Offspring of patients diagnosed with bipolar disorder and schizophrenia (Off-BDSZ) have a high genetic risk of developing a mental illness. The aim of this project is to develop and investigate the efficacy of an intervention aimed at this population, based on the concept of cognitive reserve.
MethodsThis is a multicenter randomized trial with an experimental test–retest design study with control group. Two groups will be included: a community comparison group (CC) and a Off-BDSZ group. A total of 108 Off-BDSZ and 65 CC aged between 6 and 25 years will be recruited. Off-BDSZ participants will be randomized to receive either Cognitive Reserve EnhAncement ThErapy (CREATE) (n=54), or a supportive approach (n=54). The CC group will be assessed at baseline. The duration of the intervention will be 3 months, with 12 weekly group sessions. The primary outcome will be the improvement in CR measured according to change in the Cognitive Reserve Assessment Scale in Health (CRASH) and Cognitive Reserve scale for Adolescents (CORE-A). All participants will be blindly evaluated using clinical, cognitive and neuroimaging measures at baseline, at three months (after the psychological intervention), and at twelve-month follow-up after treatment completion.
DiscussionThe results will provide insight into whether the CREATE-Offspring version may enhance cognitive reserve (CR) in child, adolescent and young adult Off-BDSZ as well as advance knowledge about changes in clinical manifestations, neuropsychological performance and brain structure and function associated with improving CR. This novel and cost-effective intervention represents an advance in the framework of preventive interventions in mental health.
Trial registrationClinicaltrials.gov, NCT03722082. Registered on 26 October 2018.
Schizophrenia and bipolar disorder, for many years, were considered as totally different diagnostic categories. However, more recently a growing number of studies have examined the relationship between conditions.1 Both disorders are considered the result of anomalous neurodevelopment, with a high heritability that revolves around 80%.2 The two disorders share clinical symptoms,3 cognitive difficulties4 and even some abnormalities at the level of brain structure5 and function.6,7 One of the most studied features in schizophrenia and bipolar disorder is cognition. Cognitive deficits are recognizable early in the trajectory of both disorders and are evident in individuals at their first manic or psychotic episode. A significant impairment during childhood and in the early adolescence before the onset of psychosis supports the role of neurodevelopmental factors in schizophrenia and for at least in a subgroup of individuals with bipolar disorder.8 In contrast to findings in schizophrenia, a number of studies have suggested normal, at times superior, cognitive abilities and school achievement in children and adolescents who develop adult bipolar disorder.9 Longitudinal studies in chronic patients with bipolar disorder and few available studies in first-episode bipolar disorder have not so far supported evidence for progressive cognitive decline in BP. These findings are rather similar to outcome of longitudinal studies in schizophrenia.9 The high hereditary component and the presence of neurodevelopmental processes in both disorders implies that the offspring of patients diagnosed with bipolar disorder or schizophrenia (Off-BDSZ) are considered a high-risk population, and therefore suitable for the study of vulnerability markers.10,11 The difficulties observed, considered as susceptibility factors for Off-BDSZ affect multiple domains, including clinical, cognitive and neuroimaging measures. Clinically, studies have detected higher rates of psychopathology in samples of child and adolescent Off-BDSZ adolescents than in controls (offspring of parents without these pathologies). Rates of psychopathology observed in Off-BDSZ range between 36 and 78%,12–17 which contrasts with the 18% rate of psychopathology found in the children of control parents18 or the 10–12% observed in general population.19,20 Specifically, Off-BDSZ have been found to show a higher prevalence of anxiety disorders, mood disorders and attention deficit hyperactivity disorder than controls.21,22
Studies conducted in Off-BDSZ samples have also documented difficulties in some cognitive domains, especially in verbal and visual memory, working memory, processing speed, attention and executive functions.17,21,23–26
Neuroimaging studies have found evidence of global and regional structural compromise in Off-BDSZ,27–29 especially in prefrontal and limbic cortices. Gray matter reduction appears to be more pronounced in Off-SZ than in Off-BP27,28 and has been associated with lifetime psychiatric disorders and general cognitive capacity,30 as well as affective31 and psychotic spectrum32 symptoms. Changes in brain functional connectivity have also been described in Off-BPSZ, both at the level of the connectome33 and more specifically in emotion regulation and reward processing circuits.34–38
Despite evidence that Off-BDSZ already show impairments in various domains during youth, no study so far has assessed the effect of psychological interventions in child and adolescent offspring of parents affected by these severe mental health conditions. This study aims to develop a preventive intervention focused on this population, based on the concept of Cognitive Reserve (CR). CR was initially developed in the field of dementia. It posits that people with the same brain damage may have different clinical manifestations depending on their ability to compensate for such damage.39 The brain cognitive reserve hypothesis states that high premorbid intelligence, education and active and stimulating lifestyle provide reserve capacity, which acts as buffer against the clinical, cognitive and brain structural and functional deficits due to the genetic environmental effects of familial risk for disease.40 Studies in bipolar disorder and schizophrenia have documented an association between greater CR and better psychosocial and cognitive functioning.41–44 While there is evidence of an association between greater CR and more preserved brain structure and function in Alzheimer's disease40 and in multiple sclerosis,45 only one study so far has analyzed this relationship in patients diagnosed with schizophrenia (and none that we are aware of in bipolar disorder), which suggested that CR may act as a modifier in age-related brain structural and cognitive decline.46 It is worth noting, however, that this study was conducted in an adult sample with a long illness duration. During the first two decades of life, the brain experiences dramatic maturational change47 and is especially sensitive to external influences due to greater plasticity.48 Therefore, some authors have suggested that psychological interventions during this period may have a greater impact on the brain than later on in life.49
Aims and hypothesesThis study aims to develop and apply a psychological approach (CREATE-Offspring program) in order to enhance CR in child, adolescent and young adult Off-BDSZ. The two main objectives will be (i) to test the effectiveness of the program vs. support treatment and (ii) to test if the observed improvements are stable over time (nine months follow-up).
We hypothesize that (i) as first outcome, Off-BDSZ participating in the CREATE program will experience an improvement in CR assessed by the CRASH or CORe-A and other questionnaires related with CR proxies, (ii) as second outcome, receiving the CREATE approach will improve cognitive functions such as sustained attention and executive function assessed by CPT-II and WCST, and will help remediate change in gray matter structure and functional connectivity associated with familial risk, especially in the prefrontal cortex and limbic structures, (iii) and, finally, as third outcome the Off-BDSZ participants in the CREATE program will present a decrease in the severity of the psychopathology.
MethodsStudy designThis is a single blind, randomized controlled clinical trial approved by Ethics Committee of Hospital Clinic and Hospital Parc Tauli. The study will take place at three different units: the Bipolar and Depressive Disorders Unit and the Child and Adolescent Psychiatry and Psychology Department at the Hospital Clínic (University of Barcelona and Depression and Anxiety Program at the Hospital Parc Tauli).
The research groups are part of the Spanish network Center for Biomedical Research in Mental Health, CIBERSAM.50 This project will be conducted in accordance with the ethical principles of the Declaration of Helsinki and Good Clinical Practice and approved by the Hospital Clinic Ethics Committee.
Sample size calculationIt has been calculated that, to achieve a size effect of 0.7 with an alpha error of 0.05, and a statistical power of 0.90, 54 subjects per group would be needed to detect clinically relevant differences between the treatments groups.
ParticipantsThe Off-BDSZ will be randomized in two groups: the experimental receiving the CREATE-Offspring intervention and the control one receiving a support intervention.
The sample will have to fulfill the following inclusion and exclusion criteria:
- 1.
Inclusion criteria for Off-BDSZ comprised: (a) parental diagnosis of schizophrenia, schizoaffective or bipolar disorder according to Diagnostic and Statistical Manual of Mental Disorders (DSM-5) criteria and (b) aged ranged between 6 and 25 years old.
- 2.
Inclusion criteria for the community comparison group: (a) absence of diagnostic of schizophrenia, schizoaffective or bipolar disorder in first and second degree relatives and (b) aged between 6 and 25 years old.
- 3.
Exclusion criteria for both groups are: (a) presence of intellectual disability (IQ≤70) with impairment in psychosocial functioning and (b) presence of neurological disease or history of traumatic brain injury with loss of consciousness.
All participants will receive extensive information on the study and, if interested, will provide written informed consent prior the inclusion by the PI's of each center. Parents or legal guardians of participants under 18 years will sign the agreement to participate in case that they are willing so. The participants will receive a financial compensation for travel and time spent on scans and assessments. The attendance to the intervention will be registered in order to monitor the adherence to the program.
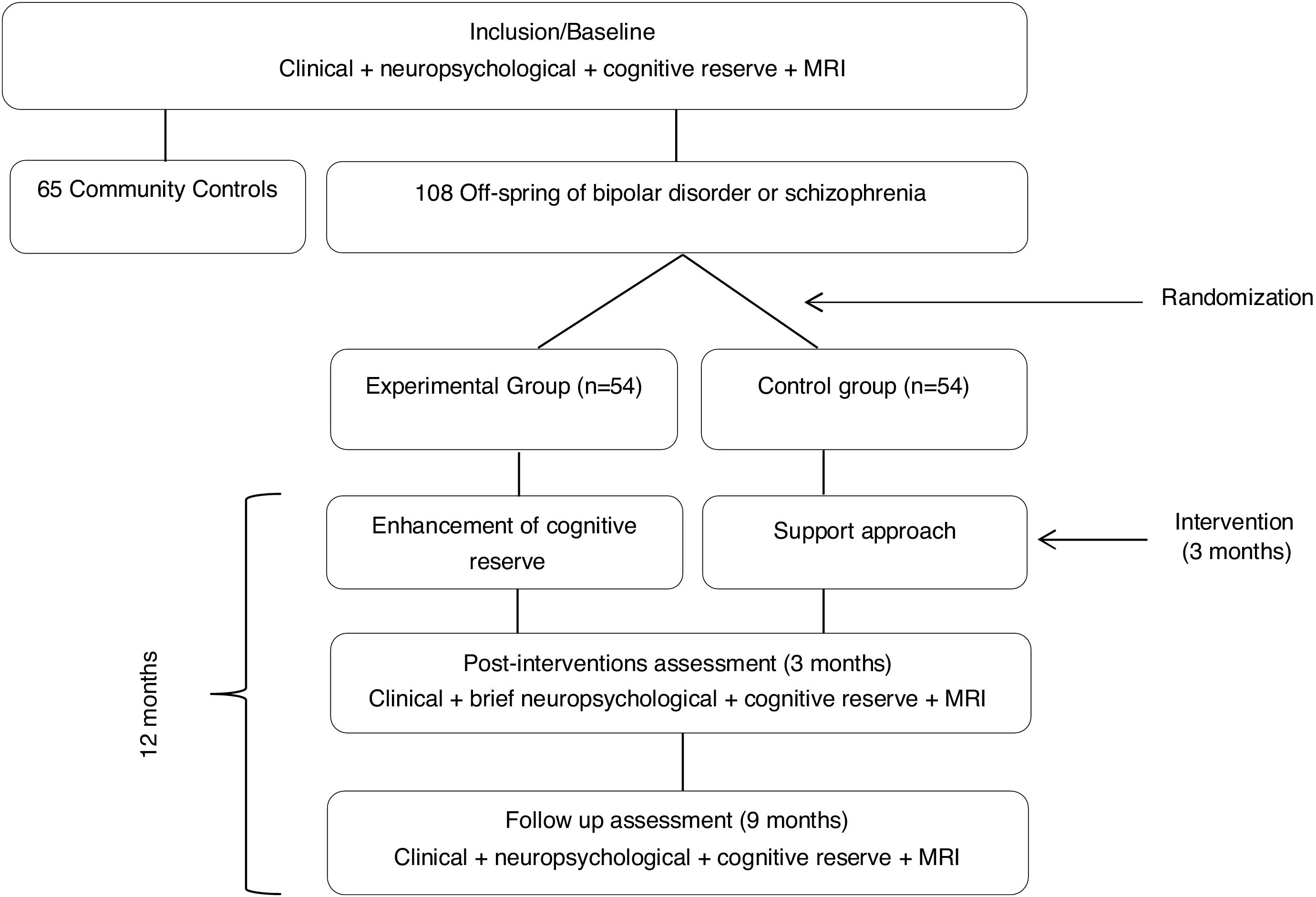
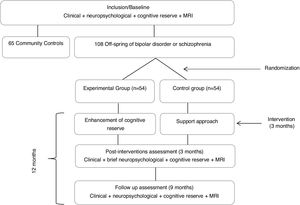
Global procedureAt baseline, all participants (Off-BDSZ and community comparison group will be assessed in clinical, neuropsychological, cognitive reserve and neuroimaging variables. After the baseline assessment, the Off-BDSZ will be randomized to the experimental group, receiving the CREATE-Offspring intervention, or to control group only receiving a support approach. Once the intervention is finished, that is 3 months later, all the Off-BDSZ will be assessed in those clinical variables and some neuropsychological domains (attention and executive function) hypothesized to improve after the intervention. Finally, at follow-up (9 months after the end of the intervention) all Off-BDSZ will be assessed again in all clinical, cognitive reserve, neuropsychological and neuroimaging variables to check the stability of the changes observed. Fig. 1 represents a flowchart of the study procedure.
Randomization and blindingOff-BDSZ will be randomized following the block randomization method balanced by a psychiatrist external to the study to the experimental or control group. Two psychologists blind to the evaluation results will perform the CREATE intervention. All assessments will be conducted for a psychologist blind to condition group (experimental or support).
Data collection- (a)
Sociodemographic and clinical assessment
After providing written informed consent, all participants will undergo through a structured clinical interview based on the SCID for DSM-551 with the exception of the children that will be interviewed with the K-SADS (Kiddie Schedule for Affective Disorders and Schizophrenia).
Socio-demographic variables such as age, gender, socioeconomic status, years of education, history of repetition academic year and type of living will be collected for all participants.
Clinical measures will be selected depending on the age range of the participants, including:
- -
Subjects under 17 years old: (i) structured interview Kiddie Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version K-SADS-PL (version of University of Washington in St. Louis, Missouri)52 (WASH-U-KSADS) and its Spanish version of semi-structured interview for affective and schizophrenia diseases at present and in the past53; (ii) Conners’ Rating Scales (CPRS-48)54 for assessing the children's behavior; (iii) Child Mania Rating Scale, (parents version) (CMRS)55 for the presence of manic symptoms.
- -
Subjects over 17 years old: i) clinical structured interview for assessing axis I diseases of DSM-IV, SCID-I y SCID-II will be fulfilled56; (ii) Hamilton Depression Rating Scale for depressive symptomatology (HDRS)57; (iii) Young Mania Rating Scale (YMRS)58 to assess the hypo/manic symptoms.
In addition to these measures common clinical assessment for both groups will include: Sexual Maturity Rating (SMR) or Tanner Stage59; the Premorbid Adjustment Scale (PAS)60; Scale of Prodromal Symptoms (SOPS) through the semi-structured interview (SIPS)61; Global Assessment Functioning (GAF)62 and Functioning Assessment Short Test (FAST)63 to evaluate psychosocial functioning a Clinical Global Impressions to measure the global clinical status (CGI).64 To assess general functioning in subjects under 18 we will use the Children Global Assessment Scale (C-GAS).65 We will also collect the drug use trough a record chart. Stressful Life Events Schedule (SLES)66 to assess stressful factors in children and adolescents, the Community Assessment of Psychic Experiences (CAPE)67 to determine positive, negative and depressive dimensions in high risk population for psychosis. Schizotypal Personality Questionnaire-Brief (SPQ-B)68 for schizotypal personality traits. Social Communication Questionnaire (SCQ)69 to detect symptoms of autism spectrum disorder. ADHD Rating Scale-IV70 to evaluate symptoms of attention deficit disorder with or without hyperactivity and. finally, Bipolar Prodrome Symptom Interview and Scale-Prospective (BPSS-P)71 to assess prodromal symptoms of bipolar disorder prospectively.
The following socio-demographic and clinical variables will be also collected from the parents affected with bipolar disorder or schizophrenia: kind of disorder (schizophrenia or bipolar disorder), presence or history of psychotic symptoms, response to treatment, comorbidity and substance use. We also conduct the clinical structured interview the SCID-I and SCID-II, PAS, GAF and FAST.
- (b)
Cognitive Reserve
Cognitive reserve in adult participants will be assessed using the Cognitive Reserve Assessment Scale in Health (CRASH)72 which is an interviewer-administered, rapid (approximately 10minutes) and easy scale designed and validated to assess CR in severe mental disorders. It consists of 23 items evaluating the 3 fundamental domains of cognitive reserve: education, occupation, and intellectual and leisure activities. This last domain is assessed taking into account different stages of the adult's life (childhood/adolescence, adulthood and last year), as well as the frequency and variability of each activity. The CRASH scale provides an overall score and a score per domain. The higher the score the higher the CR level, with a maximum of 60 points.
In children and adolescents, we will use the Cognitive Reserve Questionnaire for adolescents (CoRe-A) (Camprodon et al., submitted), which is a 13-item interviewer-administered questionnaire in which the state and functioning before the onset of the disease is evaluated from 4 domains: studies, occupation, leisure activities and social activities. Education and occupation domains are evaluated taking into account the number of years of compulsory schooling completed, the school performance prior to the onset of the disease, the educational level of the parents and other aspects of the child's development i.e. language, reading, writing and psychomotricity. The other two domains, leisure activities and social activities, are evaluated by asking parents about the intellectual interests, hobbies and social relationships of their children.
We also will gather more information about CR through the following questionnaires: Survey of eating habits, Pittsburgh Sleep Quality Index,73 Inventory of leisure activities, questionnaire of habits and study techniques (CHTE),74 Metacognitions Questionnaire-30,75,76 Mindful Attention Awareness Scale (MAAS).77 These last questionnaires will be the ones mainly used to calculate the post-intervention CR proxy (at three months) in the sample of young people because the CRASH scale covers the entire last year as a more short-term measure.
- (c)
Neuropsychological assessment
An extended cognitive battery of tests has been selected following recommendations for cognition trials in mental health by consensus of schizophrenia and bipolar disorder.78,79 The different cognitive domains tested are the following:
- (1)
Estimated Intelligence Quotient will be evaluated with the Wechsler Adult Intelligence Scale (WAIS-IV)80 calculated on the performance on the Block design, Matrix Reasoning, Similarities and Vocabulary subtest for adults and with the Wechsler Intelligence Scale for Children (WISC-IV)81 based on the performance on the Block design, Matrix Reasoning, Similarities, Figure Weights for participants under 16 years old. This measure will be evaluated only at baseline.
- (2)
Processing Speed: processing speed index from WISC-IV or WAIS-IV depending on the age of the subject will be administered, made up of the digit symbol coding and symbol search subtests.
- (3)
Working Memory: index of working memory from WISC-IV or WAIS-IV depending on age, consisting of digits and letter-number sequencing subtests.
- (4)
Attention: will be measured with the Continuous Performance Test (CPT-II)82 and Trail Making Test part-A (TMT-A).83
- (5)
Verbal memory: TOMAL (Test of memory and learning)84 assess verbal and non-verbal memory in children between 5 and 19 years old. For subjects over age 19 the logical memory and word list subtests of the Wechsler Memory Scale III (WMS-III)85 will be applied.
- (6)
Visual memory: for assessing visual memory we use the visual memory subtest of WMS-III and Rey-Osterrieth Complex Figure- immediate memory (ROCF).86,87
- (7)
Executive functions: the Stroop Color-Word Interference Test (SCWT)88 assess the inhibition response and the Wisconsin Card Sorting Test (WCST) (computerized version)89 assess cognitive flexibility capacity, TMT-B83and verbal fluency.90
The extended cognitive battery will be completed at baseline and at 12 months. However, in order to avoid possible learning effects, the post-intervention assessment (at 3 months after baseline) will be a brief cognitive battery where only attention and executive function tests are administered (CPT, TMT, WCST, verbal fluency and SCWT) which are also the domains proposed in the hypotheses for improvement.
- (d)
Neuroimaging
All participants will undergo a neuroimaging assessment at baseline and at 12-month follow-up, which will be conducted on a 3-T Siemens Prisma Magnetic Resonance scanner (Siemens Medical Systems, Berlin, Germany) at the Center for Image Diagnosis at the Hospital Clinic of Barcelona, and processed at the Medical Imaging Platform at IDIBAPS.
A high-resolution T1-weighted 3-dimensional magnetization-prepared rapid sequence will be obtained using the following parameters: 240 sagittal slices, 2300-ms repetition time, 3.01-ms echo time, 1-mm slice thickness, 900-ms inversion time, 394×240 field of view, 256×256 matrix size, and 9° flip angle. To rule out possible underlying pathology, an axial T2 structural image will be acquired and subsequently reviewed by a neuroradiologist blinded to group classification. Surface reconstruction will be visually inspected for accuracy by a rater blinded to group status.
After reorientation along the anterior and posterior commissural line, measurements of cortical thickness, and surface area and cortical and subcortical gray matter volume will be computed using the standard FreeSurfer v. 6.0 pipeline. The FreeSurfer automated procedure includes motion correction, non-uniform intensity normalization, registration to Montreal Neurological Institute stereotactic space, skull strip, white matter segmentation, definition of pial surface, and parcellation of the brain into 34 cortical regions per hemisphere using the Desikan-Killiany brain atlas. Regions previously showing structural abnormalities in Off-BDSZ will be selected for analyses.27–29 Freesurfer longitudinal processing pipeline will be used to determine longitudinal change between groups.
An 8-min resting-state fMRI sequence will also be acquired, during which participants will be instructed to keep their eyes closed and remain as still as possible for the duration of the scanning session. Acquisition parameters will be as follows: 240 volumes, TR=2000ms; TE=29ms; matrix size=480×480; slice thickness=4mm, acquisition matrix=80mm×80mm, 32 slices, voxel size 3mm×3mm×4mm. Resting-state fMRI images will be realigned, co-registered to the individual T1-weighted scan (segmented using a sample specific template), normalized to the Montreal Neurological Institute (MNI) space and smoothed using a 6-mm Gaussian kernel in SPM12. Individuals showing mean framewise displacement >.2mm will be excluded due to excessive motion.91 A seed-based intrinsic connectivity approach will be used to examine whole brain resting state functional connectivity of regions selected on the basis of showing structural–functional abnormalities in Off-BDSZ in previous studies.34,35,37,38 These regions of interest will be registered from the template to each participant's pre-processed fMRI data, and the mean time series of voxels in these regions will be extracted for each subject for use as primary regressor in a general linear model analysis of all other voxel time series, resulting in individual whole-brain maps. Group differences in these maps will be determined using linear mixed models. Seed regions that show baseline differences, within the cross-sectional sample, will be chosen as seed regions to investigate treatment-related changes in resting state fMRI connectivity within the longitudinal sample.
Setting- (a)
CREATE (Cognitive Reserve EnhAncement ThErapy) – Offpsring protocol
The CREATE-Offspring protocol will have a duration of 3 months including a total of 12 weekly group sessions lasting 60minutes approximately. This intervention is focused on improving academic skills, increasing leisure activities and improving neurocognitive functions with the ultimate goal of enhancing cognitive reserve. The content of this intervention is based on ecological tasks that will be carried out in two areas, both in the hospital and at home. To carry out the tasks we will use pencil and paper, audiovisual and virtual reality support, mobile apps and group activities. In addition, we will provide a hand-out with the content of each session and with extended information and additional resources providing further depth into the topics. The content of the sessions and the material will be adapted to the different age ranges of the attendees. In case of children (6–12 years old), the sessions will be carried out with their parents. Groups will be run separately for children, adolescents and young adults.
The 12 sessions will consist on the following content: (1 and 2) healthy lifestyle habits; (3 and 4) focused attention techniques and mindfulness; (5 and 6) leisure activities; (7) improving academic skills; (8 and 9) metacognition; (10) social abilities; (11) warning signs and (12) final session to review contents related to previous sessions (see Table 1).
Summary of the CREATE program.
| Sessions | Content |
|---|---|
| Session 1 | Healthy life habits (I). It will focus primarily on eating and sleeping habits, psychoeducation about the use of substances and physical exercise. |
| Session 2 | Healthy life habits (II). Practical and ecological tasks focused on the content of session 1. |
| Session 3 | Focused attention (I). It will focus on learning and practicing focused attention techniques. Mindfulness is a process of active, open, nonjudgmental awareness. It is paying attention in the present moment with openness, curiosity, kindness and flexibility. |
| Session 4 | Focused attention (II). More focused on practice. Inviting to dwell with awareness on one thing—kind of like gently coming home to one moment—which might include our breath, an aspect of sensation or stillness or a focus on our whole body, based on awareness of breath, walking meditation, sensory guided meditations and body scans. |
| Session 5 | Leisure activities (I). Mainly focused on regular exercise practice. Helping to identify what are the most rewarding activities for everyone, divided into indoor and outdoor activities. Checking if the difficulties to carry them and trying to integrate them in the daily schedule trying to limitate the use of screens. |
| Session 6 | Leisure activities (II). Introduction to the leisure activities of an intellectual nature enhancing attention and memory capacity. Including reading and games such as chess or puzzles. |
| Session 7 | Academic and educational activities. Introduction of study techniques applicable to academic life. |
| Session 8 | Metacognition (I). It will focus mainly on the identification of attributional styles, cognitive distortions and will be oriented to the explanation of ‘Jumping to conclusions’. |
| Session 9 | Social skills. |
| Session 10 | Problem solving. |
| Session 11 | Warning signs. Psychoeducation will be carried out on the main warning signs of general psychopathology. |
| Session 12 | Closure of the group. Session reminder of everything exposed in previous sessions and resolution of doubts. |
The criteria for discontinuation will be one or more of the following: (1) missing more than three sessions during the intervention, (2) hospitalization for any type of psychopathology and (3) withdrawal of consent.
- (b)
Control intervention
The support approach protocol includes the same number of sessions (12) and the same duration (60minutes) as the CREATE-Offspring protocol. They will also be delivered to parents and children, adolescents and young adults. The support group will consist of meetings where the participants talk about the difficulties that they may have experienced throughout the week, but without a specific psychological intervention in this regard.
Outcome measuresThe primary outcome will be the changes assessed by the CRASH or CoRe-A and other questionnaires related with CR proxies or the intervention. Other secondary outcomes will be (i) cognitive function such as sustained attention and executive functions assessed by CPT-II and WCST respectively, (ii) severity of the psychopathology assessed by HDRS, YMRS and CMRS and (iii) changes in structural magnetic resonance assessed by changes in gray matter and functional connectivity in prefrontal and limbic regions.
Statistical analysesFor descriptive purposes, continuous variables will be expressed as means, standard deviations and ranges, and frequencies and/or percentages will be used to describe the categorical variables. Taking into account that in our samples there is a percentage, although not high, of siblings, cross-sectional data will be analyzed using multilevel mixed-effect logistic regression models for categorical variables or multilevel mixed-effect linear regression models for continuous variables. Group will be included in the model as a fixed factor and the fact of having a sibling in the study as a random variable.
In order to test group's differences over time, data will be analyzed with a new multilevel mixed-effect linear regression model with group, time and interaction time x group as fixed variables and the fact of having a sibling in the study and the identification number of the subject at baseline and follow-up as random variables. Age, gender and socio-economic status will be included in both analysis cross-sectional and follow-up and the backward stepwise method will be used to decide which variables remain in the model. With this method, only variables with a p value <0.05 remain in the final model. Moreover all the analysis will be conducted applying the Bonferroni correction for multiple comparisons in order to avoid false positive results. All statistical analysis will be conducted using SPSS 23.
DiscussionTo our knowledge, the present study is the first attempt to design and test the effects of enhancing CR in a sample of offspring of patients with severe mental illness such as bipolar disorder or schizophrenia. CREATE-Offspring intervention is framed as a preventive treatment and also explores the effects of an early intervention, that is, one step before the first manifestations of the disease. The treatments available today are mainly focused on clinical, psychoeducational, cognitive or psychosocial aspects during the intermediate stages of the disease, but no interventions have been developed focused on high-risk population. Furthermore, the inclusion of longitudinal follow-up assessments will allow us to potentially identify vulnerability factors for the development of mental disease, as well as predictors of response to treatment.
Previous studies have demonstrated that CR is a good predictor of psychosocial functioning in first-episodes of psychosis,41 as well as in bipolar disorder, measured with the FAST scale.92 CR is also an important predictor of neurocognitive performance: higher CR is related with better performance in many cognitive domains such as attention, verbal memory,44 executive functions, processing speed and working memory in bipolar disorder.42 Therefore, including CR in research and clinical interventions has the potential to help prevent or reduce cognitive and functional impairment.
The aim of this study is not only to improve CR, but we also expect this intervention, directly or indirectly, to have effects on clinical symptoms and cognitive performance, which will in turn improving psychosocial functioning and quality of life. Moreover, taking into account the period of life in which the study will take place, we expect this intervention to exert changes in gray matter structure and functional connectivity in brain regions implicated in the clinical and cognitive symptoms observed in Off-BDSZ. This study has the potential to significantly advance knowledge on bipolar disorder and schizophrenia, in addition to providing a cost-effective preventive intervention strategy, especially considering the disability and burden that risk for these disorders entails.
The present project is not exempt from limitations; one of the main problems will be the loss of subjects longitudinally. However, given that the vast majority of the parents of Off-BDSZ are following treatment in the outpatient department of the Hospital Clinic of Barcelona, we think that the number of participants lost to follow-up will be minimal. The financial compensation for travel expenses and time spent during scanning and clinical and cognitive assessments will also help decrease subject loss in this group. The existence of a control group is necessary to compare the effectiveness of the intervention, due to the fact that going to therapy weekly the control group could increase their subjective perception of improvement in certain clinical variables and/or in functionality and finally, we can not exclude a possible learning effect in the neuropsychological performance after the different assessments since many tests do not have alternative forms.
Ethics approval and consent to participateThe proposed study has the approval of the Ethical Committee of the Hospital Clínic of Barcelona where the project will be carried out. The study design and the evaluation of the results will be carried out following the CONSORT criteria (Schulz et al., 2010). Al data will be collected following the current Law of Data Protection. Written informed consent will be obtained from all participants. Any important changes in the protocol will be reported to the Ethics Committee of Hospital Clínic of Barcelona. All data collected will be confidential. All Case Report Form will be anonymized with a code for each subject. Either name or any personal data that could identify to the subject will be registered in the data base. Only personal involved in the present project will have access to the data collection.
This protocol was registered in Clinicaltrials.gov (NCT03722082) on 26 October 2018 (https://clinicaltrials.gov/ct2/show/NCT03722082?id=NCT03722082&draw=2&rank=1)
This study was conducted in accordance with the ethical principles of the Declaration of Helsinki and Good Clinical Practice. The study protocol was approved by the ethics committee of the Hospital Clinic with the registration number: HCB/2017/0439.
Authors’ contributionsES, EV, JC, NC and CT conceived the study with substantial contributions from BS and AMA. ES, LM and CT wrote the first draft with substantial contributions from PC, GS, MV, MS and MR. All authors substantially participated in and approved the final draft for submission to the journal. All authors have read and approved the manuscript. The coordinator center and Parc Tauli will be responsible of the recruitment of subjects with age over 18 as well as the assessment of clinical, neuropsychological and neuroimaging variables and carry out the intervention for enhancing cognitive reserve. The CIBERSAM center will be responsible of recruitment, assessment and carry out the intervention for subjects between 6 and 18 years old. The three groups will be in charge of data processing and publications.
The results derived from this study will be disseminated through international scientific meetings and will be published in high impact international indexed journals.
FundingThe authors would like to thank the support of the Spanish Ministry of Science (PI17/00741/PI17/01066/PI18/00976/PI15/00467/PI18/00696/SLT006/17/00346) integrated into National plan of I+D+I and co-financed by Instituto de Salud Carlos III (ISCIII) and the European Regional Development Fund (ERDF); CIBERSAM; and the Comissionat per a Universitats i Recerca del DIUE de la Generalitat de Catalunya to the Bipolar Disorders Group (2017 SGR 1365/) and to the GRUPPIJ (2017SGR881), and to the Fundació Clínic Recerca Biomèdica (Ajut a la Recerca Pons Bartran, FCRB_PB1_2018). This work has also been supported with the project SLT006/17/00352, in the “Pla estratègic de Recerca i Innovació en Salut 2016-2020” (Health Department) CERCA Program/Generalitat de Catalunya). Dr. Carla Torrent is funded by the Spanish Ministry of Economy and Competitiveness, ISCIII, through a ‘Miguel Servet’ postdoctoral contract (CPI14/00175) and a Miguel Servet II (CPII19/00018).
Conflict of interestThe authors declare no conflict of interest related to this manuscript.
This work was supported by the Spanish Ministry of Science, Innovation and Universities/Economy and Competitiveness/Instituto de Salud Carlos III (CPII19/00009 contract grant to JR and all authors contributed to and have approved the final manuscript.