The 4CMenB vaccine confers protection against serogroup B invasive meningococcal disease (MenB IMD). Licensed worldwide based on immunogenicity and safety data, we now have data on its effectiveness and impact. We have exhaustively reviewed all the evidence gathered in the real world since the vaccine was licensed.
ResultsData from 7 countries provide evidence of effectiveness and impact in different settings and age groups, including national/regional immunisation programmes, observational studies, and outbreak control.
The administration of at least 2 doses of 4CMenB reduced IMD by 50–100% in individuals from 2 months to 20 years of age. Estimation of the effectiveness of the vaccine in fully vaccinated cohorts ranged from 59 to 100%. The real-world safety profile of 4CMenB corresponded with the data from the clinical trials undertaken prior to its licensing.
ConclusionMenB IMD is a rare but potentially fatal disease, with unpredictable epidemiology. The data on the effectiveness and impact data of 4CMenB support its use in the prevention of IMD. The results underline the importance of directly protecting the groups most at risk: infants/young children and adolescents. Direct protection through systematic immunisation in infancy (with salvage in young children) and systematic immunisation in adolescents could constitute the preferred model for the control of MenB disease.
A Video Abstract linked to this article is available on Figshare: https://doi.org/10.6084/m9.figshare.
4CMenB es una vacuna que confiere protección frente a la enfermedad meningocócica invasiva por el serogrupo B (EMI por MenB). Autorizada en todo el mundo a partir de los datos de inmunogenicidad y seguridad, disponemos ahora de datos de su efectividad e impacto. Hemos revisado de manera exhaustiva toda la evidencia recopilada en el mundo real desde su autorización.
ResultadosLos datos de 7 países aportan evidencia de efectividad e impacto en diferentes entornos y grupos de edad, incluidos programas de inmunización nacionales/regionales, estudios observacionales y control de brotes. La administración de al menos 2 dosis de 4CMenB redujo la EMI por MenB en un 50–100% en personas de 2 meses a 20 años. Las estimaciones de la efectividad vacunal en las cohortes completamente vacunadas oscilaron entre 59 y 100%. El perfil de seguridad de 4CMenB en el mundo real se correspondió con los datos de los ensayos clínicos previos a su autorización.
ConclusiónLa EMI por MenB es una enfermedad poco frecuente pero potencialmente mortal, con una epidemiología imprevisible. Los datos sobre efectividad e impacto de 4CMenB respaldan su uso para la prevención de la EMI. Los resultados subrayan la importancia de la protección directa de los grupos con mayor riesgo: lactantes/niños pequeños y adolescentes. La protección directa a través de la inmunización sistemática en la lactancia (con rescate de los niños pequeños) y la vacunación sistemática en la adolescencia podrían constituir la modalidad preferida para el control de la enfermedad por MenB.
En Figshare encontrará un resumen en vídeo relacionado con este artículo: https://doi.org/10.6084/m9.figshare.
Meningitis and septicemia caused by Neisseria meningitidis are uncommon but life-threating diseases that can result in death or permanent sequelae. Of the 6 serogroups that cause the majority of invasive meningococcal disease (IMD) in humans (A, B, C, W, X and Y), prevention by vaccination is available for 5 using currently licensed conjugate vaccines targeting capsular groups A, C, W and Y (MenACWY), and protein vaccines targeting group B (MenB).1 The majority of IMD in Europe, Australia, New Zealand, and much of the Americas is caused by MenB with the highest incidences observed in infants and young children.1 Adolescents and young adults are the second most affected age-group in whom an increase in IMD incidence coincides with peak prevalence of nasopharyngeal carriage.1,2
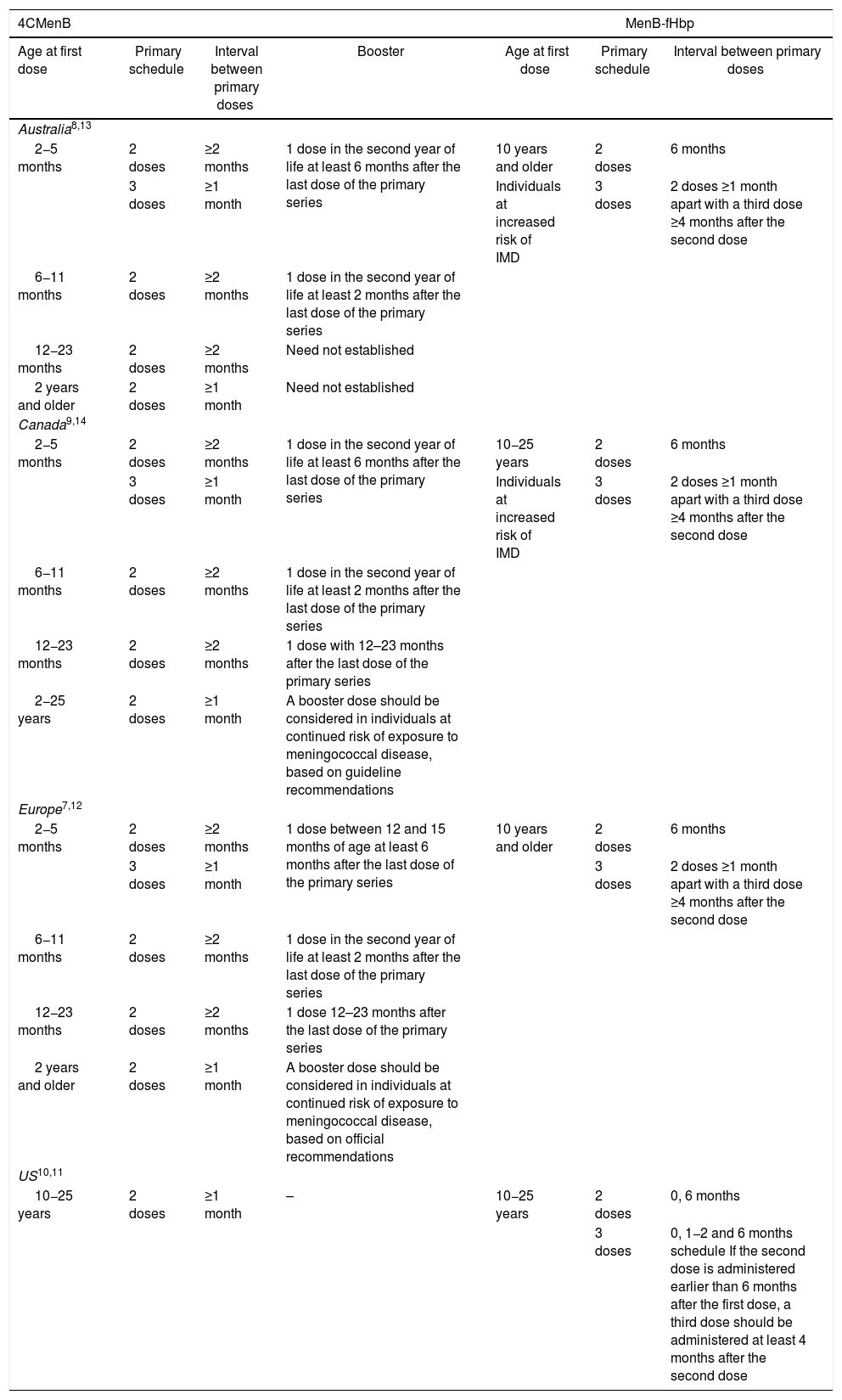
Development of broadly effective MenB vaccines was complicated due to challenges specific to the MenB capsule.3–5 The first multi-component MenB vaccine (4CMenB, Bexsero, GSK) was licensed in 2013, 14 years after availability of the first meningococcal conjugate vaccine (serogroup C).6 4CMenB was first licensed in Europe and was used in a 3 + 1 schedule beginning between the ages of 2–5 months. Since 2020, 4CMenB is approved from 2 months of age as a 2 + 1 schedule (Table 1).7 4CMenB was also licensed in Australia and Canada in 2013 for individuals from 2 months of age and older, and in the United States (US) in 2015 for adolescents and young adults aged 10−25 years as a 2-dose schedule (0, 1 month) (Table 1).8–10
4CMenB and MenB-fHbp approved vaccination schedules in Australia, Canada, Europe and the United States.
| 4CMenB | MenB-fHbp | |||||
|---|---|---|---|---|---|---|
| Age at first dose | Primary schedule | Interval between primary doses | Booster | Age at first dose | Primary schedule | Interval between primary doses |
| Australia8,13 | ||||||
| 2−5 months | 2 doses | ≥2 months | 1 dose in the second year of life at least 6 months after the last dose of the primary series | 10 years and older | 2 doses | 6 months |
| 3 doses | ≥1 month | Individuals at increased risk of IMD | 3 doses | 2 doses ≥1 month apart with a third dose ≥4 months after the second dose | ||
| 6−11 months | 2 doses | ≥2 months | 1 dose in the second year of life at least 2 months after the last dose of the primary series | |||
| 12−23 months | 2 doses | ≥2 months | Need not established | |||
| 2 years and older | 2 doses | ≥1 month | Need not established | |||
| Canada9,14 | ||||||
| 2−5 months | 2 doses | ≥2 months | 1 dose in the second year of life at least 6 months after the last dose of the primary series | 10−25 years | 2 doses | 6 months |
| 3 doses | ≥1 month | Individuals at increased risk of IMD | 3 doses | 2 doses ≥1 month apart with a third dose ≥4 months after the second dose | ||
| 6−11 months | 2 doses | ≥2 months | 1 dose in the second year of life at least 2 months after the last dose of the primary series | |||
| 12−23 months | 2 doses | ≥2 months | 1 dose with 12–23 months after the last dose of the primary series | |||
| 2−25 years | 2 doses | ≥1 month | A booster dose should be considered in individuals at continued risk of exposure to meningococcal disease, based on guideline recommendations | |||
| Europe7,12 | ||||||
| 2−5 months | 2 doses | ≥2 months | 1 dose between 12 and 15 months of age at least 6 months after the last dose of the primary series | 10 years and older | 2 doses | 6 months |
| 3 doses | ≥1 month | 3 doses | 2 doses ≥1 month apart with a third dose ≥4 months after the second dose | |||
| 6−11 months | 2 doses | ≥2 months | 1 dose in the second year of life at least 2 months after the last dose of the primary series | |||
| 12−23 months | 2 doses | ≥2 months | 1 dose 12–23 months after the last dose of the primary series | |||
| 2 years and older | 2 doses | ≥1 month | A booster dose should be considered in individuals at continued risk of exposure to meningococcal disease, based on official recommendations | |||
| US10,11 | ||||||
| 10−25 years | 2 doses | ≥1 month | – | 10−25 years | 2 doses | 0, 6 months |
| 3 doses | 0, 1−2 and 6 months schedule If the second dose is administered earlier than 6 months after the first dose, a third dose should be administered at least 4 months after the second dose | |||||
IMD, invasive meningococcal disease.
Another MenB vaccine, MenB-fHbp (Trumenba, Pfizer), was licensed in the US in 2014, originally approved for use in a 3-dose schedule (administered at 0, 1−2 and 6 months), and later as a 2-dose schedule (0 and 6 months) in 10−25 year-olds.11 MenB-fHbp was licensed in Australia, Canada and Europe in 2017 for use in a 2-dose (0, 6 months) or 3-dose (0, ≥1, and ≥4 months post-dose 2) schedule from the age of 10 years (Table 1).12–14
Both vaccines were licensed on the basis of safety and immunogenicity using serum bactericidal antibody assays in clinical trials, with the expectation that evidence of effectiveness would be gained after licensure.15,16 At the time of writing, MenB-fHbp has not been included in national immunization programs (NIP) in any country. As a result, opportunities to evaluate real-world effectiveness have been focused on publicly available evidence consisting of outbreak control experiences in the US and France.17–19 4CMenB has been implemented in routine infant NIPs in 8 countries: the United Kingdom (UK), Ireland, Italy (including San Marino), Lithuania, Malta, Czech Republic, Portugal and Andorra.20–27 A regional infant immunization program was introduced in 2019 in Castilla y León and in 2020 in the Canary Islands, Spain, and an infant and adolescent program in 2018 and 2019 respectively in South Australia.28,29 In Australia, 4CMenB was also implemented for Aboriginal and Torres Strait Islander infants up to 2 years of age from July 2020 due to the higher burden of disease in this group.30 In the US, 4CMenB is recommended for individuals from 10 years of age at risk of MenB IMD, such as persons with complement deficiencies, asplenia or during a MenB outbreak. Adolescents and young adults aged 16–23 years may also be vaccinated (recommended based on shared clinical decision making).31 Additionally, 4CMenB was used in an outbreak control program in Quebec in the Saguenay-Lac-Saint-Jean region32; in a cluster randomized study in adolescents in South Australia33; and extensively in the private market in many other countries.15 The first estimate of vaccine effectiveness in infants was published in 2016 and described the effectiveness and impact of 2 doses of 4CMenB administered in the UK infant immunization program.34 The overall effectiveness of 2 doses of 4CMenB against MenB IMD was 82.9% (95% confidence interval [CI] 24.1, 95.2).34
In this review we describe the most recently available effectiveness and impact data for 4CMenB. We refer to vaccine effectiveness (VE) as the risk of MenB IMD in vaccinated individuals compared to unvaccinated individuals, and to vaccine impact (VI) as the reduction in MenB disease at the population level over time in vaccine-eligible individuals, regardless of vaccination status.
MenB IMD outbreak control in US universitiesIndividuals aged 18–24 years who attend college in the US are at higher risk of developing MenB IMD compared to 18–24 year-olds who do not attend college, with an annual incidence between 2015 and 2017 that was more than 5-times higher than college non-attendees.35 Between 2011 and 2019, all US college-based outbreaks of IMD were caused by MenB, encompassing 14 outbreaks, 50 cases and 2 deaths.35
In the period 2013–2018, 4CMenB was used for MenB IMD outbreak control in 7 university outbreaks in the US.36 The results of 3 of these emergency vaccination campaigns have been published and reported that no further cases occurred in vaccinated individuals after the implementation of the vaccination campaign.37–39 Although suggestive of an impact of vaccination, impact is difficult to assess for outbreak settings as they are typically of short duration with small case numbers and vaccine intervention usually starts when the overall risk is already in decline.
Reactive management of outbreaks requires the rapid deployment of public health resources at high cost, and high vaccination coverage can be difficult to achieve.36 Procurement of vaccines and implementation of vaccination campaigns could be more efficient if routine vaccination programs were already in place. The onset and magnitude of the immune response to a booster dose is greater than that following primary vaccination,40 meaning that the effectiveness of a vaccination campaign is likely to be faster and higher if targeted individuals have some level of underlying vaccine-induced immunity.
MenB IMD outbreak control in QuebecQuebec experienced an outbreak of MenB IMD that began in 2003, caused by a MenB sequence type ST-269 strain with the highest incidence in the Saguenay-Lac-Saint-Jean region. In May 2014, 4CMenB was implemented in a mass vaccination campaign in Saguenay-Lac-Saint-Jean region targeting all individuals from 2 months until 20 years of age.41 4CMenB coverage for the ST-269 strain was estimated to be 95%.42 Between May and December 2014, 82% of the targeted age-group received at least 1 vaccine dose.41 No cases of MenB IMD were reported in vaccinated persons between 2015 and 2016. The relative risk (RR) of MenB IMD in the Saguenay-Lac-Saint-Jean region after the 4CMenB vaccination campaign (July 2014 to December 2016) compared to before the campaign (July 2006-June 2014) and adjusted for region, season, age and year, was 0.22 (95% CI 0.05, 0.92, p = 0.04).41
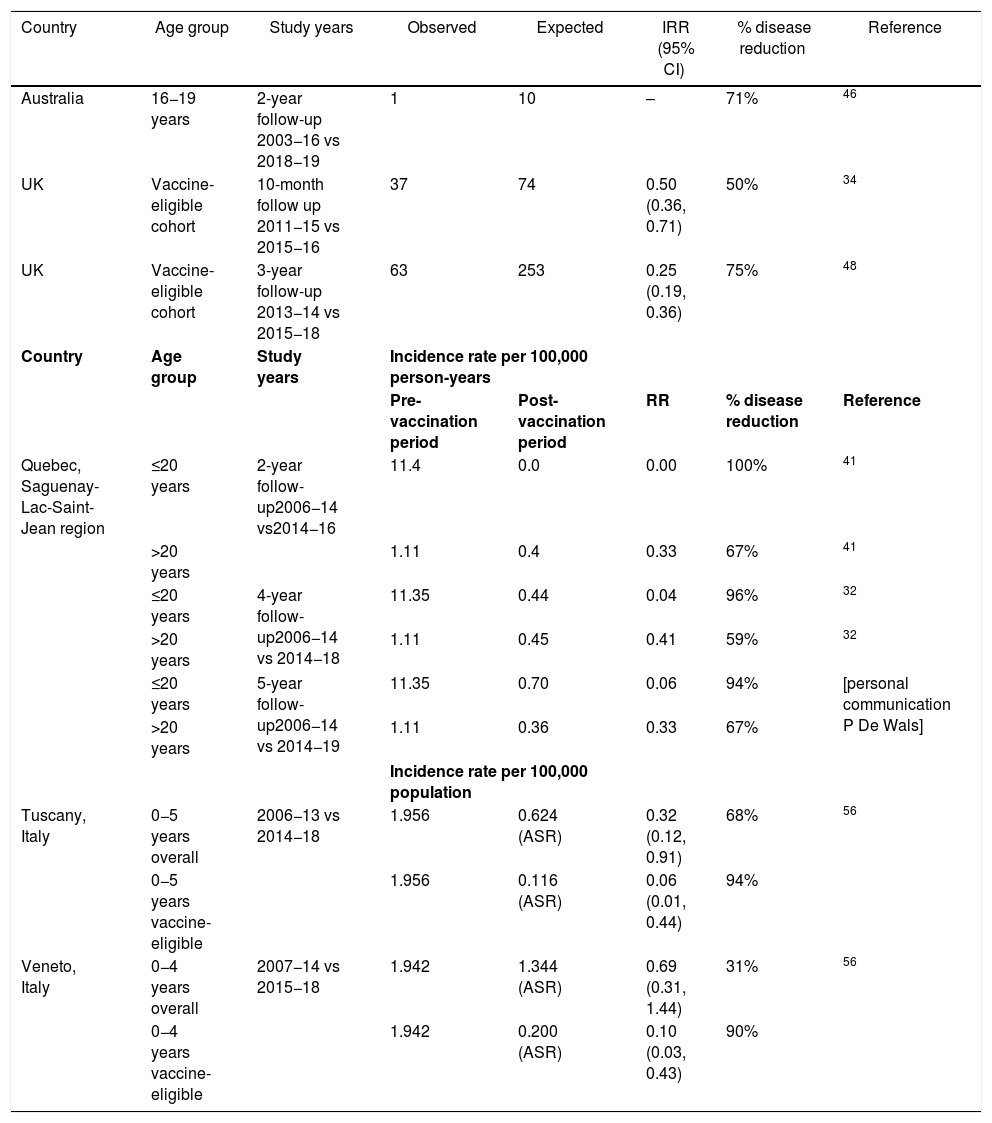
Vaccine impact was evaluated for the 5-year post-campaign period July 2014 until June 2019 compared to the pre-campaign period of July 2006 to June 2014 (personal communication Philippe De Wals) (Table 2). MenB IMD decreased in the targeted age-group from 11.4 per 100,000 person-years in the pre-vaccination period to 0.7 per 100,000 person-years in the post-vaccination period (p = 0.0001). MenB IMD incidence decreased by 100% in the vaccine-eligible population in the first 2 years of the program (p < 0.0001), by 96% in after 4 years (p = 0.0013) and 94% after 5 years (p < 0.06)32,41 (personal communication Philippe De Wals).
4CMenB vaccine impact (percent reduction in disease) against invasive meningococcal disease caused by serogroup B.
| Country | Age group | Study years | Observed | Expected | IRR (95% CI) | % disease reduction | Reference |
|---|---|---|---|---|---|---|---|
| Australia | 16−19 years | 2-year follow-up 2003−16 vs 2018−19 | 1 | 10 | – | 71% | 46 |
| UK | Vaccine-eligible cohort | 10-month follow up 2011−15 vs 2015−16 | 37 | 74 | 0.50 (0.36, 0.71) | 50% | 34 |
| UK | Vaccine-eligible cohort | 3-year follow-up 2013−14 vs 2015−18 | 63 | 253 | 0.25 (0.19, 0.36) | 75% | 48 |
| Country | Age group | Study years | Incidence rate per 100,000 person-years | ||||
| Pre-vaccination period | Post-vaccination period | RR | % disease reduction | Reference | |||
| Quebec, Saguenay-Lac-Saint-Jean region | ≤20 years | 2-year follow-up2006−14 vs2014−16 | 11.4 | 0.0 | 0.00 | 100% | 41 |
| >20 years | 1.11 | 0.4 | 0.33 | 67% | 41 | ||
| ≤20 years | 4-year follow-up2006−14 vs 2014−18 | 11.35 | 0.44 | 0.04 | 96% | 32 | |
| >20 years | 1.11 | 0.45 | 0.41 | 59% | 32 | ||
| ≤20 years | 5-year follow-up2006−14 vs 2014−19 | 11.35 | 0.70 | 0.06 | 94% | [personal communication P De Wals] | |
| >20 years | 1.11 | 0.36 | 0.33 | 67% | |||
| Incidence rate per 100,000 population | |||||||
| Tuscany, Italy | 0−5 years overall | 2006−13 vs 2014−18 | 1.956 | 0.624 (ASR) | 0.32 (0.12, 0.91) | 68% | 56 |
| 0−5 years vaccine-eligible | 1.956 | 0.116 (ASR) | 0.06 (0.01, 0.44) | 94% | |||
| Veneto, Italy | 0−4 years overall | 2007−14 vs 2015−18 | 1.942 | 1.344 (ASR) | 0.69 (0.31, 1.44) | 31% | 56 |
| 0−4 years vaccine-eligible | 1.942 | 0.200 (ASR) | 0.10 (0.03, 0.43) | 90% | |||
ASR, age-standardized incidence rate; CI, confidence interval; IMD, invasive meningococcal disease; RR, risk ratio; IRR, incidence rate ratio.
There were 6 cases of MenB IMD in the Saguenay-Lac-Saint-Jean region between July 2014 and June 2019. Two cases were reported in vaccinated individuals; 1 case of meningitis in a 6 year-old child in 2018, and 1 case of meningitis in a 13 year-old child in 2019, both of whom had received 2 4CMenB doses in 2014. Both children recovered. There was 1 case of meningitis and meningococcemia in an unvaccinated young adult belonging to the targeted age-group. The 3 remaining cases were reported in unvaccinated adults aged 44–65 years, 1 of whom died (personal communication Philippe De Wals).
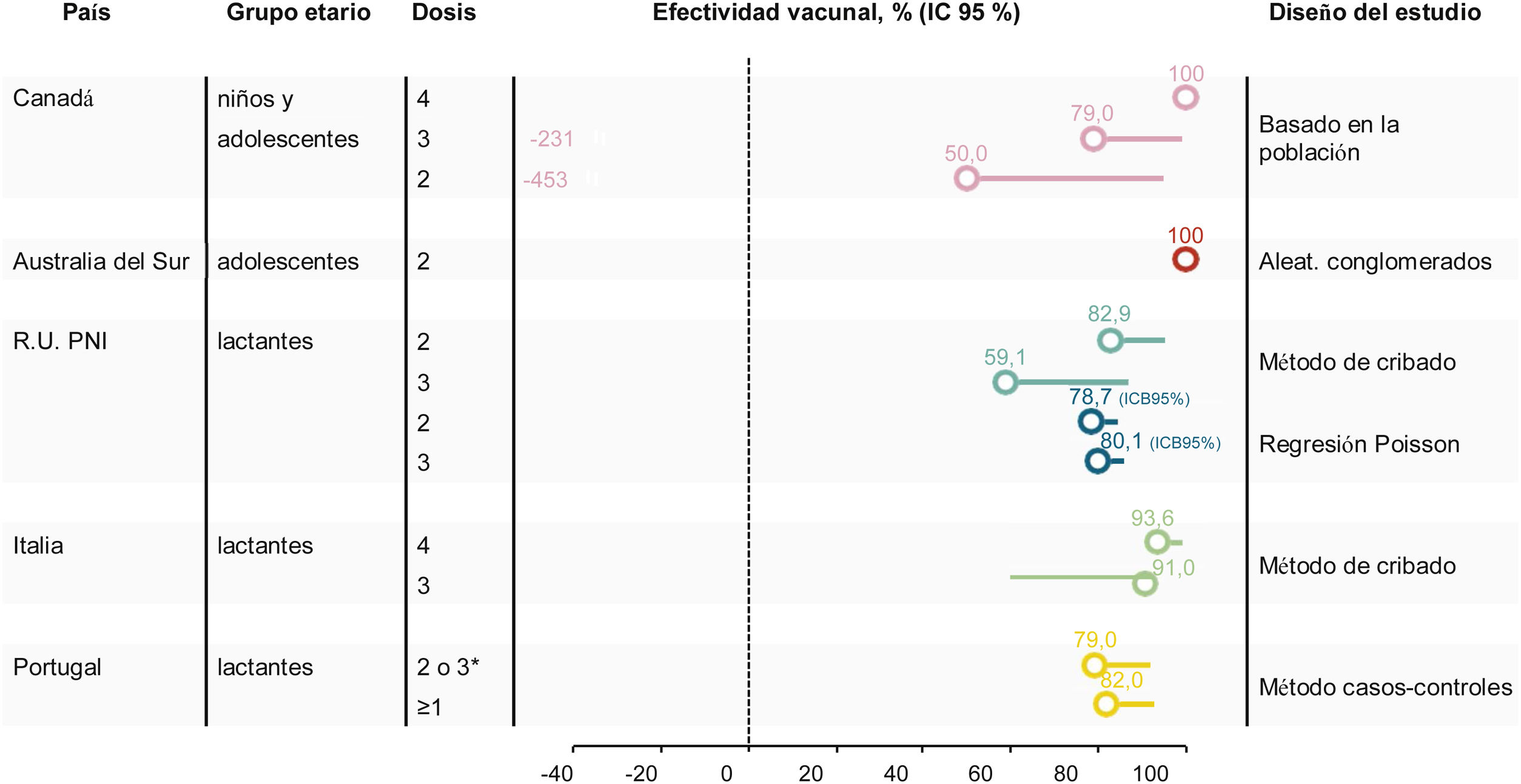
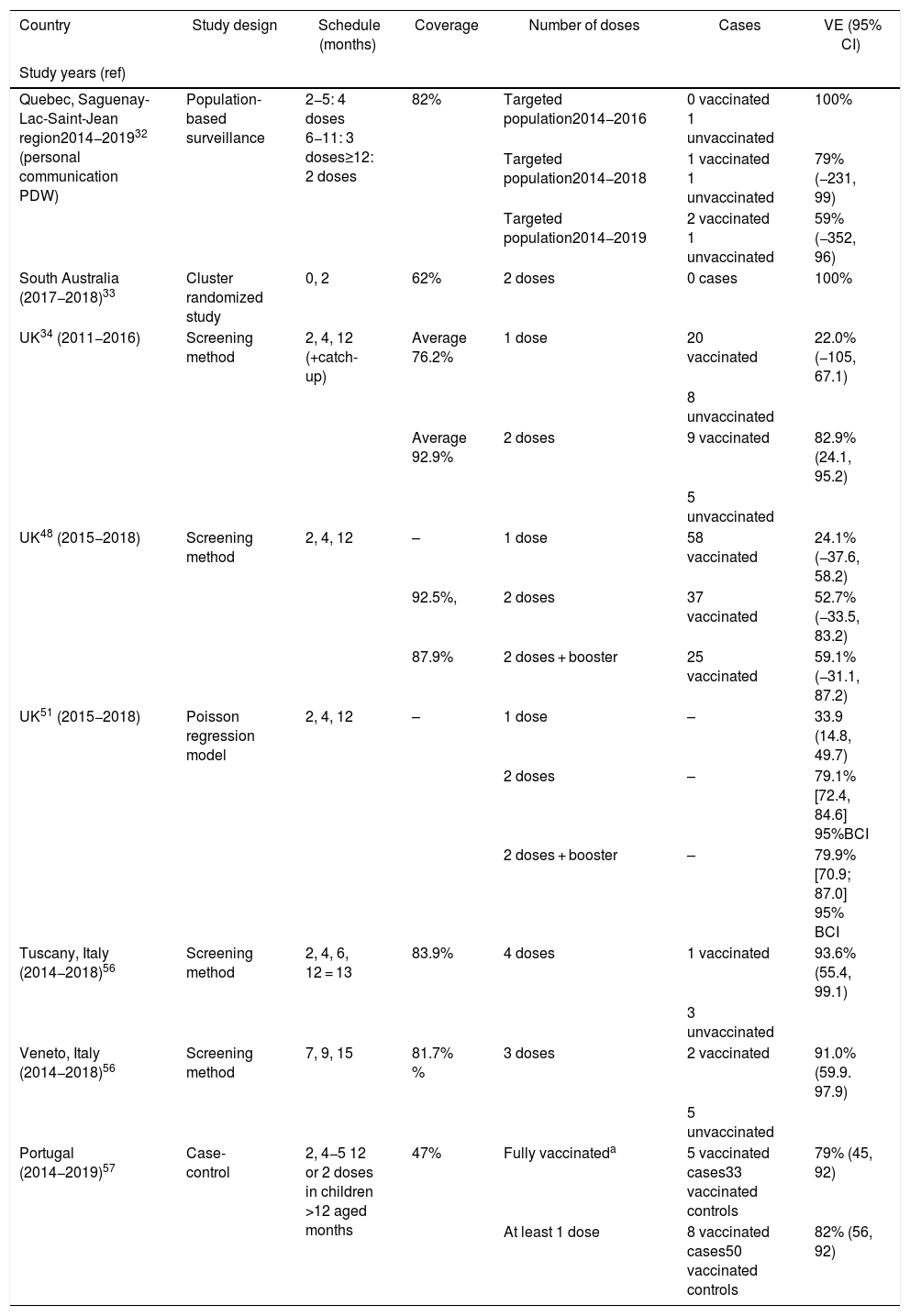
Crude VE in the target age-group was 100% during the first 2 post-campaign years (no case among vaccinated persons), 79% (95% CI −231, 99) over 4 years, and 59% (95% CI −352, 96) over 5 years (Table 3, Fig. 1)32,41 (personal communication Philippe De Wals).Taking into account age-specific inter-regional immigration and emigration rates, the adjusted 5-year VE was 50% (95% CI −453, 95) (personal communication Philippe De Wals), suggesting a duration of protection of approximately 4 years post-vaccination.
Estimates of 4CMenB vaccine effectiveness against invasive meningococcal disease caused by serogroup B.
| Country | Study design | Schedule (months) | Coverage | Number of doses | Cases | VE (95% CI) |
|---|---|---|---|---|---|---|
| Study years (ref) | ||||||
| Quebec, Saguenay-Lac-Saint-Jean region2014−201932 (personal communication PDW) | Population-based surveillance | 2−5: 4 doses 6−11: 3 doses≥12: 2 doses | 82% | Targeted population2014−2016 | 0 vaccinated 1 unvaccinated | 100% |
| Targeted population2014−2018 | 1 vaccinated 1 unvaccinated | 79% (−231, 99) | ||||
| Targeted population2014−2019 | 2 vaccinated 1 unvaccinated | 59% (−352, 96) | ||||
| South Australia (2017−2018)33 | Cluster randomized study | 0, 2 | 62% | 2 doses | 0 cases | 100% |
| UK34 (2011−2016) | Screening method | 2, 4, 12 (+catch-up) | Average 76.2% | 1 dose | 20 vaccinated | 22.0% (−105, 67.1) |
| 8 unvaccinated | ||||||
| Average 92.9% | 2 doses | 9 vaccinated | 82.9% (24.1, 95.2) | |||
| 5 unvaccinated | ||||||
| UK48 (2015−2018) | Screening method | 2, 4, 12 | – | 1 dose | 58 vaccinated | 24.1% (−37.6, 58.2) |
| 92.5%, | 2 doses | 37 vaccinated | 52.7% (−33.5, 83.2) | |||
| 87.9% | 2 doses + booster | 25 vaccinated | 59.1% (−31.1, 87.2) | |||
| UK51 (2015−2018) | Poisson regression model | 2, 4, 12 | – | 1 dose | – | 33.9 (14.8, 49.7) |
| 2 doses | – | 79.1% [72.4, 84.6] 95%BCI | ||||
| 2 doses + booster | – | 79.9% [70.9; 87.0] 95% BCI | ||||
| Tuscany, Italy (2014−2018)56 | Screening method | 2, 4, 6, 12 = 13 | 83.9% | 4 doses | 1 vaccinated | 93.6% (55.4, 99.1) |
| 3 unvaccinated | ||||||
| Veneto, Italy (2014−2018)56 | Screening method | 7, 9, 15 | 81.7% % | 3 doses | 2 vaccinated | 91.0% (59.9. 97.9) |
| 5 unvaccinated | ||||||
| Portugal (2014−2019)57 | Case-control | 2, 4−5 12 or 2 doses in children >12 aged months | 47% | Fully vaccinateda | 5 vaccinated cases33 vaccinated controls | 79% (45, 92) |
| At least 1 dose | 8 vaccinated cases50 vaccinated controls | 82% (56, 92) |
VE vaccine effectiveness; CI, confidence interval, BCI, 95% Bayesian credible interval.
Summary of vaccine effectiveness estimates in infants using 4CMenB in different healthcare settings.
% BCI, 95% Bayesian credible interval; CI, 95% confidence interval; NIP, national immunization program.
*2 doses in infants until 16 months of age, 3 doses after 16 months of age, 2 doses in children who commenced vaccination after age 12 months.
The RR in unvaccinated individuals 4 years after the campaign was 0.48 (95% CI 0.17, 1.35; p = 0.17), which does not support a herd effect of 4CMenB vaccination and reinforces the need for direct individual protection.32 Although invasive disease due to MenB ST-269 has not been eliminated in the Saguenay-Lac-Saint-Jean region, the targeted vaccination program demonstrated high effectiveness in controlling the outbreak.
4CMenB confers individual protection to adolescents in AustraliaSouth Australia has experienced a sustained disease burden due to MenB IMD with an overall incidence rate of 2.8 per 100,000 population in 0–25 year-olds. Routine MenB immunization was introduced in South Australia in 2018.43 The program consists of an ongoing infant and adolescent schedule with time-limited catch-up for children and young adults. Infants up until 12 months of age receive 3 4CMenB doses at 6 weeks, 4 and 12 months of age, and 15 year-olds receive 2 4CMenB doses through school immunization programs, with a minimum interval of 8 weeks between doses.29 Catch-up programs (2 doses with a minimum interval of 8 weeks between doses) were conducted in 1–4 year-olds and 17–20 year-olds for approximately 15 and 13 months, respectively, and have now ended.43
Meningococcal Antigen Typing System (MATS)-predicted strain coverage in South Australia was 90%.44 Convincing evidence to answer the question of whether 4CMenB has an impact on carriage of N. meningitidis was obtained in a cluster randomized controlled trial (“B Part of It”) conducted in South Australia.33 An earlier randomized clinical trial of 2,954 18–24 year-olds in the UK showed a statistically significant, though limited impact of 4CMenB on combined carriage of capsular groups B, C, W and Y, 12 months after vaccination.45 The objective of “B part of it” was to determine the effect of 4CMenB on carriage prevalence of disease-causing N. meningitidis in a large cohort of adolescents 12 months after receiving 4CMenB.33 A total of 34,489 senior school students were enrolled and schools randomly assigned to treatment (students’ received 2 doses of 4CMenB) or a control school (students received 4CMenB after the 12 month evaluation of oropharyngeal carriage). In total, 62% of secondary school students age 15–17 years in South Australia received 4CMenB, allowing observation of disease impact.33,46
4CMenB had no impact on oropharyngeal carriage of IMD-associated meningococci of any capsular group, including MenB. No cases of MenB IMD were reported in vaccinated adolescents during the trial period (2017−2018) versus 12 cases in 15–18 year-olds in the year prior (2015–2016).33 There were approximately 15 (95% CI 3, 19) fewer cases of MenB IMD in the post-vaccination period than the number of predicted cases.46
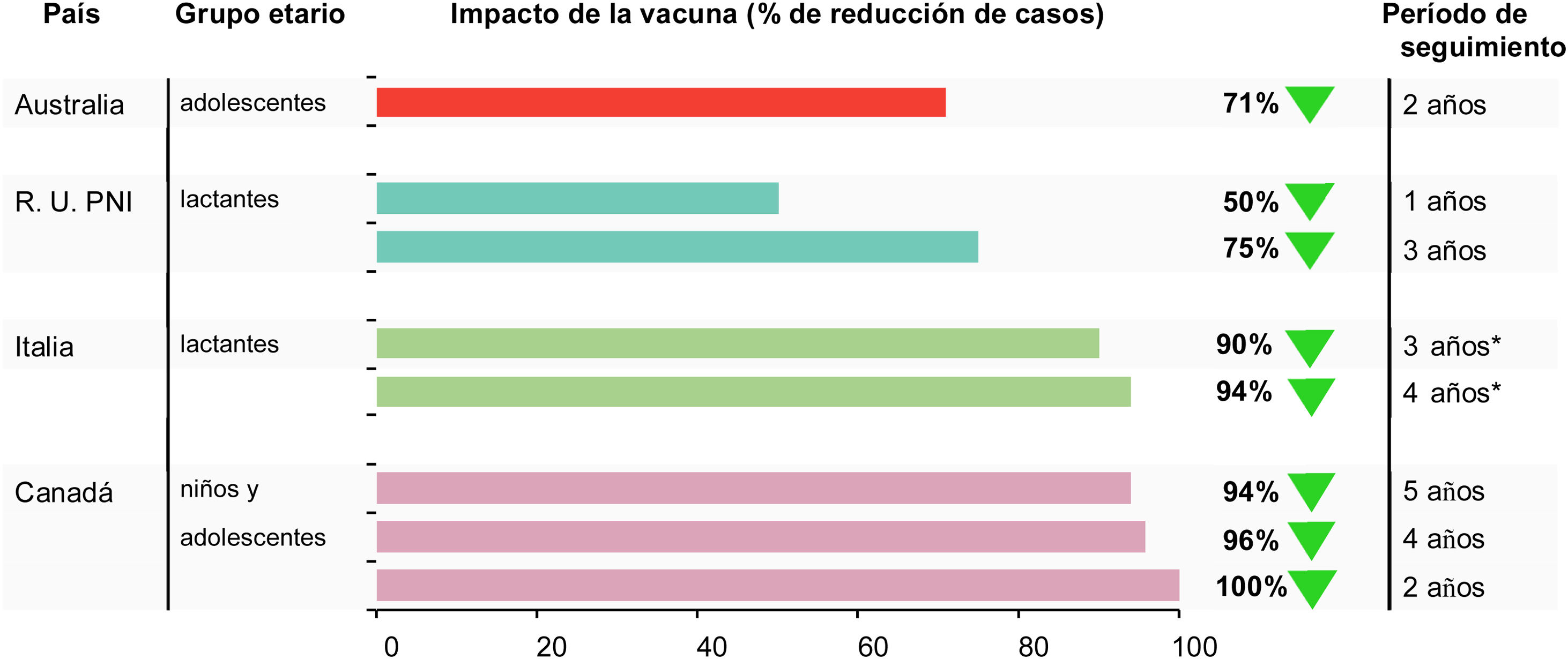
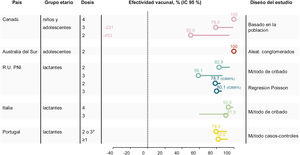
Vaccination significantly reduced MenB IMD in the targeted population by 71% (95% CI 15, 90) (Fig. 2). There was no reduction in the number of MenB IMD cases in children aged 0−4 years during the same period, suggesting no herd immunity impact.46 The data reinforce the need for individual protection against MenB IMD.
From 1 July 2020 the Australian immunization schedule has been updated to improve protection against meningococcal disease. 4CMenB will be added to the NIP for Aboriginal and Torres Strait Islander infants with a catch-up program for children <2 years of age until 2023. 4CMenB vaccine is also be funded for all persons with complement deficiencies or functional or anatomical asplenia.30
Impact and effectiveness of routine infant 4CMenB immunization in the UKThe UK was the first country to include 4CMenB in the NIP for all infants, commencing in September 2015.34 The program uses a 2 + 1 schedule administered at 2, 4 and 12 months of age with other routinely recommended vaccines.20,34 Parents are advised to give 3 consecutive doses of prophylactic paracetamol to infants when they receive vaccine doses 1 and 2.47
During the first 3 years of the program there was a 75% reduction in MenB IMD incidence in children fully eligible for vaccination, as shown by an adjusted incidence rate ratio [IRR] of 0.25 (95% CI 0.19, 0.36)48 (Table 2). The number of observed cases vaccine-eligible cohorts was 62% lower than expected, and an estimated 277 cases of MenB IMD were prevented in the first 3 years of the program,48 or 1 case approximately every 4 days. Four years after commencement of the program, this trend has continued.49 The adjusted IRR showed significant reductions in MenB IMD up to 2 years after the booster dose in vaccine-eligible cohorts (0.43, 95% CI 0.25, 0.74), translating to an ongoing reduction in disease incidence after booster vaccination of 57%.48
VE was evaluated using the indirect screening method comparing cases in vaccinated children with all children who were eligible to receive 4CMenB. VE was estimated for children born on/after 01 July 2015 and did not include children who had been vaccinated during the initial catch-up campaign, with adjustment for changes in MenB IMD incidence in <5 year-olds. Coverage of 1 4CMenB dose in 2018 was approximately 95% and 87.9% had received the booster dose. VE of 2 4CMenB doses was 52.7% (95% CI −33.5, 83.2), and VE of 3 4CMenB doses was 59.1% (95% CI −31.1, 87.2)48 (Fig. 1).
The VE estimate in this study was lower than the initial estimate after the first 10 months of the program (82.9%, 95% CI 24.1–95.2),34 possibly due to lower case numbers in the unvaccinated cohort and lack of statistical power, suggesting that other modelling approaches can be used to obtain more precise VE estimates in this setting.48,50,51 Re-estimation of VE using a Poisson regression model of MenB incidence based on real-world data found that 4CMenB VE was 79.1% [95% Bayesian credible interval 72.4, 84.6] after 2 doses and 79.9% [Bayesian credible interval 70.9, 87.0] 3 doses,51 which more closely resembles previous measures of impact.
Non-MenB effects: potential protection of 4CMenB on serogroup W (MenW) IMD in the UKThe hypervirulent N. meningitidis serogroup W ST-11 strain has caused an increase in MenW IMD cases in some countries, including the UK.52 In the UK, an emergency vaccination program targeting MenW disease was implemented in 2015 using MenACWY conjugate vaccines in adolescents at approximately the same time as implementation of the 4CMenB infant vaccination program.53 An early report of the effect of the MenACWY vaccination program on MenW disease in England found that the number of MenW IMD cases increased from 2014 to 2015 to 2015–2016 in all age-groups except for the age-group targeted for MenACWY vaccination (15–19 year-olds in whom a 31% reduction in cases was observed), and in infants less than 1 year of age targeted for 4CMenB vaccination, in whom a 35% reduction in cases was observed.53 Surveillance of MenW IMD 4 years before and after implementation of the 4CMenB vaccination program found that there were 69% fewer MenW IMD cases than expected in 4CMenB fully-eligible infant cohorts. The 4CMenB program prevented 98 cases of MenW IMD in infants and young children over the first 4 years.54 These observations are supported by 2 in vitro studies; high levels of bactericidal activity against 6 clinical MenW isolates from England and Wales in sera from 4CMenB-vaccinated individuals, suggesting cross-reactivity due to surface proteins common to both serogroups.52 A study with sera from 4CMenB-vaccinated infants demonstrated bactericidal killing of 74% of a panel of MenC, MenW and MenY clinical isolates representing a range of clonal complexes.55
4CMenB vaccine effectiveness and impact in Italy4CMenB was licensed in Italy in 2013 and was implemented into the NIP for infants in 2017. Before that, vaccination policy was decided and implemented regionally, allowing the opportunity to evaluate the VE and VI of different schedules used in similar healthcare settings. In Tuscany, 4CMenB was introduced and regionally funded from 2014 as a 4-dose schedule for infants administered at 2, 4, 6, and 12–13 months of age. Mean coverage at age 24 months was 83.9% between 2016 and 2019. Veneto in contrast, introduced 4CMenB and regional funded vaccination for older infants from 2015 as a 3-dose schedule, administered at 7, 9, and 15 months of age. Mean coverage at age 24 months was 81.7% between 2017 and 2019.56
Between 2014 and 2019 there were 4 cases of MenB IMD in Tuscany in children up to age 5 years; 1 case in a vaccinated child who had received 2 doses, and 3 cases in non-vaccinated children, giving a VE estimate of 93.6% (95% CI 55.4, 99.1) (Table 3). Between 2014 and 2019 there were 7 cases of MenB IMD in children up to age 5 years in Veneto; 2 cases in vaccinated children and 5 in non-vaccinated children, giving a VE estimate of 91.0% (95% CI 59.9, 97.9)56 (Fig. 1).
Although the estimates of VE were similar in both regions, the post versus pre-4CMenB IRR showed a reduction in disease incidence of 68% (statistically significant) in Tuscany where the vaccination schedule commenced in early infancy (IRR 0.32 (95% CI 0.12, 0.91)) vs 31% (not significant) in Veneto (IRR 0.69 (95% 0.31,1.56)) (Table 2). By contrast, when taking into account the vaccination status, evaluation of disease incidence in the vaccinated cohorts showed significant reductions in both regions that were, as expected, similar to the VE estimates; 94% (95% CI 56, 99) in Tuscany and 90% (95% CI 57, 97) in Veneto56 (Fig. 2).
4CMenB vaccine effectiveness in a case-control study in Portugal4CMenB was licensed in Portugal in 2013 but was not publicly funded initially. Although not included in the national immunization program, infant vaccination with 4CMenB was recommended by the Portuguese Society of Pediatrics and uptake by parents has been enthusiastic. Coverage of at least 2 4CMenB doses increased from 32.8% in the 2015 birth cohort to 56.7% in the 2018 cohort. 4CMenB was introduced into the infant NIP in October 2020.27
VE was estimated in a case-control study conducted in 31 pediatric hospitals throughout Portugal between October 2014 and March 2019. Cases were children at least 74 days and less than 18 years of age with laboratory-confirmed IMD. Controls (children presenting to the same hospital without IMD, usually 2 per case) were identified for each case, matched by date of birth, area of residence, gender, and date of attendance at the hospital.
There were 117 IMD cases reported during the study period, of which 82 (70%) were due to MenB. There were 69 MenB cases who were old enough to have been fully immunized. The median age of all MenB cases was 17.5 months (inter-quartile range 6.2–43.6). VE against MenB IMD was 79% (95% CI 45, 92) in fully vaccinated children, and 82% (95% CI 56, 92) in children who had received at least 1 4CMenB dose (Table 3, Fig. 1).57 These estimates were supported by sensitivity analyses including stratification by socioeconomic class, estimation of VE using the screening method, and estimation of VE after removal of the 14-day immune response window. The VE observed in the case-control study was noted to be higher than the strain coverage predicted by MATS (67.9% in isolates over 4 years from 2011−201558).
There were no deaths or long-term sequelae reported among vaccinated cases, whereas among the 87 unvaccinated cases there were 7 deaths (8%) and 16 children with sequelae (18%).
Estimated 4CMenB vaccine impact in Spain4CMenB has been available in Spain since 2013 and MenB vaccination of infants is recommended by the Spanish Pediatric Association. Although not included in the NIP nor publicly funded, moderate uptake of 4CMenB has been achieved. Coverage of 2 4CMenB doses in infants born in 2015–2016 was estimated to be 34%, with regional differences (up to 59% uptake in Galicia and 55% in Castilla y León).15
The 4CMenB impact in Spain was roughly approximated by comparing case numbers from the pre-vaccination period from 2013 to 2014 to the post-vaccination period from 2017−2018. The number of MenB IMD cases decreased by 41.7% in 0–5 month-olds and by 65.4% in 6–11 month-olds.15 These initially encouraging observations are being investigated more systematically in a case-control study in Spain.15
4CMenB is included in the immunization programs in Castilla y León and the Canary Islands since 2019, and has been announced for inclusion in the Andalucía immunization program in 2020.28
4CMenB safety experience in real-world settingsSafety surveillance from countries where 4CMenB has been widely used reported that adverse events following vaccination with 4CMenB were consistent with the established safety profile as reported in clinical trials.59–63 No safety concerns have been raised from the surveillance that encompasses diverse age-groups, countries, schedules, and use in NIPs, and in outbreak management. They include the administration of more than 3 million 4CMenB doses in infants in the UK64,65; administration to approximately 30,500 adolescents in South Australia66; passive surveillance in Australia after market introduction67; surveillance during implementation in university outbreaks the US68; and post-marketing surveillance in Italy60 and Germany.69 Post-marketing surveillance in the UK found no evidence of an increased risk of seizures, febrile seizures or Kawasaki disease after 4CMenB vaccination.64,65 No immune-mediated or neurological safety signals were identified in post-marketing surveillance in Germany.69
A potential safety signal was initially identified in Saguenay-Lac-Saint-Jean in which 4 cases of nephrotic syndrome were reported in children aged 2−5 years within the first 13 months after the first 4CMenB dose.70 The incidence rate of nephrotic syndrome in 1–9 year-olds was 16.3 per 100,000 person-years during this period and the hospitalization rate was significantly higher compared to the pre-vaccination period (RR 7.65, 95% CI 1.02, 57.09).70 This potential signal was investigated further in a substantially larger cohort by evaluation of hospitalizations for nephrotic syndrome before and after 4CMenB introduction in the UK. The results showed no evidence of an increased risk in the UK.71 Symptoms of nephrotic syndrome can initially be mild and remain undetected, preventing accurate assessment of a temporal relationship with vaccination. This, plus the lack of cases of nephrotic syndrome reported in other countries and the absence of a biologically plausible causal mechanism of action, is suggestive that the cases observed in the Saguenay-Lac-Saint-Jean region occurred by chance.70
DiscussionThe effectiveness of vaccines targeting uncommon diseases can usually only be measured after widespread implementation occurs after licensure. For 4CMenB, estimates of VE are now available from 5 countries, obtained during funded routine use in the UK and Italy, in a non-funded healthcare setting in Portugal, an observational study in Australia, and in outbreak control in the Saguenay-Lac-Saint-Jean region, Canada (Table 3). VE of at least 3 doses of 4CMenB in infants ranged from 59.1% to 93.6%, and were usually higher than predicted strain coverage rates using MATS. The 4CMenB vaccination for outbreak control in Quebec resulted in 100% VE in children and adolescents aged 2 months to 20 years in the first 2 years after vaccination and VE was sustained for 4 years. Data from routine 4CMenB infant vaccination in the UK shows that VE was sustained for 2 years after the booster dose in young children vaccinated in infancy.32,48
The impact of 4CMenB on MenB IMD incidence rates was demonstrated following infant vaccination in the UK, Italy, and Spain, and in children/adolescents/young adults in prolonged outbreaks in the Saguenay-Lac-Saint-Jean region and South Australia (Table 2). The impact of vaccination on outbreaks such as those that occur in universities in the US is much harder to measure because of the small number of cases that occur. However, the absence of breakthrough cases after vaccine implementation is suggestive of a VI. Evidence of a 4CMenB impact for outbreak control continues to accumulate. An outbreak of 5 MenB cases in a middle school and high school in France in 2016–2017 was managed with a vaccination campaign with 4CMenB in the 2 schools and 11–19 year-olds living close by.72 No further cases were reported after the vaccination campaign.
The safety profile of 4CMenB administered in real-world settings appears to reflect that established in pre-licensure clinical trials.7 No safety concerns have been raised in post-marketing surveillance.
A consistent, substantial and increasing body of real-world evidence supports the effectiveness and impact of 4CMenB in preventing MenB IMD in young infants and adolescents. The potential for 4CMenB to prevent gonococcal disease in young persons warrants further investigation of the role of 4CMenB in adolescents.73
Since licensure in 2013, real-world experience with 4CMenB used in numerous countries, age-groups, schedules and healthcare settings have become available. Some of the questions around 4CMenB implementation at the time of licensure can now be answered. The studies presented here provide detailed consistent responses to the fundamental questions of effectiveness, impact and safety; demonstrating that 4CMenB is effective in preventing MenB IMD in vaccinated individuals, reduces the disease incidence in populations, and has a safety profile that is consistent with that established in clinical trials. Real-world evidence shows that 4CMenB reduced MenB IMD in individuals from 2 months to 20 years of age, with substantial impacts on MenB IMD observed in UK, Australia, Italy, Portugal, Quebec and in US university outbreaks.32,34,37–39,41,46,48,56 VE estimates in these individual countries were usually higher than tentative predictions of coverage across MenB strains using methods such as MATS ranged between 66% and 91%.46
To date neither 4CMenB nor MenB-fHbp have shown evidence that they have an effect on meningococcal carriage32,33,74,75 suggesting that this could be a common feature of MenB protein vaccines. Experience from Italy suggests that early infant vaccination has a greater impact on reducing cases of MenB IMD than vaccination delayed until 7 months of age.56 Together, these findings have important programmatic implications. 4CMenB is likely to be most effective when administered directly to the age-groups at highest risk. 4CMenB vaccination should therefore commence prior to the onset of the highest risk period, which in most countries is in infants and young children.1 This is in contrast to meningococcal conjugate vaccines that demonstrate strong herd effects,76 and where interruption of transmission through vaccination of adolescents in whom carriage rates are highest is plausible.77
4CMenB also demonstrates a statistically significant impact in preventing MenW IMD.54 The potentially wider impacts of 4CMenB on non-MenB/non-MenW disease have yet to be quantified. Of particular interest is whether 4CMenB can also prevent infections due to N. gonorrhoeae which shares 80–90% genetic homology with MenB.78 A reduction in the number of gonorrhea cases was observed in New Zealand after a campaign using an outer membrane vesicle vaccine that is the same as a component of 4CMenB, and in the Saguenay-Lac-Saint-Jean region after the vaccination campaign described above (not statistically significant possibly due to small numbers).79,80 A retrospective case-control study in New York and Philadelphia found that 4CMenB vaccination was associated with a statistically significantly lower likelihood of gonorrhea infection, with an estimated vaccine effectiveness of 36% (95% CI 23, 52).81 These studies provide important information that expand our understanding of the potential additional benefits of 4CMenB vaccination.
In coming years we can expect to see increased implementation of 4CMenB in NIPs, availability of longer term effectiveness data to guide the need for booster doses, potentially greater understanding of the different contributions to protection conferred by the different 4CMenB components, ongoing accumulation of safety experience, and further development of pentavalent vaccines (MenABCWY) that could help prevent infection and improve coverage from all major disease-causing serogroups.82
In conclusion, IMD caused by MenB is an uncommon but life-threatening disease with potentially severe sequelae and a high case-fatality rate. Epidemiology is unpredictable due to strain evolution and outbreak potential. There was some reluctance at the time of registration to implement a MenB vaccine into national programs given the initial lack of data, evidence of long-term protection, and budgetary impacts. Since then clear evidence of 4CMenB-induced protection against MenB IMD has been demonstrated in different age-groups and healthcare settings. The results reinforce the importance of direct protection of the highest risk groups, infants/young children and adolescents. Direct protection via routine infant immunization with catch-up in young children and routine vaccination of adolescents could be the preferred option for control of MenB disease.
Trademark statementBexsero is a trademark owned by or licensed to the GSK group of companies. Trumenba is a trademark of Pfizer Inc.
Funding detailsGlaxoSmithKline Biologicals SA funded all costs associated with the development and the publishing of the present manuscript.
Role of the funding sourceGlaxoSmithKline Biologicals SA was involved in the writing the manuscript and in the decision to submit the article for publication.
Disclosure statementAB, MP, RR and RBB are employees of the GSK group of companies and hold shares as part of their employee remuneration. HM’s institution receives funding for investigator led studies from GSK, Pfizer and Sanofi-Pasteur. PDW received investigator-initiated research grants unrelated to the present work from GSK, Pfizer and Sanofi. RM has received investigator-initiated research grants unrelated to the present work from GSK. FM-T has received honoraria from GSK, Pfizer Inc, Sanofi Pasteur, MSD, Seqirus, Novavax, and Biofabri and Janssen for taking part in advisory boards and expert meetings and for acting as a speaker in congresses outside the scope of the submitted work. FM-T has also acted as principal investigator in randomized controlled trials of the above-mentioned companies except for Biofabri as well as Ablynx, Regeneron, Novartis, and MedImmune, with honoraria paid to his institution and the author declares grants from AstraZeneca outside the scope of the submitted work. FM-T receives support for research activities from the Instituto de Salud Carlos III (Proyecto de Investigación en Salud, Acción Estratégica en Salud): Fondo de Investigación Sanitaria (FIS; PI070069/PI1000540/PI1601569/PI1901090) del plan nacional de I+D+I and ‘fondos FEDER’. CA declares no conflicts of interest. All authors declare no non-financial conflict of interest.
Declaration of interestsThe authors declare the following financial interests/personal relationships which may be considered as potential competing interests
The authors thank the Modis platform for writing support, editorial assistance and manuscript coordination, on behalf of GSK. Writing support was provided by Joanne Wolter; editorial support and publication management was provided by Divya Kesters. The authors also thank Business & Decision Life Sciences for design support for the digital animations, on behalf of GSK.
Please cite this article as: Martinón-Torres F, Banzhoff A, Azzari C, de Wals P, Marlow R, Marshall H, et al. Avances recientes en la prevención de la enfermedad meningocócica B: evidencia real de la vacunación con 4CMenB. Vacunas. 2021;22:189–202.
This article was originally published in English by the Journal of Infectionhttps://doi.org/10.1016/j.jinf.2021.04.031 and has the corresponding permission for translation and publication in Vaccines.