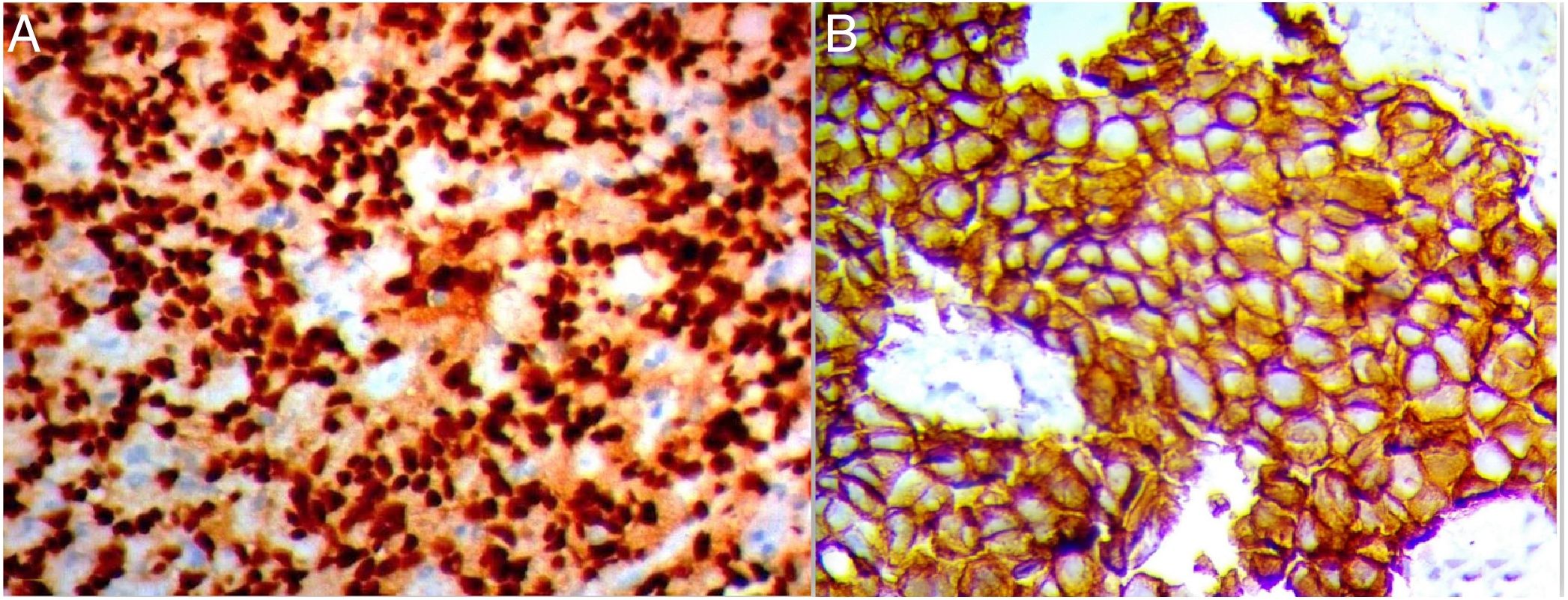
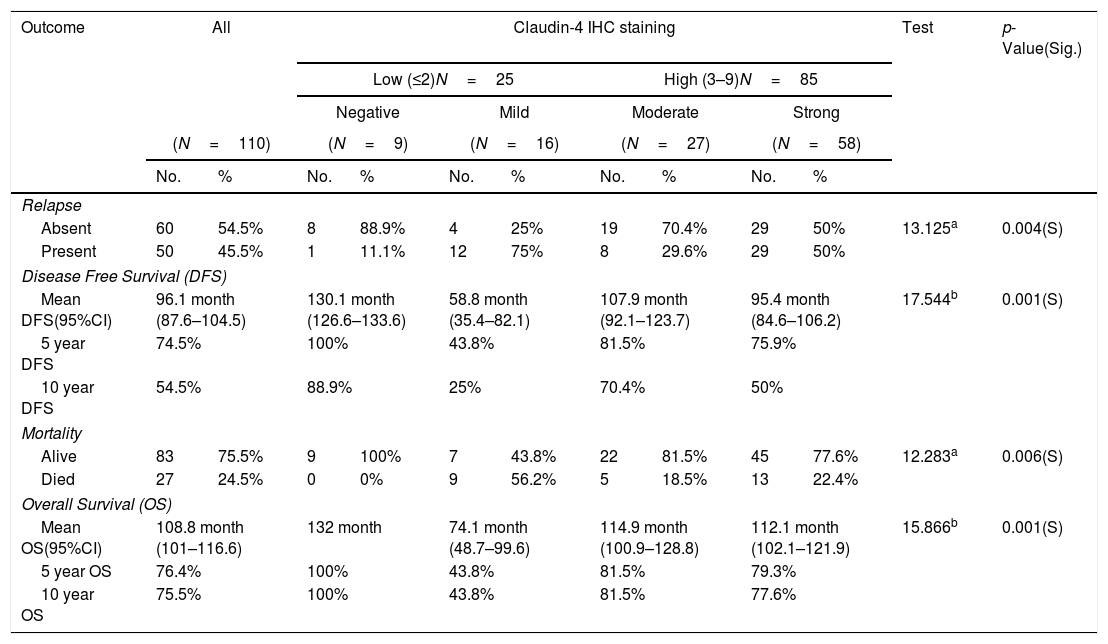
Although the molecular profile of the breast provides prognostic indicators, risk stratification in breast cancer continues to be a challenge. Therefore, it is mandatory to seek new prognostic markers that could aid the early diagnosis of potential metastases in biopsy samples from breast cancer; among these are increased Snail-1 and Claudin-4 expression.
ObjectivesThe aim of this study was to analyze the correlation between Snail-1 and Claudin-4 with other clinical-pathological parameters and distinct molecular subtypes.
MethodsThis study included 110 patients with invasive ductal carcinoma from 2009 to January 2015. Snail-1 and Claudin-4 were assessed by immunohistochemistry in formalin-fixed paraffin-embedded tissue blocks and the data were correlated with clinical-pathological data and survival.
ResultsA total of 65 patients (68.2%) were positive for Snail-1 and 85 patients (77.3%) were positive for Claudin-4. High Snail-1 and high Claudin-4 were detected in high-grade tumors and were associated with lymphovascular infiltration and lymph node metastases (p<0.001 for each). There was a highly significant correlation between Snail-1, Claudin-4 expression and the molecular subtype of breast cancer (p<0.001), with higher Snail-1 expression in TNBC and Her 2/neu cases (p=0.001). Claudina-4 expression in the Her2/neu enriched subtype, Snail-1-positivity and high Claudin-4 expression were associated with recurrence (p=0.001; 0.004 respectively) among the cases studied. Snail-1 and Claudin-4 were inversely related with overall survival (p=0.001) and disease-free survival (p=0.001).
ConclusionHigh Snail-1 and Claudin-4 levels were associated with adverse outcomes in patients with breast cancer.
Aunque el perfil molecular de la mama proporciona indicadores de pronóstico, la estratificación del riesgo de cáncer de mama sigue siendo un desafío y es obligatoria para buscar nuevos marcadores de pronóstico que puedan facilitar el diagnóstico temprano de metástasis potenciales en las muestras de biopsia de cáncer de mama; entre estos se encuentra la expresión creciente de Snail-1 y Claudin-4.
ObjetivosEl objetivo de este trabajo es estudiar la correlación de Snail-1 y Claudin-4 con otros parámetros clínico-patológicos y diferentes subtipos moleculares.
MétodosSe inscribieron 110 pacientes con carcinoma de conducto invasivo en este estudio durante el período de enero de 2009 a enero de 2015. Snail-1 y Claudin-4 fueron evaluados por inmunohistoquímica (IHC) en bloques de parafina y los datos se correlacionaron con características clínico-patológicas y de supervivencia.
ResultadosFueron positivos 75 casos (68,2%) para Snail-1 y 85 (77,3%) positivos para Claudin-4. High Snail-1 y High Claudin-4 se detectaron en tumores de alto grado y se asociaron con invasión linfovascular y metástasis en los ganglios linfáticos (p < 0,001 para cada uno). Se detectó una correlación altamente significativa entre Snail-1, la expresión de Claudin-4 y el subtipo molecular de cáncer de mama (p < 0,001), con la mayor expresión de Snail-1 en los casos de cáncer de mama triple negativo (TNBC) y Her 2/neu (p = 0,001). La expresión de Claudina-4 en subtipo Her2/neu enriched, Snail-1 positivo y alta expresión de Claudin-4 se asoció con recaída (p = 0,001; 0,004, respectivamente) entre los casos estudiados. La expresión de Snail-1 y Claudin-4 se relacionó inversamente con la SG (p = 0,001) y la SSE (p = 0,001).
ConclusiónLos niveles altos de proteínas Snail-1 y Claudin-4 se asocian con resultados adversos en pacientes con cáncer de mama.
According to the latest report of International Agency for Research on Cancer (IARC), breast cancer is the most frequent cancer in females Worldwide and ranks the second among all cancer types.1 In Egypt, Breast cancer is the most diagnosed malignant tumor and the leading cause of cancer death among females, accounting for 25% of the total cancer cases and 14% of the cancer death, estimated by National Cancer Institute.2 Invasive ductal carcinoma (IDC) represents the most common subtype of all breast cancers.3
Cellular tight junctions are composed mainly of three integral membrane proteins: occludin, claudins, and junctional adhesion molecules. Claudins form most of the backbone of tight junction protein strands. Claudins family comprises 27 members and categorized into four transmembrane protein classes with the carboxyl-terminus in the cytoplasm and two extracellular loops.4,5
Mesenchymal cells lack cell-cell contacts, express mesenchymal markers and exhibit metastatic behavior. During epithelial-to-mesenchymal transition (EMT), epithelial cells lose their specific epithelial features and gain a fibroblast-like morphology, with cytoskeletal changes, over-expression of mesenchymal markers, and enhancement of invasiveness and metastatic potential.6 EMT is thought to support tumor cell survival in the blood stream and to enhance extravasation of cells at the distant sites of metastasis.7 Monoclonal antibodies against Claudin-4 individually or in combination with anti-Claudin-38 have been shown to produce promising results in cancer treatment.
New prognostic markers could achieve early diagnosis of potential metastasis; Snail-1 and Claudin-4 are two of these hopeful markers. Hence, we purposed to study the correlation of Snail-1 and Claudin-4 expression with other clinicopathological parameters (age, grade, tumor size, hormonal status, different molecular subtypes, lymph node status and metastasis) in IDC to evaluate the prognostic utility of these markers in breast cancer and their role in metastasis and to evaluate the correlation between patient survival and Snail-1, Claudin-4 immunohistochemical (IHC) expression.
Patients and specimen selectionThis is a retrospective, cross-sectional study that was carried out at Pathology, Oncology unit of General surgery, Medical oncology, and Clinical oncology and nuclear medicine departments, Faculty of Medicine, Zagazig University and surgical oncology department Al-Ahrar Teaching Hospital. One hundred and ten patients with IDC of no special type (NST) were introduced in this study during the period from January 2009 to January 2015, follow up has occurred from the date of diagnosis as IDC until last medical visit. Institutional Review Board (IRB) of the faculty of Medicine Zagazig University confirmed the study protocol (No.4144).
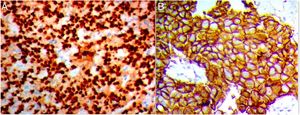
Immunohistochemistry protocolFormalin-fixed paraffin-embedded tissue (FFPE) blocks were serially sectioned into 3–5μm sections then deparaffinized in xylene, followed by rehydration in descending series of alcohols. For antigen retrieval processing, 10mM citrate buffer (pH 6.0) at the microwave for nearly 20min was used. Endogenous peroxidase activity was blocked by 3% hydrogen peroxide for 10min. After repeat and numerous washing in PBS, the slides were then incubated with anti-Snail polyclonal antibody (clone CE2C3, diluted 1:100; Santa Cruz, CA, USA); and anticlaudin-4 polyclonal antibody (A-12): sc-376643 diluted 1:50; Santa Cruz, CA, USA). Binding site of primary antibodies was visualized by using the polymer detection system; the Dako EnVision™ kit (Dako, Copenhagen, Denmark). Finally, the tissue sections were counterstained with Meyer's hematoxylin.
Interpretation of the immunohistochemistrySnail evaluationThe extent of positivity was scored as follow; 0 if positive cells was <5%; 1 when it was 5–25%; 2 when it was 26–50%; 3 when it was 51–75%; and 4 when it was >75%. The intensity was scored as 0 when no stain was detected, 1 for weak, 2 for moderate, and 3 for strong staining. The extent and intensity scores were added to get a total score, which ranged from (0 to 7). The cutoff values were 4 for Snail-1 expression in IDC tissues.10
Claudin-4 evaluationClaudin-4 was considered when showing membranous and cytoplasmic staining and evaluated in 5 high-power fields at ×400 magnification, based on combined score of the intensity (0, no stain; 1, weak; 2, moderate; and 3, strong) and the percentage of stained tumor cells (0, <5%; 1, 5–25%; 2, 26–50%; and 3, >51%). The two scores were multiplied to give total score of (0–9); 0 for negative; 1 and 2 scores were weak; 3, 4 and 6 scores were moderate; and 9 score was strong. Negative and weak expression was considered as low expression, whereas moderate and strong as high expression. So, a cut-off ≤2 was considered as negative according to Sheehan et al., 2007.11
Statistical analysisContinuous variables were expressed as the mean±SD & median (range), and the categorical variables were expressed as a number (percentage). One Way ANOVA test was used to compare between more than two groups of normally distributed variables while Kruskal Wallis H test was used for non-normally distributed variables. Percent of categorical variables were compared using Pearson's Chi-square test or Fisher's exact test when was appropriate. Overall Survival (OS) was calculated as the time from diagnosis to death. Disease Free Survival (DFS) was calculated as the time from date of surgery to date of relapse or the most recent follow-up contact that patient was known as relapse free. Stratification of OS and DFS was done according IHC staining for Snail-1 and Claudin-4. These time-to-event distributions were estimated using the method of Kaplan–Meier plot, and compared using two-sided exact log-rank test. All tests were two sided. A p-value<0.05 was considered significant. All statistics were performed using SPSS 20.0 for windows (SPSS Inc., Chicago, IL, USA).
ResultsPatients’ characteristicsThe age of patients (n=110) at the time of initial diagnosis ranged from 27 to 75 years. The mean and median ages were 48.88±13.17 years and 49 years, respectively. The majority of cases were grade II, III (36.4%, 51.8% respectively) and stage III (68.2%). Molecular classification of patients resulted in: (43 luminal A, 15 luminal B, 32 triple negative and 20 Her2/neu enriched). All clinical data were obtained from patients’ files. Also receptor status (ER, PR, and Her2/neu) was acquired from their pathology reports. The cases were selected depending on the availability of complete clinical data and paraffin blocks. Pretreatment tumor size was determined by a combination of clinical examination, mammography, and ultrasound examinations. Lymph node status was assessed. The tumors were graded according to the Nottingham modification of Bloom-Richardson system.9 Our patient's treatment planes were given according to stage and IHC. Neoadjuvant chemotherapy was used for 17 patients; adjuvant chemotherapy was given to those who indicated postsurgery. AC-T protocol (adriamycine/cyclophosphamide – taxol) was used for most patients (62/110), sequential 4 cycles of doxorubicin and 12 weeks of paclitaxel. Other protocols given were AC-docetaxel, CMF, CAF, FEC, and TC. Trastuzumab was used in 16 patients only according to files data, 58 patients were indicated for hormonal therapy either with tamoxifen, AIso (Aromatase Inhibitors), and ovarian suppression according to indications. Positive expression of Snail-1 and Claudin-4 in cases of IDC were 54.5% and 77.3%, respectively. Both Snail-1 and Claudin-4 expressions were positive in histological grade III IDC, associated with lymphovascular invasion. Most of the studied cases 75 (68.2%) were stage III.
Association of Snail-1 and Claudin-4 expression with clinic-pathological parameters are assessed and presented in Tables 1–2- -
Out of the 110 breast cancer cases, 60 cases (54.5%) were Snail-1 positive and 85 cases (77.3%) were Claudin-4 positive Fig. 1. Expression of both Snail-1 and Claudin-4 was found in 68.2% (75/110) of the cases and the expression of both markers was negative in 31.8% (35/110) of cases. There were a highly significant correlation between Claudin-4 with Snail-1 expression (p<0.001) in invasive duct carcinoma of breast.
- -
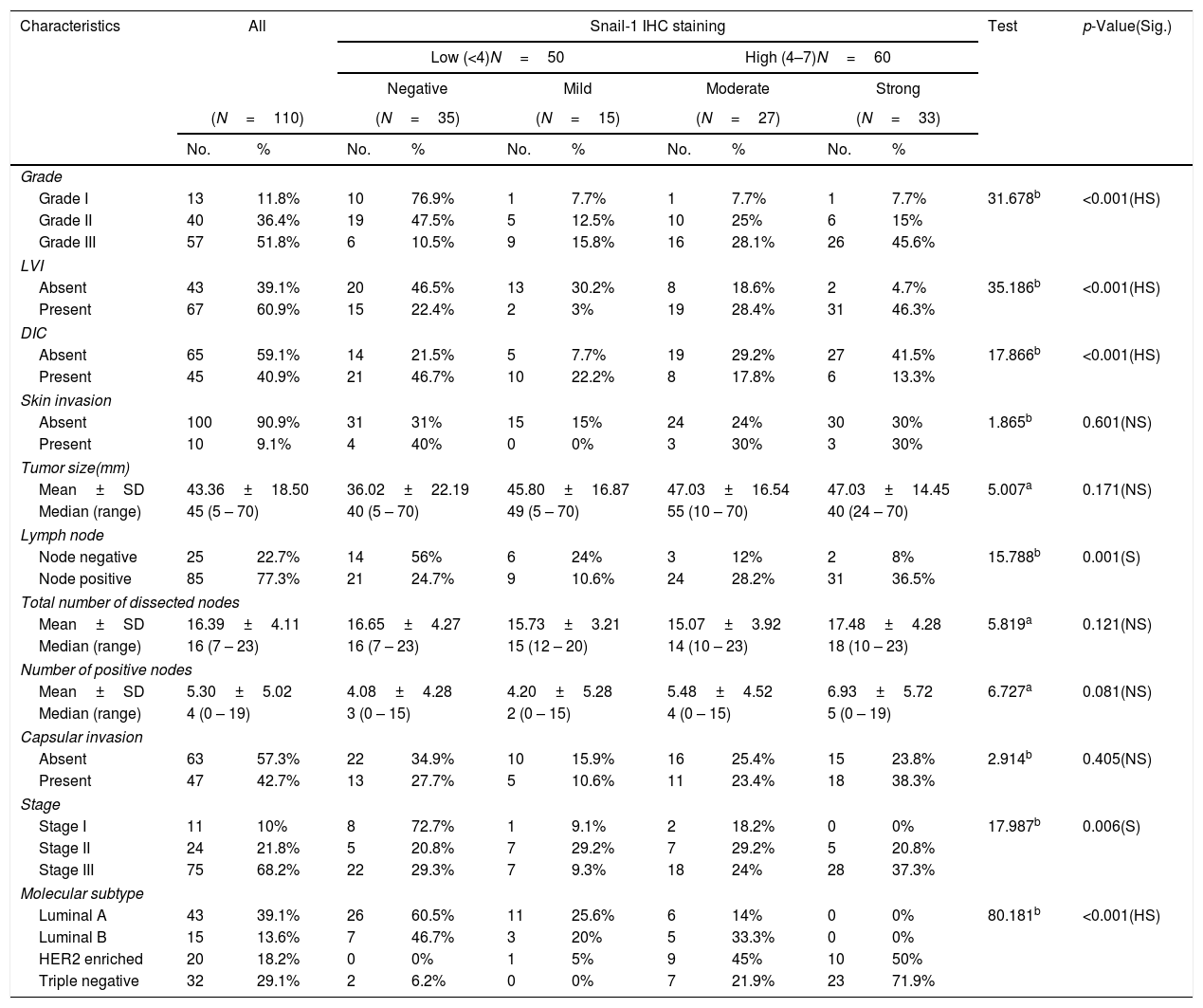
High Snail-1 expression was detected in tumor grade III with significant difference (p<0.001) and associated with lymph vascular invasion (p<0.001). There were a significant correlation of high Snail-1 expression and lymph node metastasis (p=0.001). However; there were insignificant correlation of Snail-1 expression and skin invasion (p=0.601), tumor size (p=0.171) or capsular invasion (p=0.405).
- -
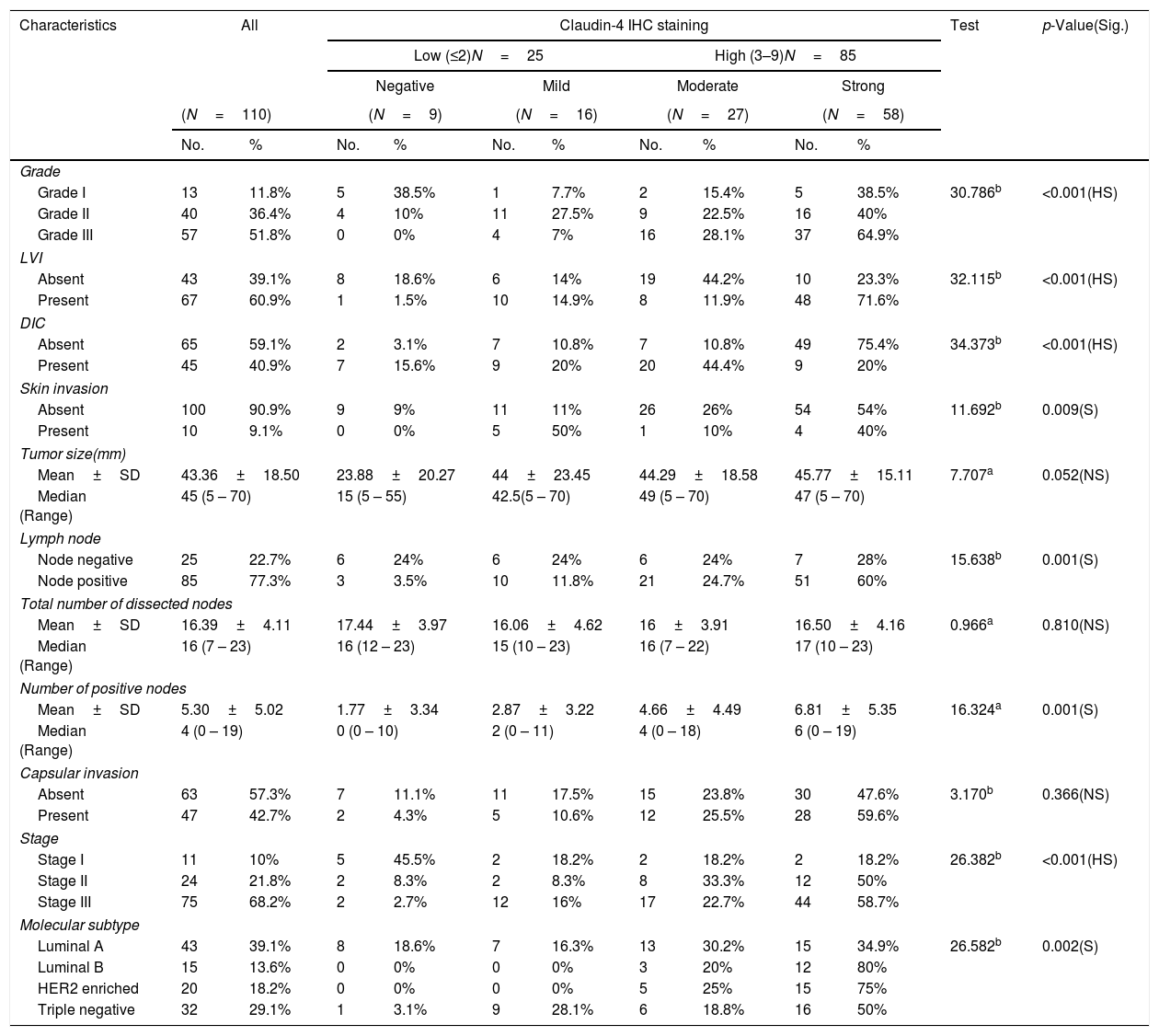
Claudin-4 expression was significantly increased with high stage (p value<0.001). There was a high significant difference in Claudin-4 expression in various tumor grades with high Claudin-4 expression in 64.9% of histological grade III tumor, and in 71.6% of cases with lymph vascular invasion. Absence of ductal carcinoma in situ component was associated with high Claudin-4 expression (p<0.001). There was a significant correlation of high Claudin- 4 expression and lymph node metastasis (p=0.001), and skin invasion (0.009). There was a significant correlation of Claudin-4 expression and tumor size (p=0.05).
A highly significant correlation was detected between Snail-1 expression with the different molecular subtype of breast cancer (p<0.001), with the highest expression in TNBC. Also, there was a significant correlation between snail-1 and Her 2/neu positive cases (p=0.001). Claudin-4 expression in tumor cells showed a significant correlation with molecular subtypes (p=0.002). Her2/neu positive subtype showed the highest expression as 75% (15/20) of cases showed high membranous Claudin-4 expression. High Claudin-4 was highly significant correlated with high Snail-1 expression (p value<0.001).
Relation between histopathological characteristics and immunohistochemical staining for Snail-1.
| Characteristics | All | Snail-1 IHC staining | Test | p-Value(Sig.) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low (<4)N=50 | High (4–7)N=60 | |||||||||||
| Negative | Mild | Moderate | Strong | |||||||||
| (N=110) | (N=35) | (N=15) | (N=27) | (N=33) | ||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | |||
| Grade | ||||||||||||
| Grade I | 13 | 11.8% | 10 | 76.9% | 1 | 7.7% | 1 | 7.7% | 1 | 7.7% | 31.678b | <0.001(HS) |
| Grade II | 40 | 36.4% | 19 | 47.5% | 5 | 12.5% | 10 | 25% | 6 | 15% | ||
| Grade III | 57 | 51.8% | 6 | 10.5% | 9 | 15.8% | 16 | 28.1% | 26 | 45.6% | ||
| LVI | ||||||||||||
| Absent | 43 | 39.1% | 20 | 46.5% | 13 | 30.2% | 8 | 18.6% | 2 | 4.7% | 35.186b | <0.001(HS) |
| Present | 67 | 60.9% | 15 | 22.4% | 2 | 3% | 19 | 28.4% | 31 | 46.3% | ||
| DIC | ||||||||||||
| Absent | 65 | 59.1% | 14 | 21.5% | 5 | 7.7% | 19 | 29.2% | 27 | 41.5% | 17.866b | <0.001(HS) |
| Present | 45 | 40.9% | 21 | 46.7% | 10 | 22.2% | 8 | 17.8% | 6 | 13.3% | ||
| Skin invasion | ||||||||||||
| Absent | 100 | 90.9% | 31 | 31% | 15 | 15% | 24 | 24% | 30 | 30% | 1.865b | 0.601(NS) |
| Present | 10 | 9.1% | 4 | 40% | 0 | 0% | 3 | 30% | 3 | 30% | ||
| Tumor size(mm) | ||||||||||||
| Mean±SD | 43.36±18.50 | 36.02±22.19 | 45.80±16.87 | 47.03±16.54 | 47.03±14.45 | 5.007a | 0.171(NS) | |||||
| Median (range) | 45 (5 – 70) | 40 (5 – 70) | 49 (5 – 70) | 55 (10 – 70) | 40 (24 – 70) | |||||||
| Lymph node | ||||||||||||
| Node negative | 25 | 22.7% | 14 | 56% | 6 | 24% | 3 | 12% | 2 | 8% | 15.788b | 0.001(S) |
| Node positive | 85 | 77.3% | 21 | 24.7% | 9 | 10.6% | 24 | 28.2% | 31 | 36.5% | ||
| Total number of dissected nodes | ||||||||||||
| Mean±SD | 16.39±4.11 | 16.65±4.27 | 15.73±3.21 | 15.07±3.92 | 17.48±4.28 | 5.819a | 0.121(NS) | |||||
| Median (range) | 16 (7 – 23) | 16 (7 – 23) | 15 (12 – 20) | 14 (10 – 23) | 18 (10 – 23) | |||||||
| Number of positive nodes | ||||||||||||
| Mean±SD | 5.30±5.02 | 4.08±4.28 | 4.20±5.28 | 5.48±4.52 | 6.93±5.72 | 6.727a | 0.081(NS) | |||||
| Median (range) | 4 (0 – 19) | 3 (0 – 15) | 2 (0 – 15) | 4 (0 – 15) | 5 (0 – 19) | |||||||
| Capsular invasion | ||||||||||||
| Absent | 63 | 57.3% | 22 | 34.9% | 10 | 15.9% | 16 | 25.4% | 15 | 23.8% | 2.914b | 0.405(NS) |
| Present | 47 | 42.7% | 13 | 27.7% | 5 | 10.6% | 11 | 23.4% | 18 | 38.3% | ||
| Stage | ||||||||||||
| Stage I | 11 | 10% | 8 | 72.7% | 1 | 9.1% | 2 | 18.2% | 0 | 0% | 17.987b | 0.006(S) |
| Stage II | 24 | 21.8% | 5 | 20.8% | 7 | 29.2% | 7 | 29.2% | 5 | 20.8% | ||
| Stage III | 75 | 68.2% | 22 | 29.3% | 7 | 9.3% | 18 | 24% | 28 | 37.3% | ||
| Molecular subtype | ||||||||||||
| Luminal A | 43 | 39.1% | 26 | 60.5% | 11 | 25.6% | 6 | 14% | 0 | 0% | 80.181b | <0.001(HS) |
| Luminal B | 15 | 13.6% | 7 | 46.7% | 3 | 20% | 5 | 33.3% | 0 | 0% | ||
| HER2 enriched | 20 | 18.2% | 0 | 0% | 1 | 5% | 9 | 45% | 10 | 50% | ||
| Triple negative | 32 | 29.1% | 2 | 6.2% | 0 | 0% | 7 | 21.9% | 23 | 71.9% | ||
Categorical variables were expressed as number (percentage).
Continuous variables were expressed as mean±SD & median (range).
Relation between histopathological characteristics and immunohistochemical staining for Claudin-4.
| Characteristics | All | Claudin-4 IHC staining | Test | p-Value(Sig.) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low (≤2)N=25 | High (3–9)N=85 | |||||||||||
| Negative | Mild | Moderate | Strong | |||||||||
| (N=110) | (N=9) | (N=16) | (N=27) | (N=58) | ||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | |||
| Grade | ||||||||||||
| Grade I | 13 | 11.8% | 5 | 38.5% | 1 | 7.7% | 2 | 15.4% | 5 | 38.5% | 30.786b | <0.001(HS) |
| Grade II | 40 | 36.4% | 4 | 10% | 11 | 27.5% | 9 | 22.5% | 16 | 40% | ||
| Grade III | 57 | 51.8% | 0 | 0% | 4 | 7% | 16 | 28.1% | 37 | 64.9% | ||
| LVI | ||||||||||||
| Absent | 43 | 39.1% | 8 | 18.6% | 6 | 14% | 19 | 44.2% | 10 | 23.3% | 32.115b | <0.001(HS) |
| Present | 67 | 60.9% | 1 | 1.5% | 10 | 14.9% | 8 | 11.9% | 48 | 71.6% | ||
| DIC | ||||||||||||
| Absent | 65 | 59.1% | 2 | 3.1% | 7 | 10.8% | 7 | 10.8% | 49 | 75.4% | 34.373b | <0.001(HS) |
| Present | 45 | 40.9% | 7 | 15.6% | 9 | 20% | 20 | 44.4% | 9 | 20% | ||
| Skin invasion | ||||||||||||
| Absent | 100 | 90.9% | 9 | 9% | 11 | 11% | 26 | 26% | 54 | 54% | 11.692b | 0.009(S) |
| Present | 10 | 9.1% | 0 | 0% | 5 | 50% | 1 | 10% | 4 | 40% | ||
| Tumor size(mm) | ||||||||||||
| Mean±SD | 43.36±18.50 | 23.88±20.27 | 44±23.45 | 44.29±18.58 | 45.77±15.11 | 7.707a | 0.052(NS) | |||||
| Median (Range) | 45 (5 – 70) | 15 (5 – 55) | 42.5(5 – 70) | 49 (5 – 70) | 47 (5 – 70) | |||||||
| Lymph node | ||||||||||||
| Node negative | 25 | 22.7% | 6 | 24% | 6 | 24% | 6 | 24% | 7 | 28% | 15.638b | 0.001(S) |
| Node positive | 85 | 77.3% | 3 | 3.5% | 10 | 11.8% | 21 | 24.7% | 51 | 60% | ||
| Total number of dissected nodes | ||||||||||||
| Mean±SD | 16.39±4.11 | 17.44±3.97 | 16.06±4.62 | 16±3.91 | 16.50±4.16 | 0.966a | 0.810(NS) | |||||
| Median (Range) | 16 (7 – 23) | 16 (12 – 23) | 15 (10 – 23) | 16 (7 – 22) | 17 (10 – 23) | |||||||
| Number of positive nodes | ||||||||||||
| Mean±SD | 5.30±5.02 | 1.77±3.34 | 2.87±3.22 | 4.66±4.49 | 6.81±5.35 | 16.324a | 0.001(S) | |||||
| Median (Range) | 4 (0 – 19) | 0 (0 – 10) | 2 (0 – 11) | 4 (0 – 18) | 6 (0 – 19) | |||||||
| Capsular invasion | ||||||||||||
| Absent | 63 | 57.3% | 7 | 11.1% | 11 | 17.5% | 15 | 23.8% | 30 | 47.6% | 3.170b | 0.366(NS) |
| Present | 47 | 42.7% | 2 | 4.3% | 5 | 10.6% | 12 | 25.5% | 28 | 59.6% | ||
| Stage | ||||||||||||
| Stage I | 11 | 10% | 5 | 45.5% | 2 | 18.2% | 2 | 18.2% | 2 | 18.2% | 26.382b | <0.001(HS) |
| Stage II | 24 | 21.8% | 2 | 8.3% | 2 | 8.3% | 8 | 33.3% | 12 | 50% | ||
| Stage III | 75 | 68.2% | 2 | 2.7% | 12 | 16% | 17 | 22.7% | 44 | 58.7% | ||
| Molecular subtype | ||||||||||||
| Luminal A | 43 | 39.1% | 8 | 18.6% | 7 | 16.3% | 13 | 30.2% | 15 | 34.9% | 26.582b | 0.002(S) |
| Luminal B | 15 | 13.6% | 0 | 0% | 0 | 0% | 3 | 20% | 12 | 80% | ||
| HER2 enriched | 20 | 18.2% | 0 | 0% | 0 | 0% | 5 | 25% | 15 | 75% | ||
| Triple negative | 32 | 29.1% | 1 | 3.1% | 9 | 28.1% | 6 | 18.8% | 16 | 50% | ||
Categorical variables were expressed as number (percentage).
Continuous variables were expressed as mean±SD & median (range).
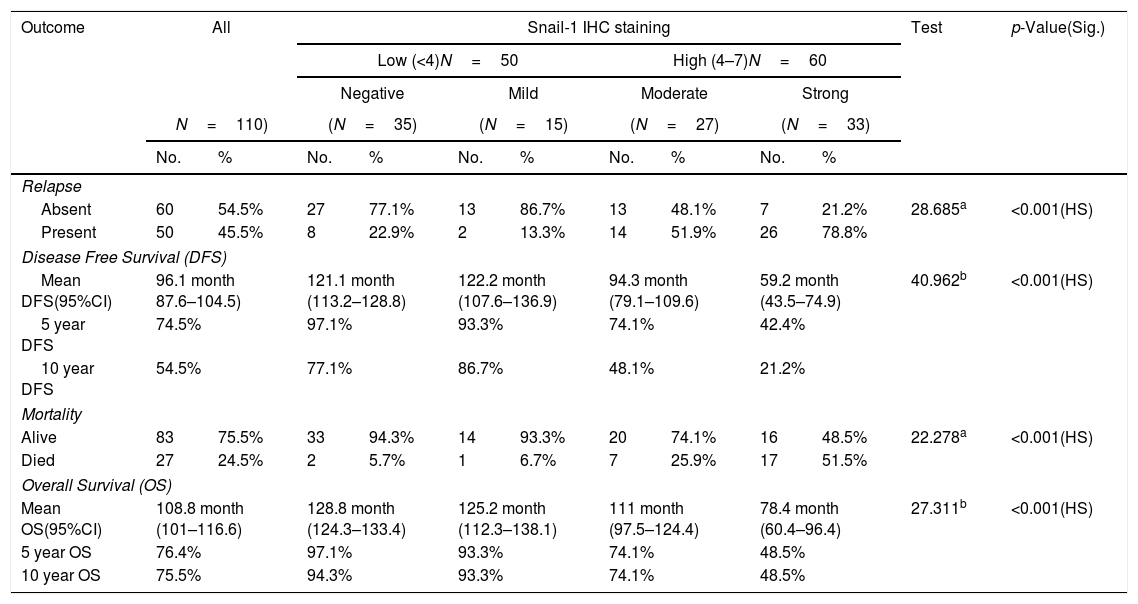
The median follow-up time was 124 months (range 12–132); during follow-up period, recurrence and/or metastasis occurred in 45.5% of patients and 24.5% of patients died. High Snail-1 and Claudin-4 expression were associated with relapse (p=0.001; 0.004 respectively) among studied cases. Survival analysis in our study, including log rank testing and Kaplan–Meier analysis demonstrated that Snail-1 and Claudin-4 expression were inversely related to OS (p=0.001) and DFS (p=0.001).
Relation between immunohistochemical staining for Snail-1 and outcome of management of breast cancer patients.
| Outcome | All | Snail-1 IHC staining | Test | p-Value(Sig.) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low (<4)N=50 | High (4–7)N=60 | |||||||||||
| Negative | Mild | Moderate | Strong | |||||||||
| N=110) | (N=35) | (N=15) | (N=27) | (N=33) | ||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | |||
| Relapse | ||||||||||||
| Absent | 60 | 54.5% | 27 | 77.1% | 13 | 86.7% | 13 | 48.1% | 7 | 21.2% | 28.685a | <0.001(HS) |
| Present | 50 | 45.5% | 8 | 22.9% | 2 | 13.3% | 14 | 51.9% | 26 | 78.8% | ||
| Disease Free Survival (DFS) | ||||||||||||
| Mean DFS(95%CI) | 96.1 month 87.6–104.5) | 121.1 month (113.2–128.8) | 122.2 month (107.6–136.9) | 94.3 month (79.1–109.6) | 59.2 month (43.5–74.9) | 40.962b | <0.001(HS) | |||||
| 5 year DFS | 74.5% | 97.1% | 93.3% | 74.1% | 42.4% | |||||||
| 10 year DFS | 54.5% | 77.1% | 86.7% | 48.1% | 21.2% | |||||||
| Mortality | ||||||||||||
| Alive | 83 | 75.5% | 33 | 94.3% | 14 | 93.3% | 20 | 74.1% | 16 | 48.5% | 22.278a | <0.001(HS) |
| Died | 27 | 24.5% | 2 | 5.7% | 1 | 6.7% | 7 | 25.9% | 17 | 51.5% | ||
| Overall Survival (OS) | ||||||||||||
| Mean OS(95%CI) | 108.8 month (101–116.6) | 128.8 month (124.3–133.4) | 125.2 month (112.3–138.1) | 111 month (97.5–124.4) | 78.4 month (60.4–96.4) | 27.311b | <0.001(HS) | |||||
| 5 year OS | 76.4% | 97.1% | 93.3% | 74.1% | 48.5% | |||||||
| 10 year OS | 75.5% | 94.3% | 93.3% | 74.1% | 48.5% | |||||||
Categorical variables were expressed as number (percentage).
Relation between immunohistochemical staining for Claudin-4 and outcome of management of breast cancer patients.
| Outcome | All | Claudin-4 IHC staining | Test | p-Value(Sig.) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low (≤2)N=25 | High (3–9)N=85 | |||||||||||
| Negative | Mild | Moderate | Strong | |||||||||
| (N=110) | (N=9) | (N=16) | (N=27) | (N=58) | ||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | |||
| Relapse | ||||||||||||
| Absent | 60 | 54.5% | 8 | 88.9% | 4 | 25% | 19 | 70.4% | 29 | 50% | 13.125a | 0.004(S) |
| Present | 50 | 45.5% | 1 | 11.1% | 12 | 75% | 8 | 29.6% | 29 | 50% | ||
| Disease Free Survival (DFS) | ||||||||||||
| Mean DFS(95%CI) | 96.1 month (87.6–104.5) | 130.1 month (126.6–133.6) | 58.8 month (35.4–82.1) | 107.9 month (92.1–123.7) | 95.4 month (84.6–106.2) | 17.544b | 0.001(S) | |||||
| 5 year DFS | 74.5% | 100% | 43.8% | 81.5% | 75.9% | |||||||
| 10 year DFS | 54.5% | 88.9% | 25% | 70.4% | 50% | |||||||
| Mortality | ||||||||||||
| Alive | 83 | 75.5% | 9 | 100% | 7 | 43.8% | 22 | 81.5% | 45 | 77.6% | 12.283a | 0.006(S) |
| Died | 27 | 24.5% | 0 | 0% | 9 | 56.2% | 5 | 18.5% | 13 | 22.4% | ||
| Overall Survival (OS) | ||||||||||||
| Mean OS(95%CI) | 108.8 month (101–116.6) | 132 month | 74.1 month (48.7–99.6) | 114.9 month (100.9–128.8) | 112.1 month (102.1–121.9) | 15.866b | 0.001(S) | |||||
| 5 year OS | 76.4% | 100% | 43.8% | 81.5% | 79.3% | |||||||
| 10 year OS | 75.5% | 100% | 43.8% | 81.5% | 77.6% | |||||||
Categorical variables were expressed as number (percentage).
Metastatic breast cancer (MBC) is present in nearly 6% of breast cancer cases at the time of first diagnosis. Since MBC is an incurable condition with median survival time of 0.5–2.2 years, depending on molecular subtype, it remains to be a challenging problem and acquires more clinical cancer research.12
The trans-differentiation of malignant epithelial cells into motile malignant mesenchymal cells, a process known as epithelial–mesenchymal transition (EMT), is an integral component in cancer progression. This switch in cellular differentiation and malignant behavior is mediated by some key transcription factors, including Snail. EMT increases the motility of individual cells and enables the development of an invasive malignant phenotype.13
Snail-1 represses epithelial genes by binding to E-box DNA sequences through their carboxy-terminal zinc-finger domains. Upon binding of E-box sequences in the proximal promoter region of the E-cadherin gene, Snail-1 recruits the Polycomb repressive complex 2 (PRC2) and make histone modifications, specifically methylation and acetylation at histone H3 Lys 4 (H3K4), H3K9 and H3K27 (REFS 48–53). H3K9 methylation and H3K27 methylation mark repressive chromatin, as seen in the E-cadherin promoter.14,15
In the present study, high Snail-1 expression was detected in high grade tumor and associated with lympho-vascular invasion. Also there were a significant correlation of high Snail-1 and lymph node metastasis (p=0.001), which was similar to previous reports,16,17 confirming the past observation of the crucial role of Snail-1 expression in tumor progression13 supposed that Snail-1 overexpression is an integral component in EMT and cancer progression.
As regarding molecular subtypes of breast cancer studied cases, there was a high significant correlation between Snail-1 expression among the different molecular subtypes (p<0.001), with the highest expression in TNBC. Also, there was a significant correlation between Snail-1 and Her2/neu positive cases (p=0.001). These finding were previously confirmed in studies done by.18 In contrast to Chang et al. (2018), who found no significant association between Snail-1 expression with the different molecular subtype of breast cancer however they stated that breast IDC patients with a combination of high Snail-1 expression, Her2/neu positive and EGFR-positive statuses, had much poor behavior with a statistically significant linear trend.
Jiralerspong et al.19 studied 334 breast cancer samples and stated that recurrence-free survival (RFS) was shorter in the high Snail-1 than low Snail-1 group in triple-negative breast cancer (HR=2.11; 95% CI 0.91–4.88; p=0.082), so Snail-1 was a marker of early relapse in triple-negative breast cancer patients.
Previous studies indicated that Snail-1 expression is positively correlated with poor overall survival in patients with the intrinsic subtypes of luminal B (p<0.0001), and triple negative, p<0.0006).18 Other studies10 indicate poor DSS (disease specific survival) and DFS in breast IDC patients with a high Snail-1 expression level was significantly dependent on the interaction between the Her2/neu status, EGFR status, and Her2/neu intrinsic subtype (p<0.012). In the current study a potent relationship between Snail-1 expression and relapse (p=0.001) among studied cases. Twenty-five (22.7%) of Snail-1 positive cases had died during the follow-up period. Survival analysis in our study, including log rank testing and Kaplan–Meier analysis demonstrated that Snail-1 expression was inversely related to OS (p=0. 001) and DFS (p=0.001).
The expression of tight junction molecules has gained great importance as a prognostic role in breast cancer.20 However, there is a controversy about Claudin-4; some studies have shown that a decreased level of Claudin-4 expression is detected in well differentiated (grade 1) IDC and high Claudin-4 expression is significantly observed in basal-like breast carcinomas, suggesting that Claudin-4 may be indicative of progressing breast carcinoma.21 Other studies identified subtypes, termed Claudin-low breast carcinomas, typically exhibits high histologic grade, with stem-cell and epithelial-to-mesenchymal transition features, low expression of cell-cell junction proteins, and poor response to therapy.22
Claudin-4 and Claudin-3 levels increase with TNBC and associated with worse prognosis. This may provide useful information for breast carcinomas; since these two CLDN members are putative therapeutic targets23 demonstrated that the single IHC evaluation of CLDN3, CLDN4 and CLDN7 is insufficient to identify the CLDN-low molecular subtype of IDC. There was a need for analysis of numerous molecular markers, such as EMT and CSC (cancer stem cell) to improve the identification of this subgroup by IHC, which also enriched in high grade carcinoma with metaplastic histopathology. In this current study the correlation between Snail-1 and Claudin-4 and other clinic pathological parameters in breast cancer were evaluated to detect their prognostic utility.
In the present study a highly significant correlation was detected between Snail-1 expression with the different molecular subtype of IDC (p<0.001), with the highest expression in TNBC. Also, there was a significant correlation between Snail-1 and Her 2/neu positive cases (p=0.001). Claudin-4 expression in tumor cells showed a significant correlation with molecular subtypes (p=0.002). Her2/neu positive subtype showed the highest expression. High Claudin-4 was significantly correlated with high Snail-1 expression. (p value<0.001).
The CLDN-low subgroup breast cancer was never detected by using immunohistochemistry (IHC), although the characteristics of this group were very well recognized: low to absent expression of luminal differentiation markers, high expression of (EMT) biomarkers, immune response genes and cancer stem cell-like features. Clinically, the CLDN-low tumors are having poor outcome, triple negative, high grade IDC, with a high percentage of metaplastic and medullary histopathology.24
Claudin-4 was the one more frequently expressed among IDC (24.8%).23 However, the big difference in the percentage of Claudin-4 expression and our work (claudin-4 was detected in 77.3% of studied cases) is due to the majority of the studies evaluating CLDNs by IHC considered cytoplasmic expression to classify a case as positive, which immediately increases the number of positive cases. As regarding IHC evaluation of CLDN expression in tumors; some authors considered membrane and cytoplasmic staining.25,26 while, others only assessed the membrane staining pattern.21
In agreement with our findings showing that Claudin-4 was correlated with a number of factors associated with more aggressive and worse disease,27 found a significant correlation between high levels of Claudin-4 expression and reduced breast cancer disease-specific survival (p<0.003), recurrence-free survival (RFS) (p<0.025) and overall survival (OS) (p<0.034). In contrast to23 who did not find any significant difference.
Although it has been reported that Claudin-4 was up-regulated in many cancers, a study has shown that Claudin-4 is downregulated in high-grade urothelial carcinoma, and that this downregulation is caused by promoter hyper methylation.28 Claudin-4 overexpression effects vary between different tumors. In ovarian cancer, Claudin-4 overexpression in an immortalized ovarian epithelial cell line was found to increase progression.29 Conversely, Claudin-4 protein overexpression in a pancreatic cancer led to favorable prognosis.30 These diverse effects may be dependent on the expression of other members of the Claudin protein family, or on the signaling environment within the cell.
ConclusionHigh levels of Snail-1 and Claudin-4 proteins are associated with adverse outcome in IDC and correlated positively with tumor grade, stage, TNBC and Her2/neu positive cases.
LimitationsThe retrospective study depends totally on collected data from patients’ files, so it is almost always criticized due to insufficient or incomplete data. Also, the small sample size of our work may represent an obstacle to get more powerful results.
FundingThis research received no specific grant from any funding agency in the pubic, commercial, or not-for-profit sectors.
Ethical considerationsThis study was approved by the Zagazig Ethics Committee.
Conflict of interestThe authors declare that they have no conflict of interest.