The epidemiological characteristics of patients with tuberculosis (TB) in European hospitals have changed in recent years.
MethodsA prospective study of patients with culture-proven pulmonary TB admitted to our institution from 1997 to 2008 is shown.
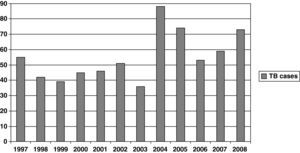
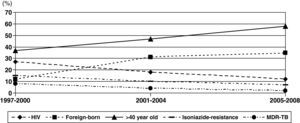
ResultsWe analyzed 661 patients with pulmonary TB. An increase in the incidence of TB was confirmed during the study period (P<.001). The proportion of patients with HIV infection decreased from 26% during 1997-2000 to 12% during 2005-2008. However, the proportions of older (>40 years old) and foreign-born patients increased significantly, from 37% to 59% and from 12% to 35%, respectively. Multivariate analysis confirmed previous antituberculous therapy and immigration as factors associated with resistance to isoniazid and to isoniazid+rifampin. After the year 2000, mortality was independently associated with extrapulmonary TB (OR: 3.1; CI 95%: 1.4-7.2), hepatitis C virus infection (OR: 6.0; CI 95%: 2.2-16.3), and diabetes (OR: 6.4; CI 95%: 2.4-16.8).
ConclusionImmigration from countries with high rates of TB infection has replaced HIV infection as the most relevant risk factor associated with TB. The increase in the number of older patients with TB and the presence of specific comorbid conditions, especially chronic liver dysfunction, could explain the more difficult management and increased mortality.
La epidemiología de los pacientes con tuberculosis ha cambiado en los hospitales europeos en los últimos años.
MétodosPresentamos un estudio prospectivo en pacientes hospitalizados en nuestra institución con tuberculosis confirmada por cultivo entre los años 1997 y 2008.
ResultadosSe analizaron 661 pacientes con tuberculosis pulmonar. Durante el período de estudio se confirmó un incremento de la incidencia (p < 0,001). El porcentaje de pacientes con infección por VIH disminuyó desde el 26% en los años 1997-2000 al 12% en los años 2005-2008. Sin embargo, la proporción de pacientes mayores de 40 años e inmigrantes se incrementó de forma significativa, desde el 37 al 59% y desde el 12 al 35% respectivamente. El análisis multivariante confirmó el tratamiento antituberculoso previo y la inmigración como factores asociados a resistencia a isoniazida y a isoniazida-rifampicina. Después del año 2000 la mortalidad se asoció de forma independiente con la presencia de tuberculosis extrapulmonar (OR: 3,1; IC 95%: 1,4-7,2), infección por virus de la hepatitis C (OR: 6,0; IC 95%: 2,2-16,3), y diabetes (OR: 6,4; IC 95%: 2,4-16,8).
ConclusiónLa inmigración desde países con elevadas incidencias de tuberculosis ha reemplazado a la infección por VIH como factor de riesgo de tuberculosis en nuestros pacientes. En los últimos años, el incremento de la edad y la presencia de otros factores de comorbilidad, especialmente hepatopatía crónica, pueden justificar una mayor morbi-mortalidad en estos pacientes.
According to the World Health Organization (WHO), tuberculosis (TB) remains the leading cause of death from curable infectious disease among adults. There are an estimated 8.8 million new cases annually —including 200,000 HIV-infected individuals— and 1.6 million deaths.1 Although estimates suggest that the number of new cases and deaths from TB is falling, recent increases in rates of drug-resistant TB have the potential to reverse these gains.2
In the region of Madrid, Spain, a TB rate of 16.9 cases/100,000 people was reported in 2007, although TB incidence had been falling since 1995.3 The decrease in the prevalence of TB has slowed down in recent years due to increased immigration from TB-endemic areas.4 In Western Europe, people born in other countries (foreign-born) account for nearly 30% of patients with TB, although geographic differences are significant, ranging from more than 60% in Sweden, Denmark, and the Netherlands to less than 15% in Finland and Southern Europe (Greece, Portugal, and Spain).5
In recent years, Spain has become the second destination, after the United States, for foreign-born from Latin America.6
The incidence of drug-resistant TB has increased. Treatment of multidrug-resistant TB (MDR-TB) is more than 100 times as costly as that of drug-susceptible TB and requires intensive management of its prolonged (18-24 months) and more toxic treatment course.7
While the average number of cases of pulmonary TB has declined in recent years, some authors have reported a slower reduction in extrapulmonary forms.8 The rate of miliary TB has remained unchanged.9
Changes in the epidemiology of TB during the last decade have modified the presentation and outcome of TB in western European hospitals, and the way the disease is managed. However, the impact of specific factors such as resistance profiles and clinical presentation (including extrapulmonary TB and outcome) have not been sufficiently analyzed. We present a prospective study of all adult patients with culture-proven TB admitted to a respiratory isolation unit.
Patients and methodsThis study was carried out at the Ramón y Cajal Hospital in Madrid, Spain, a 1000-bed university hospital providing medical care to a population of 500,000 inhabitants. In 1995, a respiratory isolation unit was created for patients referred from different departments. Patients admitted to this unit between January 1997 and December 2008 and attended by the infectious diseases department were studied prospectively. Only adults patients admitted in the respiratory isolation unit with a suspect of pulmonary TB were included in the study. Pediatric cases (<14 years old) are not usually admitted to this unit and were excluded from the analysis. Other forms of tuberculosis, mainly non-pulmonary forms not admitted in this unit, were not analyzed.
Diagnosis of tuberculosis was microbiologically confirmed by culture. Clinical samples were processed according to Tacquett & Tison method and inoculated on solid media, Lowenstein Jensen and Coletsos media (Bio Medics SL, Tres Cantos, Madrid, Spain) and from 1996 in liquid medium (Veersa TREK system, formerly ESP culture System II). The Mycobacterium tuberculosis complex strains isolates were identified using DNA probes (Gen Probe, San Diego, California, USA) and phenotypic tests. In vitro susceptibility tests were performed against fist-line drugs according to Canetti¿s method on Lowenstein Jensen medium and 7H10 agar medium until 1996 and later in liquid medium with antibiotic concentrations according to the manufacturer¿s protocol (Versa TREK system, formerly ESP culture System II). Susceptibility tests against second-line drugs were performed on 7H10 medium until 2006 and later in liquid medium (Versa TREK system) against capreomycin (2.5μg/ml), ofloxacin (2.0μg/ml), amikacin (1μg/ml) and linezolid (1μg/ml).
TB was defined as drug-susceptible when the patient had M. tuberculosis isolates at diagnosis and a positive susceptibility result for isoniazid and rifampin, independently of resistance to other first-line or second-line drugs. MDR-TB was defined as disease that was resistant to at least isoniazid and rifampin. MDR-TB with resistance to a fluoroquinolone and a second-line injectable drug was considered XDR-TB (extensively drug-resistant TB).
TB was considered to be pulmonary if M. tuberculosis was isolated in culture of respiratory samples or if the radiograph was suggestive of pulmonary TB and M. tuberculosis was isolated in non-pulmonary samples. TB was considered to be extrapulmonary if M. tuberculosis was isolated from a non-pulmonary source (including pleural fluid or pleural biopsy) or was histologically confirmed-in patients with TB proven by culture. TB was considered to be miliary (disseminated) if the chest x-ray showed characteristic features (a distinctive pattern of many tiny spots distributed throughout the lung fields with an appearance similar to that of millet seeds) and proven TB by culture of pulmonary or non-pulmonary samples.
TB was considered to have been cured if correct therapy and follow-up were confirmed and clinical and radiological features showed a favorable outcome. In the case of patients who had abandoned previous therapy and patients who had received more than one course of therapy, only the last episode was included for analysis.
The epidemiology of the disease and changes occurring during the study period were analyzed. Risk factors associated with isoniazid-resistance, MDR-TB, or XDR-TB were analyzed. Finally, factors related to mortality were analyzed globally or according to the period. The Student t test was used to compare continuous data and the Mantel-Haenszel extended chi square test to compare categorical data. The Fisher exact test was used when the expected number of cases per cell was below five. Odds ratio (OR), 95% confidence intervals (CI), and P values were calculated. Multiple logistic regression analysis was used to determine the independent risk factors associated with different types of resistance and mortality. All variables with a P<.1 in the univariate analysis were entered in the multivariate model.
ResultsWe diagnosed 661 cases of TB in patients suspected with pulmonary TB admitted to the respiratory isolation unit. Pulmonary TB was confirmed in 590 patients (89.3%). In the remaining 71 patients (10.7%), despite suspecting a pulmonary TB, extrapulmonary TB was confirmed and negative culture resulted. These patients remained in the isolation unit until pulmonary TB was ruled out. In addition, an extrapulmonary form was confirmed in 117 patients with pulmonary TB (total rate of patients with extrapulmonary forms: 28.4%, 188/661).
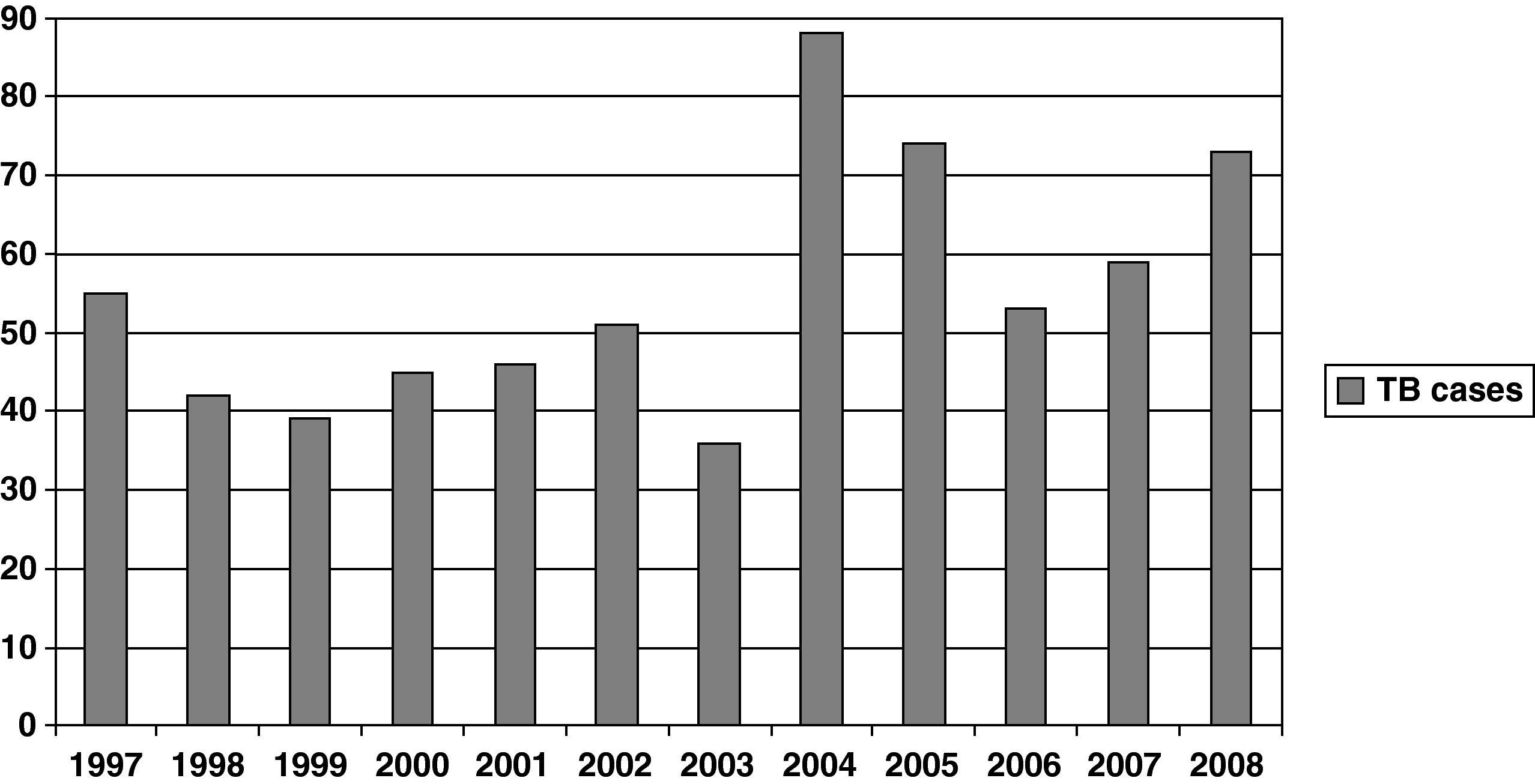
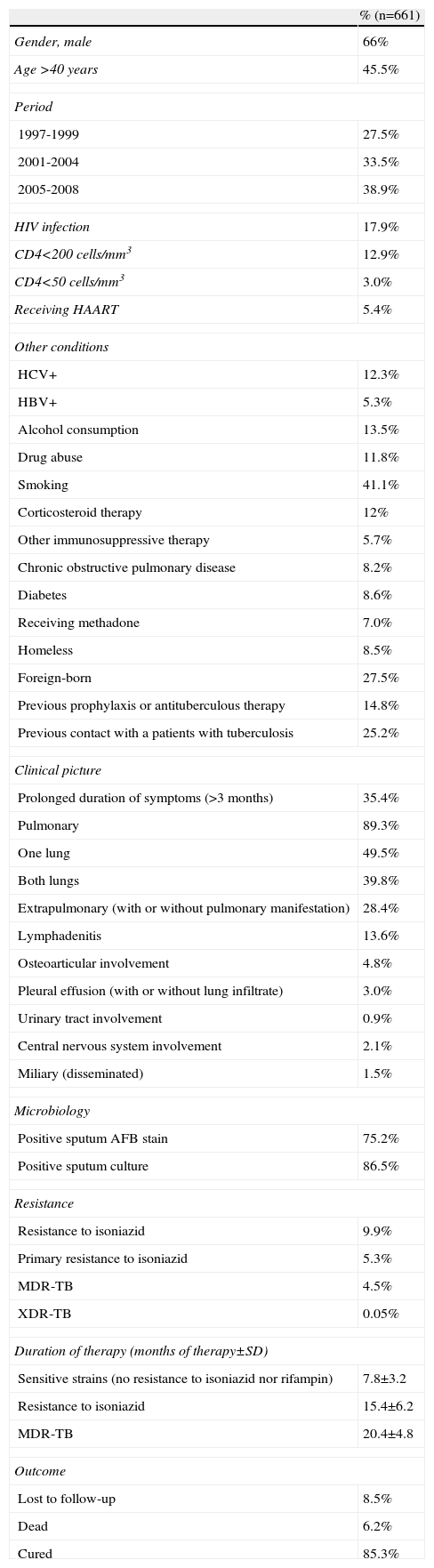
Figure 1 shows the rate of TB by year. A significant increase was observed during the study period (P<.001). When patients were grouped by period —1997-2000, 2001-2004, and 2005-2008— the percentages of patients with TB in each period were 27.5%, 33.5%, and 38.9%, respectively (Table 1). Percentage of patients aged >40 years, HIV infection patients and foreign-born patients were 45.5%, 17.9%, and 27.5%, respectively (Table 1). The regions of origin were Latin-America (17.2%), Africa (5%), Western Europe (3.5%), and other (1.8%).
Characteristics of patients with tuberculosis.
| % (n=661) | |
| Gender, male | 66% |
| Age >40 years | 45.5% |
| Period | |
| 1997-1999 | 27.5% |
| 2001-2004 | 33.5% |
| 2005-2008 | 38.9% |
| HIV infection | 17.9% |
| CD4<200 cells/mm3 | 12.9% |
| CD4<50 cells/mm3 | 3.0% |
| Receiving HAART | 5.4% |
| Other conditions | |
| HCV+ | 12.3% |
| HBV+ | 5.3% |
| Alcohol consumption | 13.5% |
| Drug abuse | 11.8% |
| Smoking | 41.1% |
| Corticosteroid therapy | 12% |
| Other immunosuppressive therapy | 5.7% |
| Chronic obstructive pulmonary disease | 8.2% |
| Diabetes | 8.6% |
| Receiving methadone | 7.0% |
| Homeless | 8.5% |
| Foreign-born | 27.5% |
| Previous prophylaxis or antituberculous therapy | 14.8% |
| Previous contact with a patients with tuberculosis | 25.2% |
| Clinical picture | |
| Prolonged duration of symptoms (>3 months) | 35.4% |
| Pulmonary | 89.3% |
| One lung | 49.5% |
| Both lungs | 39.8% |
| Extrapulmonary (with or without pulmonary manifestation) | 28.4% |
| Lymphadenitis | 13.6% |
| Osteoarticular involvement | 4.8% |
| Pleural effusion (with or without lung infiltrate) | 3.0% |
| Urinary tract involvement | 0.9% |
| Central nervous system involvement | 2.1% |
| Miliary (disseminated) | 1.5% |
| Microbiology | |
| Positive sputum AFB stain | 75.2% |
| Positive sputum culture | 86.5% |
| Resistance | |
| Resistance to isoniazid | 9.9% |
| Primary resistance to isoniazid | 5.3% |
| MDR-TB | 4.5% |
| XDR-TB | 0.05% |
| Duration of therapy (months of therapy±SD) | |
| Sensitive strains (no resistance to isoniazid nor rifampin) | 7.8±3.2 |
| Resistance to isoniazid | 15.4±6.2 |
| MDR-TB | 20.4±4.8 |
| Outcome | |
| Lost to follow-up | 8.5% |
| Dead | 6.2% |
| Cured | 85.3% |
AFB, acid-fast bacillus; HBV, hepatitis B virus; HCV, hepatitis C virus; MDR-TB, multidrug-resistant tuberculosis; XDR-TB, extensively drug-resistant TB.
Prophylaxis with isoniazid was administered to 4.4% of patients, and 10.4% had previously received antituberculous treatment. According the risk of resistance to isoniazid these patients (14.8%) were analyzed together. In the case of patients who had abandoned previous therapy and patients who had received more than one course of therapy, only the last episode was included in the analysis.
Diagnosis of pulmonary TB was confirmed by sputum culture in 97% of patients for whom a sputum sample was available. Urine culture was positive in 28% of patients with extrapulmonary disease
The rate of extrapulmonary TB among HIV-infected patients was 52%, which was significantly higher than that observed in non-infected patients (23%) (P<.001). This rate was also higher in patients with lower CD4+ T cell counts (58% in patients with <200 cells/ml and 65% in patients with <50 cells/ml, P: ns).
The mean duration of therapy in patients with susceptible isolates, resistance to isoniazid, and MDR-TB was 7.8, 15.4, and 20.4 months, respectively.
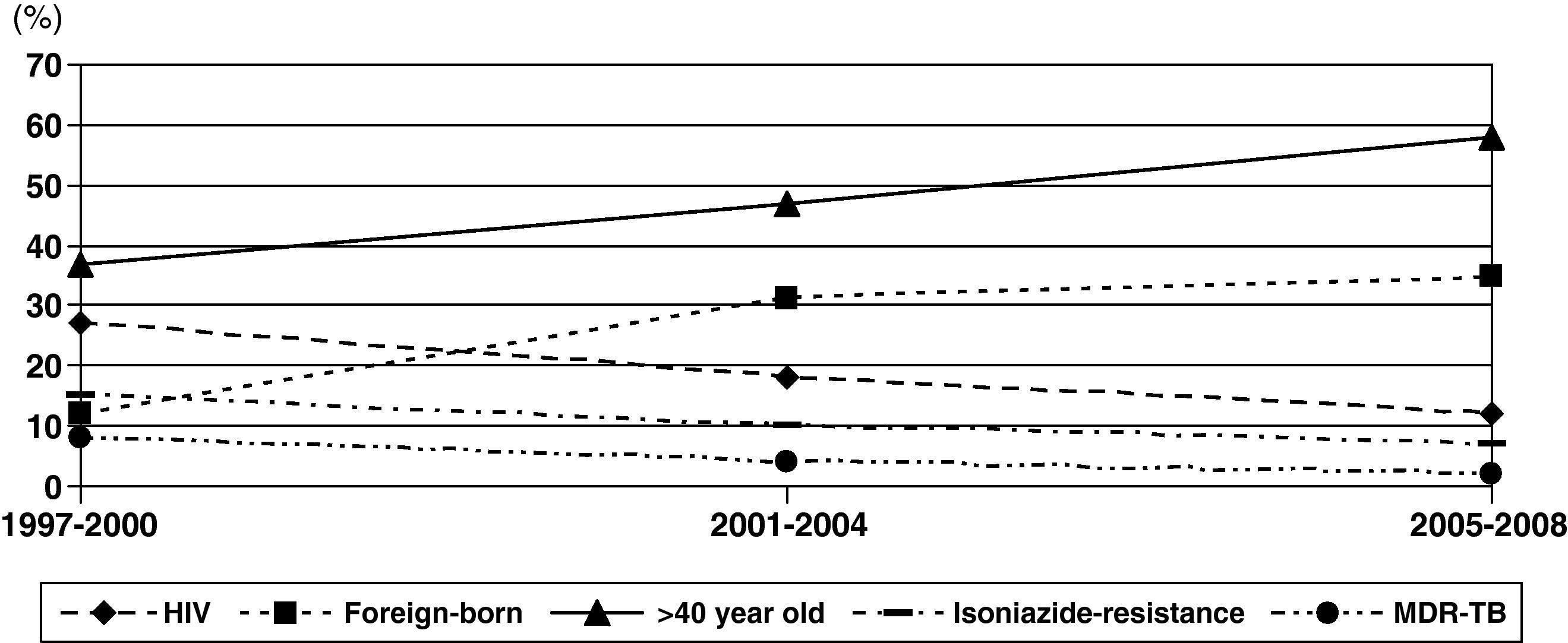
Changes in rates were observed during these periods (Fig. 2). The proportion of patients with HIV infection decreased slowly from 26% during 1997-2000 to 12% during 2005-2008. However, the percentage of patients aged >40 years old and foreign-born increased significantly, from 37% to 59% and from 12% to 35%, respectively.
Isoniazid-resistant TB and MDR-TB were observed in 9.9% and 4.5% of patients, respectively (Table 1). Only 3 cases of XDR-TB were observed during the study period. Resistance to isoniazid and MDR-TB decreased from 14% and 8% to 7% (P=.03) and 2% (P=.004), respectively (Fig. 2). Isoniazid-resistant TB and MDR-TB among autochthonous patients were 7.6% and 2.8%. They were higher in immigrant patients: 16.5% (P<.001) and 9.1% (P<.001), respectively. Worthy of note are the high rates of resistance to isoniazid in isolates of patients from Peru (28% [10/35]) and Romania (17% [3/17]). Primary isoniazid-resistance (excluding patients that previously received prophylaxis with isoniazid or antituberculous treatment) was 5.3% (4.0% (17/422) in autochthonous patients and 8.5% (14/164) in foreign-born patients, P=.047).
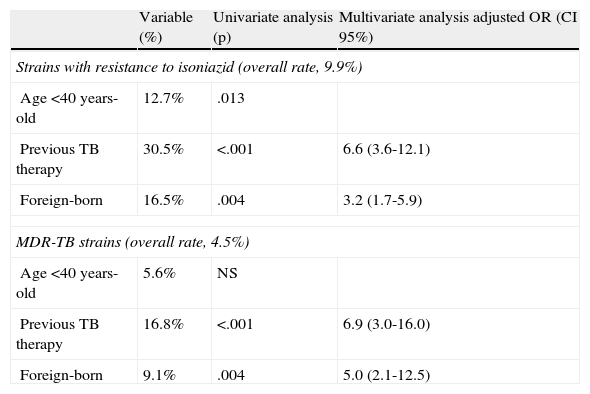
Multivariate analysis revealed the same factors to be associated with resistance to both isoniazid and isoniazid + rifampin (Table 2), namely a history of previous antituberculous therapy and immigration.
Risk factors associated with resistance to isoniazid and rifampin.
| Variable (%) | Univariate analysis (p) | Multivariate analysis adjusted OR (CI 95%) | |
| Strains with resistance to isoniazid (overall rate, 9.9%) | |||
| Age <40 years-old | 12.7% | .013 | |
| Previous TB therapy | 30.5% | <.001 | 6.6 (3.6-12.1) |
| Foreign-born | 16.5% | .004 | 3.2 (1.7-5.9) |
| MDR-TB strains (overall rate, 4.5%) | |||
| Age <40 years-old | 5.6% | NS | |
| Previous TB therapy | 16.8% | <.001 | 6.9 (3.0-16.0) |
| Foreign-born | 9.1% | .004 | 5.0 (2.1-12.5) |
CI, confidence interval; MDR-TB, multidrug-resistant tuberculosis; NS, non-significant; OR, odds ratio.
A sub-analysis performed among patients with a diagnosis of TB before 2000 showed similar results. Immigration (OR: 8.3; CI 95%: 1.5-50) and previous TB therapy (OR: 7.9; CI 95%: 2.2-28.2) were independently associated with resistance to isoniazid. Moreover, previous TB treatment (OR: 8.5; CI 95%: 1.9-38.0) was the only factor independently associated with MDR-TB during this period.
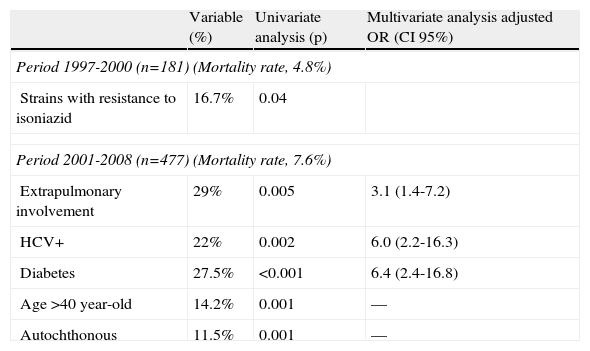
A complete follow-up was possible in 92% of patients, and mortality associated with TB was confirmed in 6.9%. Mortality was 4.9% during 1997-2000, 6.7% during 2001-2004, and 8.6% during 2005-2008 (P=NS).
A separate analysis of mortality was performed according to the different patient profiles during two study periods (1997-200 and 2001-2008) (Table 3). The only significant variable associated with higher mortality during 1997-2000 was resistance to isoniazid; mortality in patients with resistance to isoniazid was 16.7% (P=.04). During 2001-2008, the variables independently associated with higher mortality were extrapulmonary TB (OR: 3.1; CI 95%: 1.4-7.2), hepatitis C virus (HCV) infection (OR: 6.0; CI 95%: 2.2-16.3), and diabetes (OR: 6.4; CI 95%: 2.4-16.8). Overall mortality among patients with HCV infection in this period was 27.3% (9/33). Although HIV infection was not associated with higher crude mortality, HIV-HCV co-infection did seem to be associated with higher mortality. After the year 2000, mortality in co-infected patients (HIV and HCV and tuberculosis) reached 71.4% (5/7 patients), while patients with an HCV infection only (no-HIV and HCV and tuberculosis) had a lower mortality rate (15.4%, 4/26 patients).
Factors associated with mortality.
| Variable (%) | Univariate analysis (p) | Multivariate analysis adjusted OR (CI 95%) | |
| Period 1997-2000 (n=181) (Mortality rate, 4.8%) | |||
| Strains with resistance to isoniazid | 16.7% | 0.04 | |
| Period 2001-2008 (n=477) (Mortality rate, 7.6%) | |||
| Extrapulmonary involvement | 29% | 0.005 | 3.1 (1.4-7.2) |
| HCV+ | 22% | 0.002 | 6.0 (2.2-16.3) |
| Diabetes | 27.5% | <0.001 | 6.4 (2.4-16.8) |
| Age >40 year-old | 14.2% | 0.001 | –– |
| Autochthonous | 11.5% | 0.001 | –– |
In many European countries, the profile of patients with TB has changed in the last decade, mainly in the south-western European area. HIV infection as the main risk factor for TB has been replaced by immigration from TB-endemic areas. Spain is the second destination, after the United States, for foreign-born from Latin America.6 Migration flows could affect the epidemiology of some infections, for example Chagas disease, in the host country.10 These findings underscore the need to change current policies for the control of TB in our setting.
Immigration, aging, and drug resistance are the main characteristics of TB in western European hospitals. HIV infection was present in only 12% of patients with TB in the period 2005-2008, and, today, the number of patients older than 40 years with TB has increased 1.6-fold compared with the period 1997-2000; the incidence of TB among foreign-born has increased 1.9-fold. While patients from Africa and Western Europe are usually younger, patients from Latin America are older.A study performed at our center in 2003 showed that, despite an increase among foreign-born (from 1% in 1990 to 27% in 2002), the annual incidence of tuberculosis (TB) decreased from 141 in 1990 to 73 in 2002, due to a better control of HIV infection.11 However, we found that the progressive increase in immigration in our region is responsible for the overall increase in the global number of cases of TB treated during the last 12 years in our center. Our hospital is a national reference center for tropical medicine and probably attends more patients with TB than other centers. However, the epidemiology of TB in other hospitals in Madrid or other cities in Spain is similar.12–14
Since 1995, immigration has increased dramatically in Spain, which is sometimes a temporary destination for Africans and Latin Americans in transit to other European countries.15 Data obtained from the Spanish Employment and Immigration Department show that the number of foreign-born rose from 540,000 (1.37% of the population) in 1996 to 5,600,000 (12%) in 2009. Foreign-born now represent 16.4% of the population of the Autonomous Community of Madrid.16 As in other parts of the world, the decrease in the number of autochthonous TB cases has been driven mainly by appropriate control of HIV infection and AIDS-related complications since the introduction of highly active antiretroviral treatment in 1996.17
Immigration was associated with drug resistance in the present series. A paradoxical feature is the decrease in the incidence of isoniazid-resistant TB and MDR-TB observed during the study period despite an increase in the incidence of TB among foreign-born. One possible explanation could be the change in types of drug resistance. Before 2000, most cases of drug-resistant TB were secondary, probably due to poor adherence or social problems. In the last few years, we have observed an increase in primary drug resistance in treatment-naïve patients and patients from countries with a high incidence of resistance.
Efforts to ensure worldwide control of TB infection have been frustrated by the spread of HIV/AIDS in TB-endemic regions and by the global emergence of strains of M. tuberculosis that are resistant to current antituberculous drugs (MDR-TB and XDR-TB). The Global Project on Anti-TB Drug-Resistance estimated that the number of incident MDR-TB cases in 2006 was 489,000, namely, 4.8% of the total number of estimated incident TB cases in 185 countries.18 The highest rates of MDR-TB were reported in the former Soviet Union and some provinces of China. The rate of MDR-TB in Spain was 0.1%.18 A recent study, focused on sociodemographic and clinical changes in tuberculosis among HIV-infected patients in New York, confirmed a significant reduction in multidrug-resistant tuberculosis from 16% to 4%, particularly from pre-HAART to early HAART.19
Treatment of MDR-TB is challenging. WHO guidelines recommend a minimum of 18 months of directly observed therapy after culture conversion. A recent meta-analysis has confirmed this recommendation.20
In the present series, resistance to isoniazid was the only factor associated with mortality before the year 2000; this could be due to poor adherence. The dropout rate was significantly high, and 13% of patients during this period had a history of previous TB with suboptimal therapy. Poor adherence could explain the worse outcome of TB during this period. After 2000, therapy was suboptimal in only 5% of patients.
After the year 2000, mortality was associated with comorbid conditions such as HCV infection and diabetes, or with the severity of TB (extrapulmonary and disseminated disease). Mortality in extrapulmonary cases was 29%, reaching 35% in cases with central nervous system involvement.
Outcome was more complicated in patients with TB and chronic liver disease (e.g., HCV infection) due to the potential hepatotoxicity of many TB drugs.21 The risk of decompensated cirrhosis or death is significantly higher in HCV/HIV-co-infected patients. Hepatotoxicity is a concern in HIV/hepatitis C virus (HCV) co-infected patients due to their underlying liver disease. The impact of co-infection includes greater morbidity and mortality, with higher rates of opportunistic disease, development of cirrhosis, and death. Therapy in this scenario is challenging.
The relationship between diabetes and unfavorable outcome in TB patients is well documented. One systematic review identified 13 relevant, age-adjusted, quantitative observational studies associating diabetes mellitus with an increased risk of TB.22 This risk is particularly high in young people and in countries with a high background incidence of TB.23 Diabetic patients sometimes experience conditions such as impaired cell-mediated immunity, renal failure, micronutrient deficiency, and pulmonary microangiopathy, all of which predispose to pulmonary TB. Harries et al24 showed that diabetic patients with TB often present with infiltrates of the lower lobes (similar to patients with HIV/AIDS) and that outcomes can be worse with regard to smear and culture conversion, treatment failure, and mortality.
The worse prognosis associated with the extrapulmonary clinical forms of TB observed in this series has been described elsewhere. The predictors of mortality among patients with TB are the presence of disseminated disease and central nervous system/meningeal extrapulmonary TB, especially among HIV-infected patients.25 Miliary disease is more common in children, with peaks in adolescents, young adults, and older patients. Incidence and mortality are higher in immunosuppressed patients (e.g., patients with AIDS or patients receiving chemotherapy).26 In the present series, although the role of HIV infection among TB patients is less relevant, especially in recent years, its contribution to more severe forms of TB has been confirmed, and half of HIV-infected patients presented extrapulmonary TB (two-thirds of patients with <50 lymphocytes/ml).
In summary, the incidence of TB has increased in our setting in recent years and the epidemiological profile of the disease has changed. HIV infection as the main risk factor for TB has been replaced by immigration from TB-endemic areas. MDR-TB remains an important challenge, and unsuspected primary resistance not associated with poor adherence may complicate outcomes. Increased patient age and the presence of specific comorbid conditions, especially chronic liver disease, could make management more difficult and lead to an increase in the prevalence of severe forms with higher mortality.
FundingAll authors declare that no specific funding has been received in the preparation of this paper. Data have been generated as part of the routine work of the Infectious Disease Department of the Ramón y Cajal Hospital, Madrid.
Conflict of interestsThe authors declare no conflicts of interest related to this study.
We thank Thomas O¿Boyle for manuscript preparation.
Presented in part at the 49th ICAAC (Interscience Conference of Antimicrobial Agents and Chemotherapy), San Francisco, USA, sept 2009.