0.002 |
| 4 | 11009338 | 30/12/2010 | M/82 | Internal Medicine | Acute diarrheaFeverAbdominal pain | CholangitisCholangiocarcinoma | No | No | Mz | None | 1.5 | 4 | 0.19 | 1 | 8 | 1 | <0.002 |
To define episode# and severity* of CDI we considered Refs. 5,12 respectively.
CDI, Clostridium difficile infection; E, erythromycin; Cd, clindamycin; Mz, metronidazole; V, vancomycin; Imp, imipenem; Mox, moxifloxacin; R, rifampin.
Case 1: The first case of Cd027 infection in our institution was a Spanish woman living in the UK, where she was being treated for alveolar proteinosis and systemic lupus erythematosus. She returned to Spain to undergo lung transplantation. During her stay in our medical ICU she developed 2 different episodes of severe12C. difficile infection (considering as different 2 episodes of C. difficile diarrhea separated more than 8 weeks5). Detection of C. difficile toxin A and B antigens in feces was positive (Immunocard, Meridian Bioscience), cytotoxicity assay and toxigenic culture were positive too. Five different isolates of Cd027 were obtained (Table 1). The patient survived her episodes of C. difficile infection but died soon afterwards from other complications of her underlying diseases. Despite the fact that the first episode occurred 20 days after admission to our ICU and 3 months after the last hospitalization in the UK, the epidemiological history led us to consider the Cd027 infection as imported. Data from this case were partially reported in 2008.13
Case 2: A healthy pregnant laboratory technician working with the C. difficile isolates of case 1 developed C. difficile diarrhea shortly after being treated with oral fosfomycin for urinary tract infection. C. difficile toxin A and B antigens were detected (Immunocard, Meridian Bioscience), and the cytotoxicity assay and toxigenic culture from feces were also positive. This case was previously reported as a laboratory-acquired C. difficile infection.13
Case 3: A British tourist was admitted to our hospital emergency department in March 2010 with clinical features of acute appendicitis. She had developed acute diarrhea during the previous 5 days. Diarrhea persisted during her post-surgical stay, and a study of C. difficile was performed. C. difficile toxin A and B antigens were not detected (Immunocard, Meridian Bioscience) and the results of the direct cytotoxicity assay was negative. The patient's previous medical history was unclear, although she appears to have had chronic diarrhea for more than 20 years and underwent several operations in the UK more than 10 years ago (cholecystectomy, hysterectomy, and colonic resection by rectocele). We do not know if this patient had recent contact with health-care institutions in UK. This case was also considered to be imported.
Case 4: An 82-year-old Spanish man whose only recent contact with a health care institution had been with our center for acute cholecystitis (30 days previously) and complicated urinary tract infection (10 days previously), received ertapenem followed by amoxicillin/clavulanic acid to treat both infections. The patient was admitted to the emergency department of our hospital in December 2011 with a 3-day history of fever, watery diarrhea, and abdominal pain. Glutamate dehydrogenase was positive in stools, but toxins A and B were negative (Techlab C. diff Quik Chek Complete, Blacksburg Va). GeneExpert C. diff (Cepheid, CA, USA) was then performed given positive results for toxigenic C. difficile presumptive 027 strain. The result of the direct cytotoxicity assay in cell culture was also positive. Stool cultures for enteropathogens were negative, as was detection of rotavirus and adenovirus antigen. C. difficile was isolated after 48h of incubation in agar CLO (bioMérieux). Toxigenicity was confirmed by the cytotoxicity assay and multiplex PCR. Ribotyping confirmed Cd027. The patient was treated with metronidazole, and the episode resolved with no further recurrence. His only travel abroad had been to attend a football match in Vienna in 2009.
All patients but the first one had non-severe diarrhea, and none developed important complications related to Cd027 infection.12 Only the first patient had recurrence of C. difficile infection and only the first two cases were related in time (Table 1).
Characterization of isolatesAll our Cd027 isolates were susceptible to metronidazole. The isolates for the first 2 patients were highly resistant to erythromycin, clindamycin, moxifloxacin, rifampin, and imipenem, although the Cd027 isolates of the last 2 patients were not so (Table 1). The vancomycin MIC varied from 0.75μg/ml to 2μg/ml.
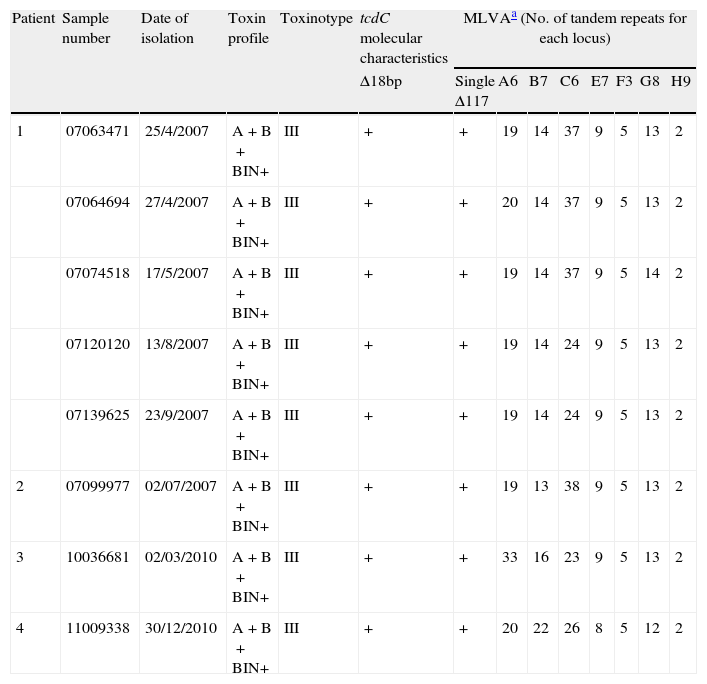
MLVA typing of Cd027 isolates revealed 4 different patterns (Table 2). The strains from the first episode of patient 1 and the strain from patient 2 were grouped in the same clonal cluster (STRD≤2). Strains from patients 3 and 4 were genetically unrelated to each other and to strains from patients 1 and 2 (STRD≥10). Surprisingly, MLVA typing demonstrated that the strains from the 2 episodes of patient 1 were different and did not belong to the same clone (STRD≥10), probably reflecting sequential isolation of different Cd027 strains from an initial polyclonal infection acquired in the hospital of origin in the UK.
Molecular characteristics of the Cd027 isolates studied.
| Patient | Sample number | Date of isolation | Toxin profile | Toxinotype | tcdC molecular characteristics | MLVAa (No. of tandem repeats for each locus) | |||||||
| Δ18bp | Single Δ117 | A6 | B7 | C6 | E7 | F3 | G8 | H9 | |||||
| 1 | 07063471 | 25/4/2007 | A+B+BIN+ | III | + | + | 19 | 14 | 37 | 9 | 5 | 13 | 2 |
| 07064694 | 27/4/2007 | A+B+BIN+ | III | + | + | 20 | 14 | 37 | 9 | 5 | 13 | 2 | |
| 07074518 | 17/5/2007 | A+B+BIN+ | III | + | + | 19 | 14 | 37 | 9 | 5 | 14 | 2 | |
| 07120120 | 13/8/2007 | A+B+BIN+ | III | + | + | 19 | 14 | 24 | 9 | 5 | 13 | 2 | |
| 07139625 | 23/9/2007 | A+B+BIN+ | III | + | + | 19 | 14 | 24 | 9 | 5 | 13 | 2 | |
| 2 | 07099977 | 02/07/2007 | A+B+BIN+ | III | + | + | 19 | 13 | 38 | 9 | 5 | 13 | 2 |
| 3 | 10036681 | 02/03/2010 | A+B+BIN+ | III | + | + | 33 | 16 | 23 | 9 | 5 | 13 | 2 |
| 4 | 11009338 | 30/12/2010 | A+B+BIN+ | III | + | + | 20 | 22 | 26 | 8 | 5 | 12 | 2 |
MLVA patterns interpreted considering STRD (summed tandem repeat differences) as previously described.11
To our knowledge, we report the first autochthonous case of C. difficile infection caused by Cd027 in Spain. The patient had no previous contact with health care institutions in Spain or abroad; therefore, we were unable to track the origin of the strain and we ignore if this patient had contact with people (households, relatives, etc.) from environments where Cd027 is frequent.14 The review of the other 3 episodes shows that Cd027 mainly appears as imported cases. The first 2 cases of Cd027 in our hospital were detected in 2007. The first was considered to be imported from the UK; the second involved horizontal transmission to a laboratory technician handling the first patient's stool samples.13
Between 2000 and 2006, rates of C. difficile infection increased markedly in the USA and Canada,15,16 mainly because of the epidemic Cd027 strain.1 Although some outbreaks of Cd027 have been described in Europe,17,18 a surveillance study performed in 34 European nations in 2008 showed a mean incidence of nosocomial C. difficile infection of 4.1 cases per 10,000 patient-days (range 0.0–36.3); only 5% were caused by Cd027.5 Similarly, a pan-European Cd027 survey revealed a relatively low prevalence in Europe that varied from country to country.19 Cd027 outbreaks in Europe were contained thanks to awareness of the American experience and the introduction of strict control measures in several European countries.
It is difficult to know the real importance of Cd027 in Spain. Our results indicate that Cd027 infection could be distinctly uncommon in Spain. Although, it is necessary to consider that not all clinical microbiology laboratories in Spain currently culture C. difficile, and diagnosis of C. difficile infection is based mainly on the results of enzyme immunoassay as demonstrated in a recent paper by our group.20 Besides, Spain does not have a national surveillance program to investigate C. difficile infection or a reference laboratory where hospitals could send C. difficile isolates for further characterization. These limitations would determine that Cd027 could go undetected mainly considering that only a few hospitals perform molecular characterization of C. difficile.
These limitations are overcome in a recently reported prospective nationwide diagnostic study, in which confirmatory cultures and molecular characterization of C. difficile isolates were performed on all diarrheic stools arriving at 118 Spanish microbiology laboratories. An incidence of 3.8 cases of C. difficile infection per 10,000 patient-days was reported without isolation of Cd027.4 In other studies recently conducted in Spanish hospitals, Cd027 was also not detected.21,22 After intensive searching at our center, we detected a very low incidence of Cd027 strains. The reasons for this finding are both unclear and unexpected, since, annually, Spain receives millions of tourists from all over the world.
The incorporation in the routine diagnosis of C. difficile infection of easy-to-use systems such as GeneExpert C. diff® (Cepheid, CA, USA), which detects toxigenic C. difficile and presumptive Cd027, could improve detection of Cd027 strains in Spain, although laboratories should culture C. difficile in order to have a stock of isolates for epidemiological studies.
In conclusion, although Cd027 does not seem to be a major problem in Spain, it is important to recommend that clinical microbiology laboratories perform C. difficile culture, at least from toxin-positive stools or in severe infections, to enable epidemiological studies to know the real situation of Cd027 and other epidemic strains.
FundingThis project was partially financed by a grant (project number PS09-02389) from the FIS (Fondo de Investigaciones Sanitarias). Fragment analysis to obtain MLVA patterns and sequencing of tcdC were performed in a 3130×1 Genetic Analyzer that was financed in part by grants from the FIS (IF01-3624 and IF08-36173).
Conflict of interestThe authors declare no conflict of interest.
We are indebted to Thomas O’Boyle for editorial assistance.
This study was partially presented at the 22nd ECCMID (poster n° 2229, 31 March–3 April 2012, London, UK).