Breast and prostatic carcinomas show some striking and surprising similarities. The absence of myoepithelial cells in breast carcinoma and the basal cells in prostatic adenocarcinoma is well established. However, there is a reported case of metastatic prostatic carcinoma in a lymph node showing presence of basal cells. We studied the histopathological morphology of both primary and ipsilateral nodal metastatic breast cancer.
MethodsWe retrospectively examined 100 cases of metastasizing infiltrating duct carcinoma to analyze the morphological relationship between the primary and the axillary metastasis using microscopic examination of both H&E stained slides and two myoepithelial immunohistochemical stains (P63&SMMHC).
ResultsAll the 100 cases of axillary lymph node metastatses are negative for any evidence of accompanying myoepithelial cells, as well as their primary tumors. Three cases changed to higher grade (from G2 to G3) in the node and two cases changed to lower grade (from G3 to G2).
ConclusionWe verify that the metastatic IDC in the regional lymph nodes looks like the primary tumor with regards to the absence of the myoepithelial layer; however upgrading & downgrading can occur with the metastasis, so more studies about the clinical significance of reporting nodal tumor grade is recommended.
Los cánceres de mama y próstata muestran algunas similitudes llamativas y sorprendentes. La ausencia de células mioepiteliales en el cáncer de mama, y de células basales en el adenocarcinoma de próstata está claramente establecida. Sin embargo, existe un caso reportado de cáncer de próstata metastásico en un ganglio linfático, que muestra la presencia de células basales. Estudiamos la morfología histopatológica de las metástasis primaria y ganglionar ipsilateral en el cáncer de mama.
MétodosEstudiamos retrospectivamente 100 casos de carcinoma ductal infiltrante (CDI) metastásico para analizar la relación morfológica entre las metástasis primaria y axilar, utilizando el examen microscópico de los portaobjetos teñidos con hematoxilina y eosina, así como 2 tinciones mioepiteliales inmunohistoquímicas (P63 y SMMHC).
ResultadosLos 100 casos de metástasis del ganglio linfático axilar fueron negativos para cualquier evidencia de presencia de células mioepiteliales, así como sus tumores primarios. En 3 casos el grado del ganglio se incrementó (pasando de G2 a G3), y en 2 casos se redujo (pasando de G3 a G2).
ConclusiónVerificamos que el CDI metastásico en los ganglios linfáticos regionales se asemeja al tumor primario en cuanto a la ausencia de capa mioepitelial; sin embargo, con la metástasis se puede producir incremento o reducción del grado, por lo que se recomienda la realización de más estudios sobre la significación clínica de reportar el grado tumoral del ganglio.
Breast cancer is the most frequent cancer in women and a worldwide predominant cause of cancer related death.1 It is a heterogeneous neoplasm made up of different cell clones, each one endowed with diverse rates of growth and metastatic potential.2 According to the multiclonal tumor progression model, the metastasization is the result of a multistep process leading to loss of the differentiated properties and loss of the proper tissue compartmentalization.3
The presence of breast tumor metastasis, tumor size and the histological grade (HG) can provide important information for the initial approach to the treatment of the cancer including the introduction of neoadjuvant chemotherapy; The HG is considered a very good indicator of the future disease development.4 Another important characteristic is the axillary lymph node involvement; it is a very important prognostic factor for invasive ductal carcinoma, whereas patients with no axillary node metastasis have a better prognosis for both disease-free survival and overall survival.5 Breast cancer and prostate cancer have biological and morphological similarity; they are more similar than different.6 The hallmarks of progression of breast duct carcinoma in situ (DCIS) to infiltrating duct carcinoma (IDC) progression are loss of the basement membrane and the myoepithelial layer, then invasion of tumor cells into the stroma and surrounding tissue.7 In 2016 Varma et al., reported a rare case of metastatic prostatic carcinoma in a lymph node showing retention of the basal cell pattern of immunoreactivity for basal cell markers, which provides conclusive evidence that rarely basal cells may be present inprostate cancer, though this basal cell pattern of basal cell marker immunoreactivit was never reported before, in a metastatic prostate cancer.8 This study aimed to verify the status of the myoepithelial cells in the regional lymph node metastasizing ductal breast carcinoma and assessment of the morphological similarity between the metastatic ductal breast cancer in the axillary lymph nodes and the primary tumor in the breast.
Materials and methodsThis is a hospital-based retrospective study, where we reviewed our histopathology archive to identify female patients with metastatic breast duct carcinoma to the axillary lymph nodes who underwent mastectomy with axillary clearance at Al-Azhar University Hospital and a private lab between April 2018 and January 2020. Paraffin blocks of the tumor and the positive lymph nodes of 100 cases were selected and recut for making Hematoxylin and Eosin (H&E) stained slides. Positive charged slides were used for both Smooth Muscle Myosin Heavy Chain (SMMHC) & p63 immunohistochemistry to confirm the presence or absence of a myoepithelial layer.
Each case (tumor and one positive lymph node) was reviewed by the author and blindly by another pathology consultant for histological grading (using the Nottingham modification of the Scarff-Bloom-Richardson grading system), then assessed for the presence or absence of the myoepithelial layer in both the primary and the metastatic tumor by examination of the H&E stained slides along with the SMMHC & p63 immunohistochemistry. We excluded cases in which the reviewer grading report of the primary tumor was different from the previously reported grade (the metastatic grade was not reported in any of the studied cases). Patients consent forms were not applicable. Ethical approval was done by the local ethical committee (IRB approval 1201/2019).
Statistical Analysis: SPSS version 19 (SPSS Inc., Cary, NC) was used for the statistical analysis. Descriptive analysis was used including mean±standard deviation (SD) for quantitative data and frequency tables for qualitative data. Independent sample T-test was used to compare the difference of means in quantitative data. Chi square test was used for qualitative data. A P-value of less than 0.05 was considered statistically significant.
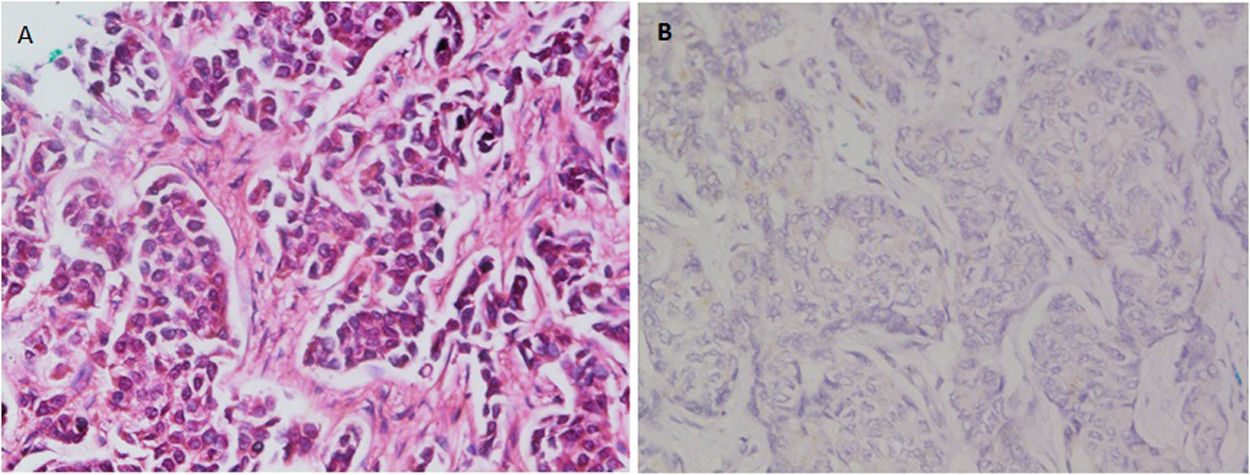
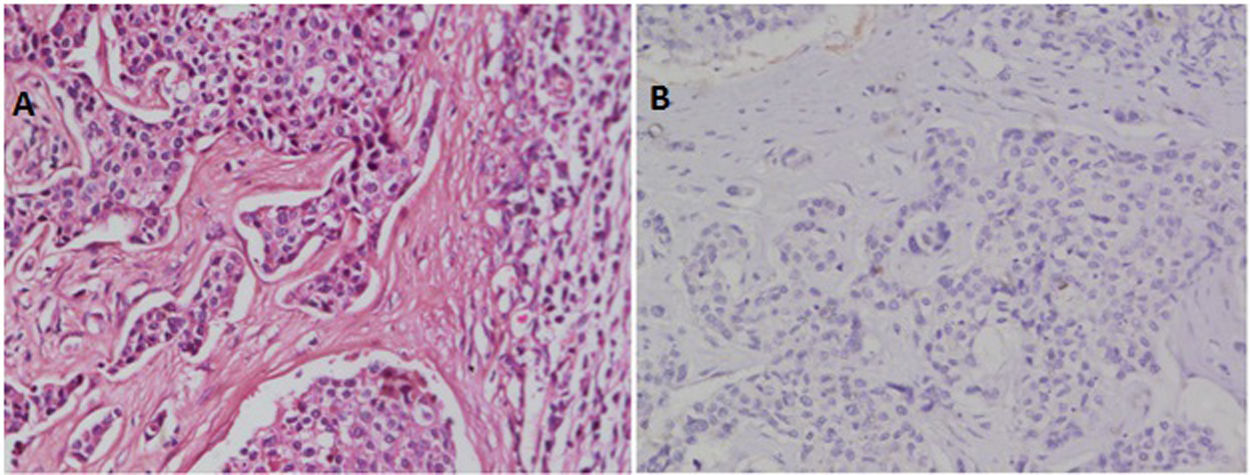
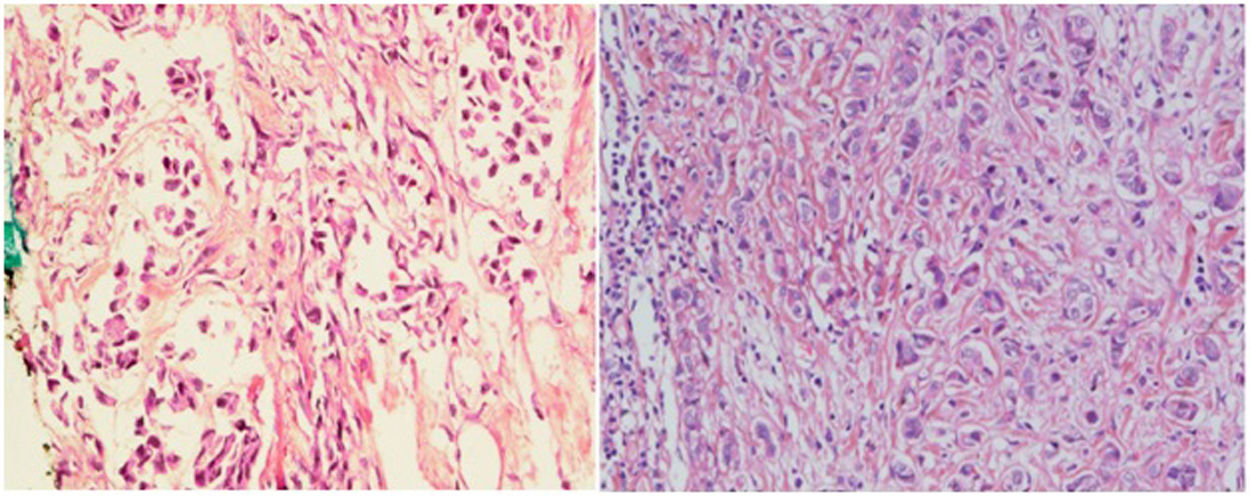
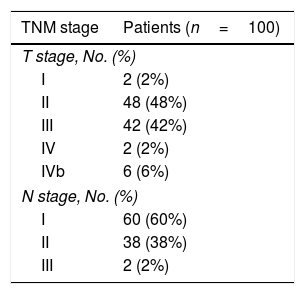
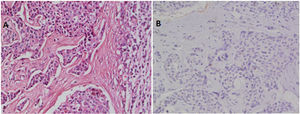
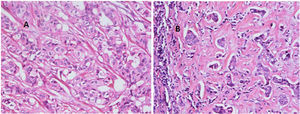
ResultsRecords of 100 female patients with metastatic infiltrating breast duct carcinoma to the axillary lymph nodes who underwent mastectomy with axillary clearance were retrieved. The mean age of the included patients was 53.46±12.1 years old. All of the 100 primary tumors show absence of the myoepithelial layer which appeared in the H&E stained slides and confirmed by negative staining of SMMHC and p63 by Immunohistochemistry (Fig. 1). All the 100 metastatic tumors show absence of the myoepithelial layer which appeared in the H&E stained slides and confirmed by negative staining of SMMHC and p63immunohistochemical marker (Fig. 2). The most common T stage was stage II (48%), followed by stage III (42%), and stage 4b (6%). On the other hand, the most common N stage was stage I (60%), followed by stage II (36%) (Table 1). All cases were Mx stage.
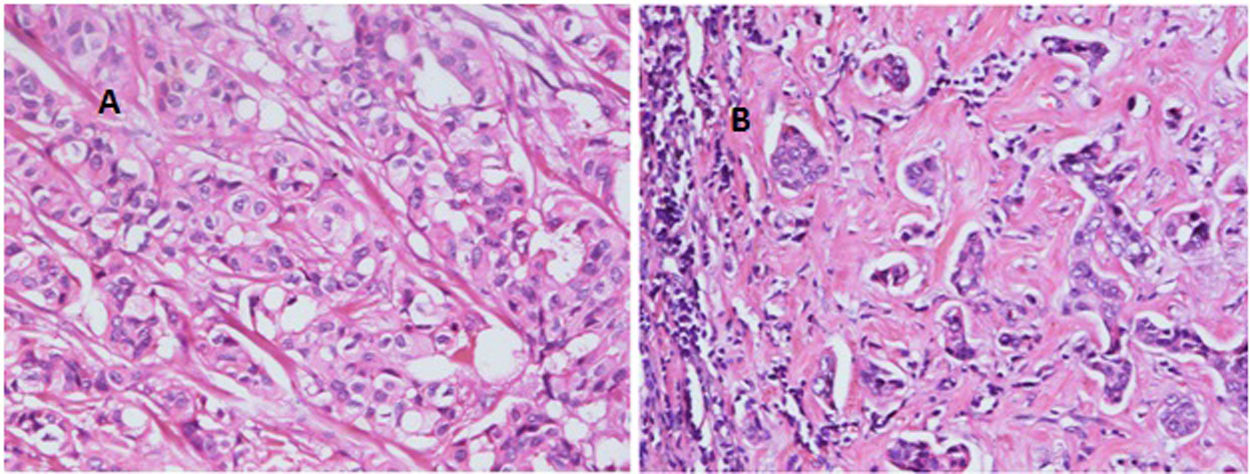
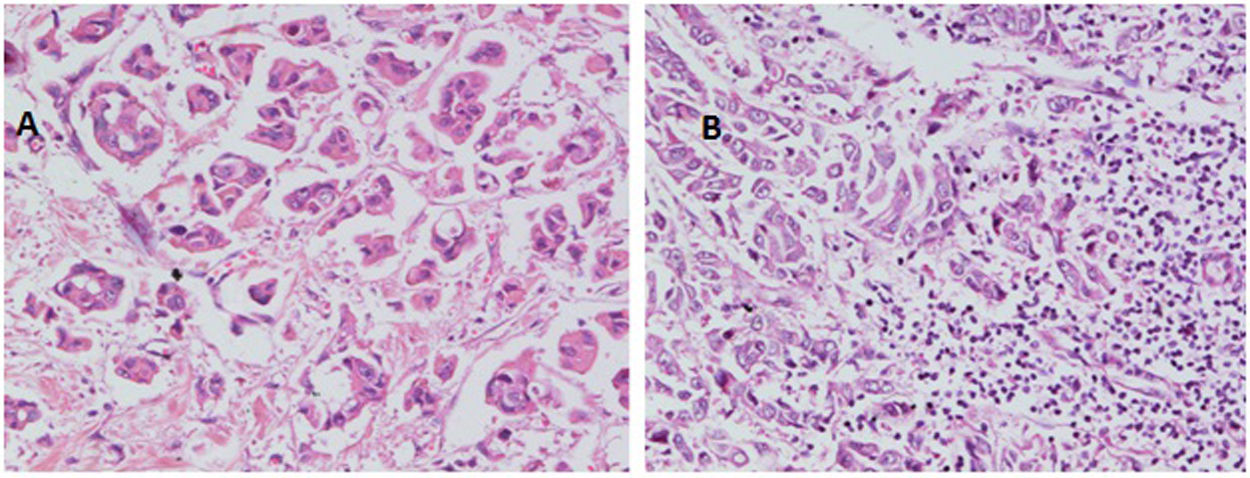
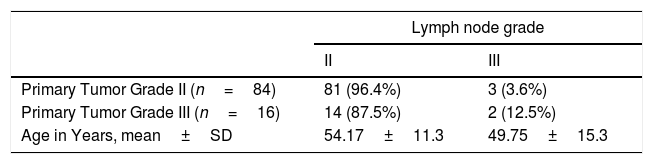
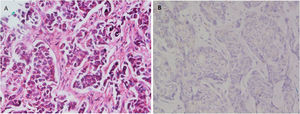
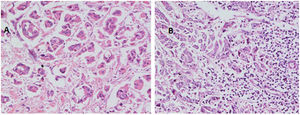
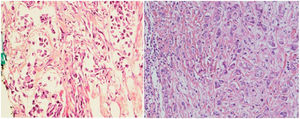
With regard to tumor grade, 84 out of 100 tumors were infiltrating duct carcinoma grade II and 16 cases were grade III Out of the 84 primary tumor grade II cases, 81 metastatic tumors in axillary lymph nodes showed the same grade of the primary (Fig. 3), whereas 3 metastatic tumor shows higher grade, grade III (Fig. 4). Likewise, out of the sixteen primary tumor grade III cases, seven metastatic tumors in axillary lymph nodes showed the same grade of the primary, whereas two metastatic tumors show lower grade, grade II (Fig. 5). The association analysis demonstrated a significant difference between primary tumors and metastatic lymph nodes regarding tumor grade but no statistically significant association between grade of lymph node and age (Table 2).
The two most common invasive carcinomas in men and women are prostate cancer and breast cancer respectively. Both malignancies have some striking and surprising similarities.9 Although these cancers arise in organs that are anatomically and physiologically different, both organs require steroids from gonads for their development, and the tumors that arise from both are mostly epithelial and hormone-driven showing remarkable underlying biological similarities.6 The similarity between the function and structure of the prostate and breast has been established for a long time.10 Normal basal cells separate the secretory luminal cells of prostate from the basement membrane and loss of those basal cells is one of the most important foundations for the histological diagnosis of prostatic adenocarcinoma.11
A few studies have reported positive basal cell marker staining, high molecular weight cytokeratin (HMWCK) staining and p63 staining, in invasive prostate cancer, but all of them were tumor cells stained in a non-basal cell distribution.12–14
The absence of myoepithelial cells in breast IDC and basal cells in prostatic adenocarcinoma is well established. However, those cells may not identifiable on H&E sections due to similarity with the fibroblasts and the compressed tumor cells. Hence, immunohistochemistry using HMWCK and p63 is used to establish an accurate diagnosis of invasive prostatic adenocarcinoma and in breast carcinoma.8,15 One study reported retained basal cells in a metastatic prostatic carcinoma in lymph node.8 p63 is expressed as a nuclear staining in several carcinomas.16,17
In breast, p63-positive myoepithelial cells have been known to surround benign epithelial lesions and a rim around the epithelial cells is seen in carcinomas in situ (CIS). No staining has been noted in any infiltrative carcinomas.15 We used p63 marker as it is nuclear and so it is easily assessed. In addition to its high specificity since neither vascular smooth muscle neither cells nor stromal fibroblasts are stained. SMMHC marker stains nearly 100% of the myoepithelial cells.18 To our knowledge, the presence or absence of the myoepithelial cells in metastatic breast carcinoma has not been studied at the time of writing. In this study, we found that all the 100 studied cases of axillary lymph node metastatic breast carcinomas are negative for any evidence of accompanying myoepithelial cells.
Regarding difference of tumor grade between the primary breast carcinoma and the axillary node metastasis, Cserni studied the tumor grade (G) of 43 cases of breast cancer metastasizing to axillary lymph nodes revealing an upgrade (worsening) of 5 cases in the nodal metastasis (1 case was changed from G1 in breast to G2 in the node, 1 case from G1 to G3, 3 cases from G2 to G3). Three cases were downgraded in the node, showing a change from G3 to G2. The remaining 35 cases (81.4%) showed no grade change between the primary and the regional nodal metastasis.12 These results are very similar to those in a study done at Guy's Hospital in London in 1998, in which 102 metastases to axillary nodes were compared with the primary breast carcinoma.13 Our study revealed 95 out of 100 cases showing similar grade between the primary breast IDC and the regional nodal metastasis, two cases with downgrade from G3 to G2 and three cases with upgrade from G2 to G3.
In conclusion, this study appears to verify that metastatic IDC in the regional lymph nodes has similar features to the primary tumor in regard to the absence of the myoepithelial layer. However, the tumor grade may progress to a higher grade or be downgraded in the axillary lymph node to a lower tumor grade. More studies about the clinical significance of reporting the tumor grade of the metastasis in breast cancer, as in the primary tumor, is recommended.
Confidentiality of dataThe authors declare that they have followed the protocols of their Center on the publication of patient data.
Funding sourcesThis research did not receive any specific grant from funding agencies in the public, commercial, or not for-profit sectors.
Conflict of interestNone declared.
The authors would like to thank Dr Murali Varma (Consultant of Cytopathology, University Hospital of Wales) for his priceless opinion and invaluable advice for preparing the study. Also thanks to Dr Jonathan Shank (Consultant of Histopathology, Christie Hospital NHS Trust in Manchester) for his proof reading and great helpful comments.