Food allergy can be considered a failure in the induction of oral tolerance. Recently, great interest has been focused on understanding the mechanisms and the contributing factors of oral tolerance development, hoping for new definitive interventions in the prevention and treatment of food allergy. Given that food processing may modify the properties and the nature of dietary proteins, several food processing methods could affect the allergenicity of these proteins and consequently may favour oral tolerance induction to food allergic children. Indeed, effective thermal food processing regimens of altering food proteins to reduce allergenicity have been recently reported in the literature. This article is mainly focused on the effect of selective thermal processing regimens on the main infant allergenic foods, with a potential clinical relevance on their allergenicity and therefore on oral tolerance induction. In the light of recent findings, the acquisition of tolerance in younger age and consequently the ability of young children to “outgrow” food allergy could be achieved through the application of selective thermal processing regimens on certain allergenic foods. Therefore, the ability of processed foods to circumvent clinical disease and at the same time to have an impact on the immune system and facilitate tolerance induction could be invaluable as a component of a successful therapeutic strategy. The opening in the new avenues of research in the use of processed foods in clinical practice for the amelioration of the impact on the quality of life of patients and possibly in food allergy prevention is warranted.
The gastrointestinal (GI) mucosa is daily exposed to an array of food proteins. The norm is that they are tolerated through a mechanism known as oral tolerance. Hence the prevalence of food allergy is kept remarkably low considering the enormous number of ingested food proteins. Despite this, the impact of food allergy is substantial. Its treatment consists of strict allergen avoidance and self-injectable epinephrine availability, both resulting in anxiety, stress and reduced health-related quality of life.1 Recently, great interest has been focused on understanding the mechanisms and the contributing factors of oral tolerance development, hoping for new definitive interventions in the prevention and treatment of food allergy.2
Allergy to common food allergens, such as cow's milk, egg, and wheat, begins predominantly before the second year of life. Over time, most food allergies are usually outgrown, but sometimes they may persist through childhood and adolescence into adulthood, and may become more severe.1 Thus, there is a need for new therapeutic interventions and strategies aiming at the development of tolerance and acceleration of the time of food allergens reintroduction.
Although food processing techniques are primarily designed for the stabilisation and preservation of food, while enhancing its digestibility, it has been long recognised that these techniques can alter food proteins allergenicity.3 Hence, effective food processing regimens of altering food proteins to reduce allergenicity have been recently reported in the literature.4 Thermal heating is a common processing technique of major importance on food allergens since it can bring substantial changes in their allergenic nature.
This article is mainly focused on the effect of selective thermal processing regimens on the main infant allergenic foods, with a potential clinical relevance on their allergenicity and therefore on oral tolerance induction.
Original research studies published in English between 1985 and 2011 were selected (PubMed and Scopus). Computer searches used combinations of key words relating to “food allergy” and “tolerance” and “food processing”. In addition, the reference lists of the retrieved articles helped in the search for other relevant articles which were not found during the searching initial procedure. Thus 42 studies were selected and discussed here (22 cross-sectional, 13 case–control and 7 population based studies). The potential factors which may bias the findings of this review are restriction to articles in English, together with database, and citation bias.
Food allergy in infancyThe annual incidence of doctor-diagnosed food allergy decreases from an average of 4.7% per year during the first two years of life to an average of 1.2% for the fifth and sixth years of age5 because of the naturally acquired tolerance to the majority of allergenic foods.
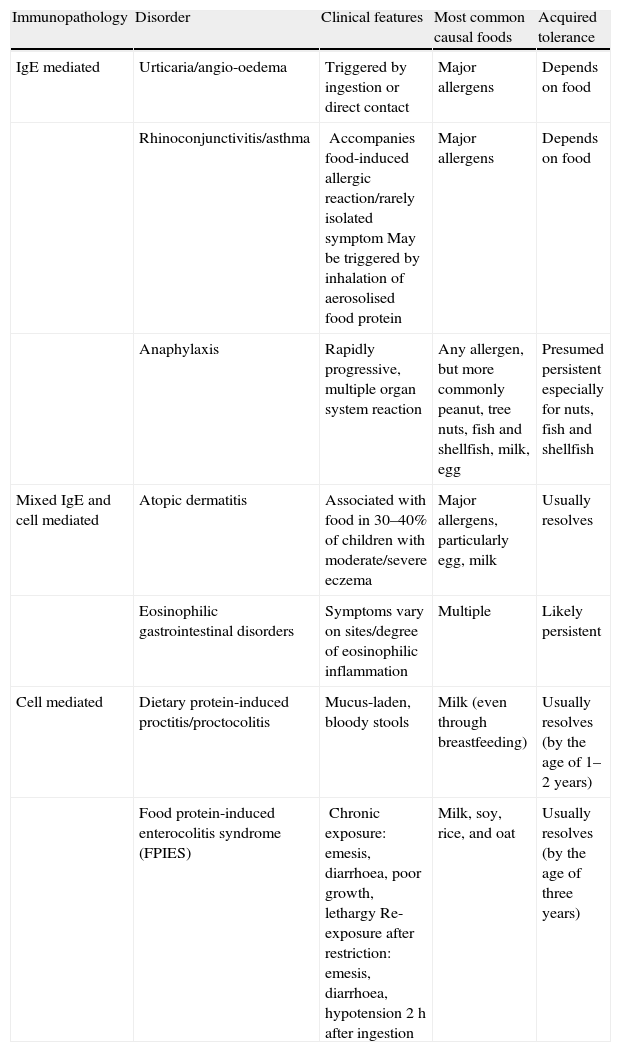
Given that food allergies are immunologically mediated, it is helpful to conceptualise them into three categories based on their underlying immunopathology: IgE-mediated reactions, mixed IgE- and cell-mediated reactions, and cell-mediated reactions (Table 1). The acquired tolerance varies among the different allergens of these three categories.6
Food-induced allergic disorders in infancy (classified based on underlying immunopathology).6
| Immunopathology | Disorder | Clinical features | Most common causal foods | Acquired tolerance |
| IgE mediated | Urticaria/angio-oedema | Triggered by ingestion or direct contact | Major allergens | Depends on food |
| Rhinoconjunctivitis/asthma | Accompanies food-induced allergic reaction/rarely isolated symptomMay be triggered by inhalation of aerosolised food protein | Major allergens | Depends on food | |
| Anaphylaxis | Rapidly progressive, multiple organ system reaction | Any allergen, but more commonly peanut, tree nuts, fish and shellfish, milk, egg | Presumed persistent especially for nuts, fish and shellfish | |
| Mixed IgE and cell mediated | Atopic dermatitis | Associated with food in 30–40% of children with moderate/severe eczema | Major allergens, particularly egg, milk | Usually resolves |
| Eosinophilic gastrointestinal disorders | Symptoms vary on sites/degree of eosinophilic inflammation | Multiple | Likely persistent | |
| Cell mediated | Dietary protein-induced proctitis/proctocolitis | Mucus-laden, bloody stools | Milk (even through breastfeeding) | Usually resolves (by the age of 1–2 years) |
| Food protein-induced enterocolitis syndrome (FPIES) | Chronic exposure: emesis, diarrhoea, poor growth, lethargyRe-exposure after restriction: emesis, diarrhoea, hypotension 2h after ingestion | Milk, soy, rice, and oat | Usually resolves (by the age of three years) |
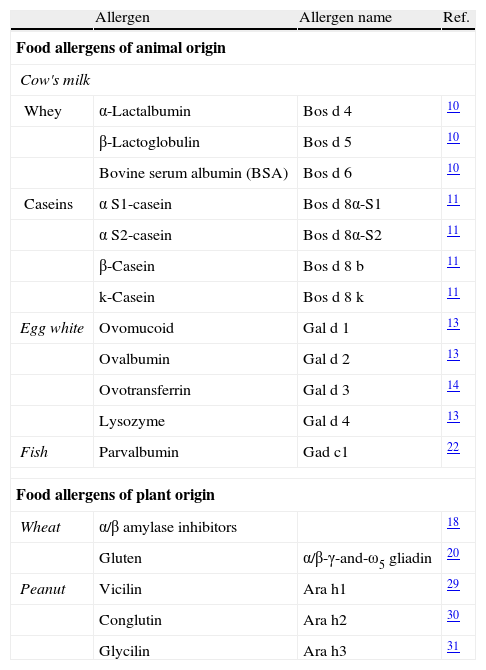
The majority of food allergies are induced by specific types of foods.7 The allergenic fraction is generally comprised of heat-stable and water-soluble glycoproteins ranging in size from 10 to 70kDa. As depicted in Table 2, the allergenic proteins in many foods have been identified, isolated, sequenced, and cloned. A summary of the most important allergens in these different foods is given below.
Major food allergens that have been isolated and characterised.
| Allergen | Allergen name | Ref. | |
| Food allergens of animal origin | |||
| Cow's milk | |||
| Whey | α-Lactalbumin | Bos d 4 | 10 |
| β-Lactoglobulin | Bos d 5 | 10 | |
| Bovine serum albumin (BSA) | Bos d 6 | 10 | |
| Caseins | α S1-casein | Bos d 8α-S1 | 11 |
| α S2-casein | Bos d 8α-S2 | 11 | |
| β-Casein | Bos d 8 b | 11 | |
| k-Casein | Bos d 8 k | 11 | |
| Egg white | Ovomucoid | Gal d 1 | 13 |
| Ovalbumin | Gal d 2 | 13 | |
| Ovotransferrin | Gal d 3 | 14 | |
| Lysozyme | Gal d 4 | 13 | |
| Fish | Parvalbumin | Gad c1 | 22 |
| Food allergens of plant origin | |||
| Wheat | α/β amylase inhibitors | 18 | |
| Gluten | α/β-γ-and-ω5 gliadin | 20 | |
| Peanut | Vicilin | Ara h1 | 29 |
| Conglutin | Ara h2 | 30 | |
| Glycilin | Ara h3 | 31 | |
Cow's milk is one of the most common causes of food allergy in the first years of life, with around 2.5% of infants showing hypersensitivity reactions to cow's milk. IgE-mediated reactions account for about 60% of milk-induced allergic disorders.8 Symptoms of cow's milk allergy (CMA) range from mild to severe reactions and involve the skin, respiratory tract, gastrointestinal tract and, in the worst case, appear as life-threatening systemic reactions.9
Cow's milk contains numerous proteins of which seven have been characterised as allergens. They include α-lactalbumin (Bos d 4), β-lactoglobulin (Bos d 5), bovine serum albumin (BSA) (Bos d 6), and lactoferrin from the whey fraction, aS1-casein (Bos d 8 a-S1), aS2-casein (Bos d 8 a-S2), b-casein (Bos d 8 b), and k-casein (Bos d 8 k) from the casein fraction (Bos d 8).10 γ-Caseins are present in milk in minute quantities, while they are abundant in cheeses characterised by proteolytic ripening (such as Grana cheese).11 Whey proteins are less abundant (20% of the total protein) and β-lactoglobulin is the main component (up to 50% of whey proteins). Since β-lactoglobulin is absent from human milk, this protein was formerly considered to be the most important cow's milk allergen, but it was later shown that other proteins, including caseins, are also critical milk allergens.12
Hen's eggEgg white of the domestic chicken (Gallus domesticus) represents the albumin fraction of the egg and contains more allergenic proteins than the yolk. Egg white contains more than 20 different glycoproteins, most of which have been purified. Ovomucoid13 (OVM) (Gal d 1: 11% of egg protein total content), ovalbumin13 (OVA) (Gal d 2, 54%), ovotransferrin14 (Gal d 3, 12%) and lysozyme (Gal d 4, 3.4%)13 have been identified as major allergens. Studies in humans utilising Radio Allergo Sorbent Test (RAST) reported the order of allergenicity as ovomucoid>ovalbumin>ovotransferrin>lysozyme.15 Although OVA is the most abundant protein in egg white, OVM has been shown to be the dominant allergen in egg.16 OVM is a highly glycosylated molecule containing 186 amino acid residues and is known to exhibit a trypsin inhibitor activity.16
WheatWheat is a major nutrient source and it is among widely used cereals. Wheat proteins can be categorised into four fractions on the basis of their solubility in a series of solvents: water (albumins), dilute salt solutions (globulins), aqueous alcohol (gliadins) and dilute alkali or acid.17 The water/salt-soluble albumins and globulins are mainly structural proteins and metabolically active enzymes, such as a- and b-amylases, and their inhibitors.18 The water/salt-insoluble gliadins and glutelins, together known as prolamins or gluten, are the major storage proteins of the wheat grain. They are responsible for the ability of wheat flour to be baked into bread by creating a visco-elastic dough capable of holding gas.19 The ethanol-soluble gliadin group comprises over 40 structurally similar proteins, which can be further categorised into a-, g-, and o-types on the basis of their electrophoretic mobility.20 Wheat prolamins share a great degree of sequence and structural homology with each other and with the corresponding proteins in rye and barley.19 Glutelins exhibit about 70–80% of the specific IgE-binding activity, responsible for food allergies in children.21
FishThe prevalence of fish allergy is low in children (≤0.2%),5 although, in countries where the consumption is high, it might be up to 13% of the children.22 Parvalbumins, the second largest animal food allergen family – after tropomyosins –, act as a major fish allergen and are believed to be similar in structure to those present in many fish species.23 Parvalbumin is a calcium-binding sarcoplasmic protein with a molecular mass of about 12kDa, resistant to heat, chemical denaturation, and proteolytic enzymes.24 Parvalbumins are present in high amounts in white muscle of lower vertebrates and in lower amounts in fast twitch muscles of higher vertebrates.25 Dark muscle contains lower levels of parvalbumins, thus these fish species are expected to be of lower allergenicity.26
PeanutPeanut allergy is a common food allergy in the United States and in European countries, affecting around 1% of the population but in some studies much higher.27 As peanut and tree nut allergy cause most food-related anaphylactic reactions in children, and cross-reactivity between nuts is commonly reported, it is common practice for paediatric allergy clinics to advise that, even where a single nut allergy is diagnosed, these patients avoid all nuts in their diets.28 Eleven peanut proteins were identified that elicit allergic reactions and/or are recognised by IgE antibodies from patients with peanut ingestion. Notably, most of the Ara h-type allergens form part of the major storage proteins of the peanut.29–31 Recent studies indicate that peanut-allergic patients are most frequently sensitised to the proteins Ara h 2 and Ara h 6, followed by Ara h 1, Ara h 3 and Ara h 7.32 Ara h 6 and Ara h 7 show around 55% and 40% amino acid sequence homology with Ara h 2, respectively, suggesting that these three proteins could share a number of identical epitopes.33
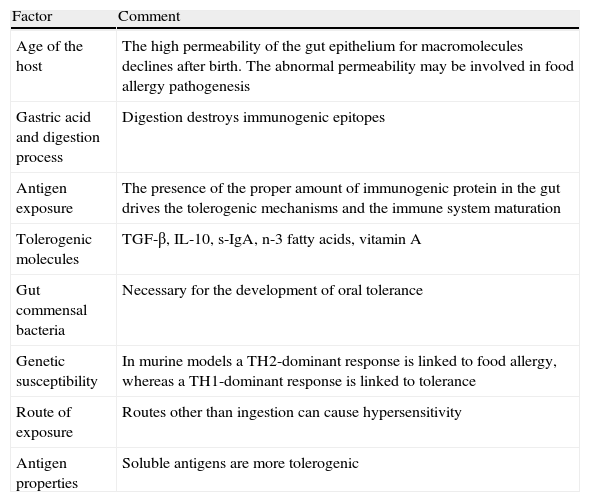
The development of oral toleranceThe key question is how the gastrointestinal immune system regulates the response to food antigens that have bypassed complete digestion, and the answer is by oral tolerance development. Oral tolerance can be defined as the active, antigen-specific non-response to antigens administered orally. The development of the oral tolerance depends on following parameters (Table 3).
Factors affecting oral tolerance induction.
| Factor | Comment |
| Age of the host | The high permeability of the gut epithelium for macromolecules declines after birth. The abnormal permeability may be involved in food allergy pathogenesis |
| Gastric acid and digestion process | Digestion destroys immunogenic epitopes |
| Antigen exposure | The presence of the proper amount of immunogenic protein in the gut drives the tolerogenic mechanisms and the immune system maturation |
| Tolerogenic molecules | TGF-β, IL-10, s-IgA, n-3 fatty acids, vitamin A |
| Gut commensal bacteria | Necessary for the development of oral tolerance |
| Genetic susceptibility | In murine models a TH2-dominant response is linked to food allergy, whereas a TH1-dominant response is linked to tolerance |
| Route of exposure | Routes other than ingestion can cause hypersensitivity |
| Antigen properties | Soluble antigens are more tolerogenic |
Age of exposure seems to be important in the development of tolerance. In mice the progression from sensitivity to tolerance appears to shift gradually with age, with tolerance becoming the norm by the time they are normally weaned.34 In humans the relatively high permeability of the intestinal epithelium for macromolecules declines soon after birth, a process that is called gut closure.35 When the gut permeability was assessed in food allergic infants by examining the lactulose/mannitol ratio in urine, it was noted that the food allergic infants had increased permeability when compared with normal healthy subjects.36
Gastric acid and the process of digestionAfter ingestion, gastric acid and digestive enzymes degrade dietary proteins resulting in the destruction of immunogenic epitopes. In animal models, disrupting the process of digestion can disrupt tolerance and lead to hypersensitivity.37,38 The potential role in the increasing prevalence of food allergy of the wide use of gastric acid blockers in infancy, for the management of the gastro-oesophageal reflux during the last years, is a subject of ongoing investigation.39
Contact of proteins and bacteria with the GI epithelial surfaceThe gastrointestinal epithelial surface and the gut-associated lymphatic tissue are in constant contact with numerous foreign dietary proteins and commensal bacteria. Hence, a state of local and systemic immunologic tolerance is usually developed. These tolerogenic interactions prevent damaging active immune responses on subsequent repeated antigen encounter and moreover exert a major stimulatory effect on immune system maturation.
The significance of the interaction between dietary proteins and commensal bacteria and the immune system has been shown in experimental animal models. More specifically, mice receiving a balanced amino-acid, protein-free diet had a poorly developed gut-associated lymphatic tissue and reduced numbers of B and T lymphocytes.40 Moreover germ-free reared mice showed a defect in T regulatory cells (Tregs) induction and oral tolerance process.41,42 In addition, there is evidence that some microbiota might be associated with more efficient tolerance than others and new DNA-based methodology has identified the probably underestimated role of non-culturable gut bacteria in the maturation of the immune system.43
There are dietary proteins which are not digested in the gut lumen, remain intact and consequently contact the epithelium and interact with the mucosal immune system in various manners. These dietary antigens are taken up by different cells of the gastrointestinal tract, including dendritic cells, microfold cells (M-cells) and epithelial cells which process and present the antigens to the gut-associated lymphatic tissue (GALT), mainly to primed T-cells. Low doses of antigen favour tolerance by Tregs activation. The activation of Tregs suppresses immune responses through the production of soluble or cell-surface associated down-regulatory tolerogenic cytokines such as Interleukin-10 (IL-10) and Transforming Growth Factor-β (TGF-β). Antigen-specific Tregs migrate to lymphoid organs, where they inhibit the generation of effector cells as well as to target organs where they release their down-regulatory cytokines.44,45 High doses of antigen favour lymphocyte anergy or clonal deletion-driven tolerance and apoptotic T-cells release TGF-β.46,47 Actually, TGF-β is a tolerogenic molecule of great significance. Besides being produced by T cells and other immune cells such as macrophages, it is found in high amounts in breast milk. Experiments in a mouse model have shown that the induction of tolerance upon antigen transfer through breast milk required the presence of TGF-β in maternal milk.48 Current data suggest that defect in Tregs activity may have a central role in the development of food allergy and increases in Tregs have been associated with food allergy outgrown.49
Genetic susceptibilityIn murine models of oral tolerance induction, genetics can contribute to the development of food allergy. In various strains of mice, food allergy is linked with a Th2-dominant response, whereas Th1-dominant response is linked to tolerance.50 There may even be a role for genetics in determining the development of allergy to a particular food.51
Nature of the antigen and route of exposureThe form of the antigen is also crucial to its ability to induce tolerance. Soluble antigens are more tolerogenic than particulate antigens.52 Many particulate antigens are degraded into soluble ones by the process of digestion.
The route of antigen exposure seems important because antigen exposure through a route other than ingestion can cause hypersensitivity. Current data suggest that skin exposure enhances allergic sensitising and prevents tolerance.53,54
According to studies, food processing techniques may modify the properties and the nature of dietary proteins. Hence food processing can affect the allergenicity of these proteins and consequently may favour oral tolerance induction to food allergic children.55,56
Food allergen processing and protein allergenicityAccording to the literature, food processing and gastrointestinal degradation are fundamentally important for food protein allergenicity.57 The term “food processing” may be defined as any manipulation that a particular food undergoes from the time it is harvested until the time it reaches the consumer.58 There are several food processing methods which are used exclusively or in combination with others.59 Both the nature of the food process and the time and intensity of the process affect this impact.60
Mode of actionFood processing induces several physical, chemical and biochemical changes that are known to potentially impact the allergenic potential of proteins.61 Generally, food proteins present in a processed food will be in a denatured state, aggregated in protein networks, or interacting with carbohydrates (e.g., Maillard reactions) and lipids, which leads either to the reduction of allergen content and thus potentially reducing its sensitising potential or to the formation of neoepitopes.61
In particular food processing can decrease protein allergenicity in several ways including the destruction of predominantly conformational epitopes, with limited effect on sequential epitopes, and chemical reactions between proteins with fat and sugars in the food matrix that account for limited availability of protein to the immune system. Food processing might potentially increase protein allergenicity by the formation of neoepitopes and by the effect of food matrix leading to decreased protein digestibility in the stomach and preservation of allergenic epitopes for interactions with the immune system in the intestine.62
Effect of heatingEach food is a mixture of allergenic proteins that differ in their physicochemical properties, stability to heat and digestion, and the potential to induce IgE sensitisation and IgE-mediated hypersensitivity reactions. The so-called classic, type 1 or complete food allergens (e.g. in cow's milk, egg white, wheat and peanut) that have the capacity to induce IgE sensitisation via the mucosa of the gastrointestinal tract, are heat-stable and acid-stable, water-soluble glycoproteins ranging in size from 10 to 70kDa. The class 1 food allergens (e.g. Gal d 1 in egg white, or Ara h 2 in peanut) are less readily affected by food processing, although recent findings underscore the importance of conformational epitope modification in cow's milk and egg allergy.63
In contrast, class 2 or incomplete food allergens (cross-reacting allergens, such as the cross-reactive birch and celery allergens Bet v 1 and Api g1) are postulated to lack the capacity to induce IgE sensitisation via the gastrointestinal tract exposure due to their susceptibility to thermal processing and gastric digestion.64 Since the class 2 food allergens are more or less heat susceptible, it is of interest to focus in more detail on the effect of heating on class 1 food allergens.
High temperature reduces allergenicity, presumably by altering the conformation of heat-labile proteins that results in loss of conformational epitopes. In cow's milk, the caseins and serum albumin have higher heat stability than the whey proteins, a-lactalbumin, b-lactoglobulin, and lactoferrin. Casein bands were preserved in the SDS-PAGE gel even after 120min of boiling at 100°C. Serum albumin band became progressively weaker after 10min of boiling but was still visible at 120min. In contrast, a-lactalbumin band disappeared after 30min, b-lactoglobulin disappeared after 15min, and lactoferrin disappeared after 10min of boiling.65
In the context of egg allergy, OVA is sensitive to thermal denaturation with a resultant decrease in allergenicity. In contrast, OVM is heat resistant and remains soluble after extensive heating; purified OVM heated for 1h at 100°C retained its antibody-binding activity.62 However, recently conducted studies showed that extensively heated egg could reduce allergenicity of heated egg white proteins as a result from altered digestion and absorption in the gastrointestinal tract.66,67 Heating of allergens prevented transport across human intestinal epithelial cells in a form capable of triggering basophile activation or T-cell activation.57
In peanuts, Maillard reaction products of peanut processing have been implicated in enhancing the allergenicity of peanut proteins, by approximately 100-fold compared with that of the native protein, through the formation of higher molecular weight aggregates and increasing resistance to digestion in the gut.68 In particular, Ara h 2, one of the most important peanut allergens as it is recognised by serum immunoglobulin E from more than 90% of peanut-allergic individuals, contains cores that are highly resistant to proteolytic digestion and to temperatures of up to 100°C.69 However, a recently conducted study showed that boiling (100°C 15min) resulted in the partial loss of Ara h 1 secondary structure and formation of rod-like branched aggregates with reduced IgE-binding capacity and impaired ability to induce mediator release. Moreover, heating of Ara h 2/6 at 110°C resulted in extensive denaturation, hydrolysis and aggregation of the protein, resulting in decreased IgE reactivity and functionality of Ara h 2/6.70 Thus, given the loss of Ara h 2/6 from boiled peanuts, the hypothesis that boiling reduces the allergenicity of peanuts could be supported.71
Additionally, the allergenicity of wheat gluten proteins might actually be increased by cooking,72 although the results from another recently conducted study showed that salt-soluble proteins from wheat-derived foodstuffs show lower allergenic potency than those from raw flour.73
Moreover, fish proteins, due to their stable structure, retain their allergenicity even after the effect of pepsin digestion and heat treatment (boiling and frying).74 However, IgE binding activity was reduced 100- to 200-fold in canned tuna and salmon as a result of extreme temperature and pressure during canning.75 Moreover, from the findings of a recently conducted study authors concluded that High Pressure Steaming (HPS) was the most effective method to accelerate the digestion of tropomyosin (TM) in gastrointestinal digestion. In particular the reactivity of IgG/IgE-binding of TM was reduced, demonstrating that proper processing of crab could decrease the incidence of crab hypersensitivity in humans.76 Taking into consideration that process-induced changes in fish protein immunogenicity are more dependent on process rather than species, although individual responses varied,77 these findings could have significant implications in clinical practice.
The so-called matrix effect includes protein interactions with other ingredients such as other proteins, fats and sugars in processed foods which are also important, in general resulting in decreased availability of protein for interaction with the immune system. Heating of β-lactoglobulin results in the formation of intermolecular disulphide bonds and subsequent binding to other food proteins, making β-lactoglobulin less allergenic.61 Another study78 demonstrated a marked decrease in the solubility of OVM when egg white was mixed with wheat flour and wheat gluten and then heated at 180°C for 10min, mimicking the process of bread making. Immunoblotting suggested that OVM polymerises and forms high-molecular weight complexes with gluten leading to aggregation and insolubilisation of OVM. An alternative approach to introducing food protein modified by high temperature and by interactions with wheat matrix to the diet of children with milk and egg allergy was recently explored.
Several studies66,67,79 reported that the majority of tested children were able to ingest baked products with milk or egg without any immediate symptoms. Introduction of baked products was associated with increasing levels of milk and egg-specific IgG4 antibody levels and decreasing skin prick test wheal sizes. In addition, children tolerant of baked milk had more regulatory T cells in the peripheral blood at baseline; following introduction of baked milk protein, the fraction of peripheral T regulatory cells decreased, suggesting possible migration of these cells to the sites of the contact with the dietary antigen (GI tract).80 Furthermore, the long-term effects of inclusion of dietary baked milk products such as muffin, baked cheese (pizza) into children's diets have been recently investigated. In particular, authors suggested that tolerance of baked milk is a marker of transient IgE-mediated cow's milk allergy, whereas reactivity to baked milk portends a more persistent phenotype.81 Therefore, authors concluded that the addition of baked milk products to the diet of children tolerating such foods appears to accelerate the development of unheated milk tolerance compared with strict avoidance.81
Oral tolerance induction by thermal food processingHeating either alone or in combination with other methods of food processing has different effects on food allergens. Structural homology does not reliably predict the effect of processing on allergenicity, and individual food allergens have to be tested.62 Interactions with other proteins, fat, and carbohydrates in the food matrix are complex and poorly understood. Better characterisation of these aspects of food allergy is critical for elucidation of food protein interactions with the gut-associated lymphoid tissue, the ability to induce IgE sensitisation, the potential to trigger hypersensitivity reactions, and different clinical phenotypes of food allergy with regard to severity and persistence.
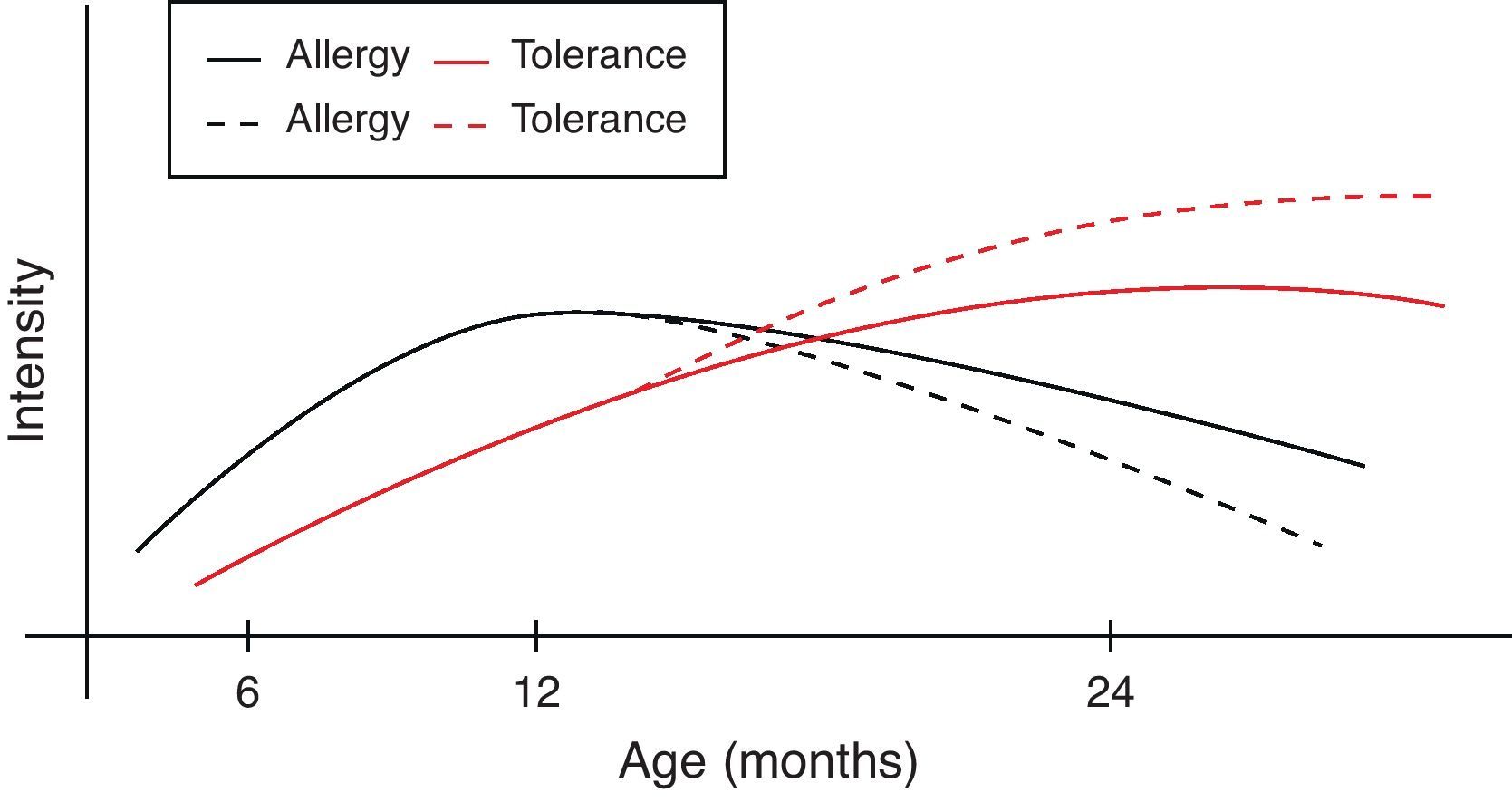
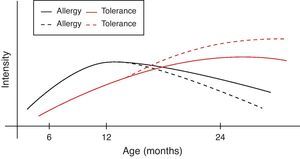
However, during the last years it has become clear that early intervention may modulate the natural course of atopic disease.82 In a broader context and from the perspective of oral tolerance, steady oral exposure of the patients to the antigen they are sensitised to, goes a long way in assisting tolerance induction to it.83–85 This is not always applicable in clinical practice if the patient experiences manifestation of clinical symptoms upon ingestion. Within this conceptual frame, the introduction of extensively heated milk and egg proteins becomes of apparent and cardinal importance, as these two represent the commonest and major food allergens in infancy, given that well-conducted studies66,67,79 confirm the safe ingestion of baked milk and egg products. Additionally, the observed humoral and cellular immunological changes parallel the changes reported in the oral immunotherapy trials, suggesting that introduction of baked milk and egg products may represent an alternative pathway of accelerating development of tolerance.66,79,80 Although wheat, peanut and fish proteins seem to retain or increase their allergenicity after the heating process, however in the light of recent studies70,71,73,76 some promising findings are revealed (Table 4). Thus the acquisition of tolerance in younger age and the subsequent ability of young children to “outgrow” food allergy could be achieved through the application of selective thermal processing regimes on certain allergenic foods. In a broader context and from the perspective of prevention of already established disease, the burden of food allergy will begin to decline at an earlier stage than expected (Fig. 1).
Effect of high temperature on the common food allergens.
| Food | Effect of mode of action as a result of heating | Positive outcome with clinical relevance |
| Cow's milk | Decreased allergenicity of β-lactoglobulin results from the formation of disulphide bonds among β-lactoglobulin and other proteins in the food matrix | Tolerance of baked milk products by children with IgE-mediated cow's milk allergy is a favourable prognostic indicator for development of tolerance to unheated milk. The addition of baked milk products to the diet appears to markedly accelerate the development of tolerance to unheated milk compared with a strict avoidance diet.81 Up to 70% of milk-allergic children tolerate milk baked with wheat matrix (muffin, waffle). |
| Egg white | Ovomucoid polymerises and forms high-molecular weight complexes with gluten in baked foods. Extensively heated egg could reduce allergenicity of heated egg white proteins57 | Up to 70% of egg-allergic children tolerated egg baked with wheat matrix. Reduced allergenicity of heated egg white proteins partially resulting from altered digestion and absorption in the gastrointestinal tract may explain the clinical tolerance of extensively heated egg in the majority of children with egg allergy. |
| Wheat | Salt-soluble proteins from wheat-derived foodstuffs show lower allergenic potency than those from raw flour73 | Potential changes in allergenic properties induced by the heat treatment and other industrial processing to produce wheat-derived foodstuffs. Some wheat-allergic patients may tolerate industrially processed wheat-derived foodstuffs. |
| Fish | Canned tuna and salmon have significantly decreased IgE-binding capacity. High Pressure Steaming may reduce the reactivity of IgG/IgE-binding of tropomyosin76 | Some fish-allergic patients may tolerate industrially processed canned tuna and salmon and crab. |
| Peanut | Boiling (100°C 15min) resulted in the partial loss of Ara h 1 secondary structure and formation of rod-like branched aggregates with reduced IgE-binding capacity and impaired ability to induce mediator release71Heating of Ara h 2/6 at 110°C resulted in extensive denaturation, hydrolysis and aggregation of the protein, resulting in decreased IgE reactivity and functionality of Ara h 2/670 | Given the loss of Ara h 2/6 and Ara h1 from boiled peanuts, the hypothesis that boiling reduces the allergenicity of peanuts could be supported with significant clinical implications |
Food processing can fundamentally alter the ability of food protein allergens to trigger reactions not only by interfering with their IgE binding, but also by altering their degradation and absorption within the GI tract.
The fact that the above-mentioned processes, perhaps even combined, affect different protein fractions in several ways, further complicates the identification of the most effective method of altering food proteins to reduce allergenicity. For some proteins the epitopes are destroyed, but for others they remain unaltered. Furthermore, for some proteins, IgE reactivity is increased subsequent to processing, which suggest a potential increase in allergenicity.
Therefore, the ability of processed foods to circumvent clinical disease and at the same time to have an impact on the immune system and facilitate tolerance induction could be invaluable as a component of a successful therapeutic strategy. The opening of new avenues of research in the use of processed foods in clinical practice for the amelioration of the impact on the quality of life of patients and possibly in food allergy prevention is warranted.
Conflict of interestThe authors have no conflict of interest to declare.
Ethical disclosuresConfidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.