Since 2008 many studies have reported short-term clinical improvement after acute ketamine administration; however, follow-up studies for more than 8 weeks are very limited or are focused on ketamine monotherapy1 time until relapse varying widely between studies (16–168 days).2,3 Available information regarding treatment with ketamine raises a large number of questions4: (1) What can we expect from ketamine in a real clinical setting? (2) Can ketamine be an effective alternative treatment for patients not responding to ECT? (3) Are tolerance and dependence limiting its use? (4) What is the best maintenance treatment after initial response?
In our study, a compassionate and protocolized intravenous treatment schedule is prescribed in patients suffering from a Severe Mayor Depressive Episode (MADRS>35) without therapeutic alternative after lack of response after bilateral ECT. All patients received a 0.5mg/kg dose of ketamine in intravenous (i.v.) infusion for 40min, two or three times per week until at least 6 sessions were completed. Clinical evolution was measured by using MADRS (Montgomery-Asberg Depression Rating Scale), FAST (Functional Assessment Staging Test) and CGI (Clinical Global Impression) scales.
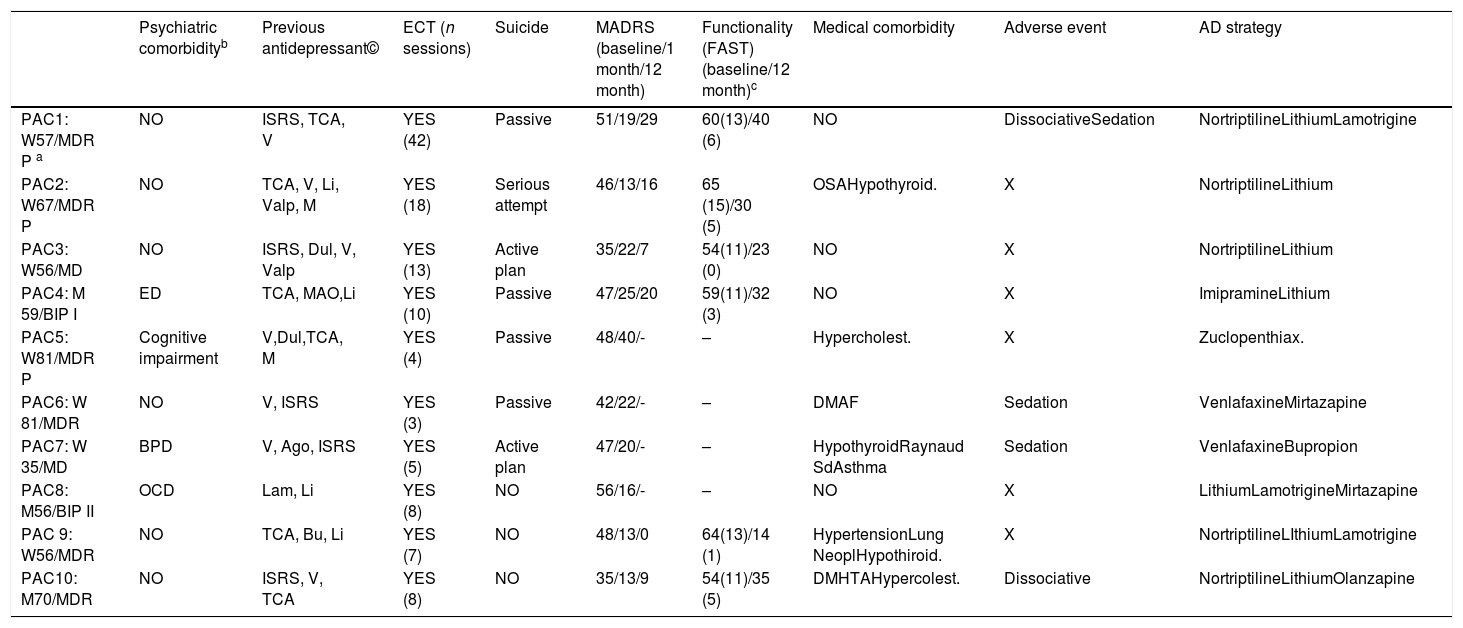
10 patients with depression resistant to pharmacological treatment and ECT were treated with ketamine i.v. The sample includes 7 women and 3 men with a mean age of 61.5 years (SD: 13.84) and age range between 35 and 81 years (Table 1). Mean MADRS score prior to ketamine treatment was 45.50 (SD: 6.58). The mean value for CGI was 5.80 (SD: 0.92), with a mode of 6 (severely ill).
Demographic and clinical description. Main outcomes.
| Psychiatric comorbidityb | Previous antidepressant© | ECT (n sessions) | Suicide | MADRS (baseline/1 month/12 month) | Functionality (FAST) (baseline/12 month)c | Medical comorbidity | Adverse event | AD strategy | |
|---|---|---|---|---|---|---|---|---|---|
| PAC1: W57/MDR P a | NO | ISRS, TCA, V | YES (42) | Passive | 51/19/29 | 60(13)/40 (6) | NO | DissociativeSedation | NortriptilineLithiumLamotrigine |
| PAC2: W67/MDR P | NO | TCA, V, Li, Valp, M | YES (18) | Serious attempt | 46/13/16 | 65 (15)/30 (5) | OSAHypothyroid. | X | NortriptilineLithium |
| PAC3: W56/MD | NO | ISRS, Dul, V, Valp | YES (13) | Active plan | 35/22/7 | 54(11)/23 (0) | NO | X | NortriptilineLithium |
| PAC4: M 59/BIP I | ED | TCA, MAO,Li | YES (10) | Passive | 47/25/20 | 59(11)/32 (3) | NO | X | ImipramineLithium |
| PAC5: W81/MDR P | Cognitive impairment | V,Dul,TCA, M | YES (4) | Passive | 48/40/- | – | Hypercholest. | X | Zuclopenthiax. |
| PAC6: W 81/MDR | NO | V, ISRS | YES (3) | Passive | 42/22/- | – | DMAF | Sedation | VenlafaxineMirtazapine |
| PAC7: W 35/MD | BPD | V, Ago, ISRS | YES (5) | Active plan | 47/20/- | – | HypothyroidRaynaud SdAsthma | Sedation | VenlafaxineBupropion |
| PAC8: M56/BIP II | OCD | Lam, Li | YES (8) | NO | 56/16/- | – | NO | X | LithiumLamotrigineMirtazapine |
| PAC 9: W56/MDR | NO | TCA, Bu, Li | YES (7) | NO | 48/13/0 | 64(13)/14 (1) | HypertensionLung NeoplHypothiroid. | X | NortriptilineLIthiumLamotrigine |
| PAC10: M70/MDR | NO | ISRS, V, TCA | YES (8) | NO | 35/13/9 | 54(11)/35 (5) | DMHTAHypercolest. | Dissociative | NortriptilineLithiumOlanzapine |
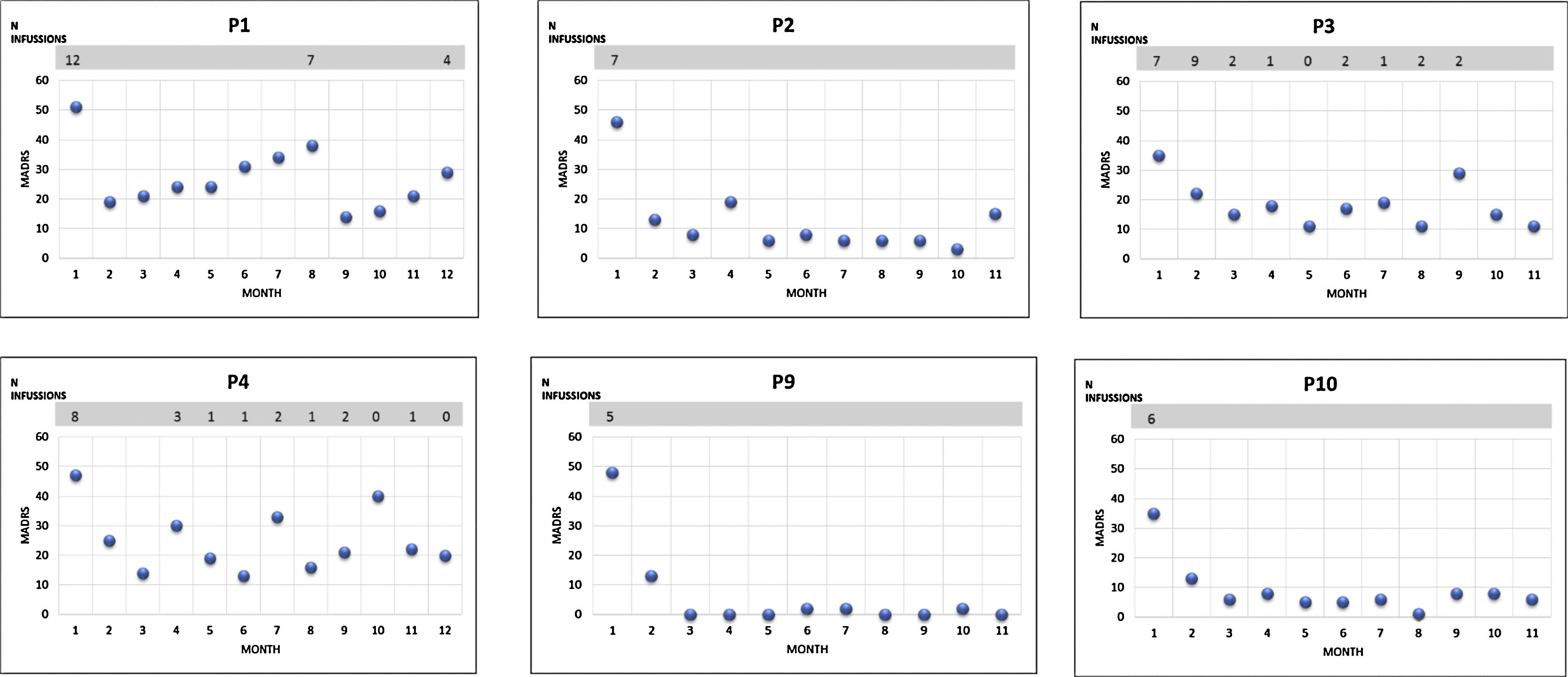
Acute intensive ketamine treatment response was assessed during hospitalization. An average of 7.4 (SD: 3.92) ketamine infusions were administered during the acute phase of treatment. The mean reduction of the MADRS scale after completing this phase was 25.2 points (SD: 10.09) (p=0.005). The proportion of respondent patients was 40%. All patients exhibited at least partial response, although none reached remission. The CGI value decreased to 2.7 (SD: 1.337) (p=0.005). 6 patients completed the follow-up, 3 of them (50%) continued in remission without requiring more ketamine infusions (Fig. 1). The 3 (50%) remaining patients required occasional ketamine infusions due to exacerbation of depressive symptoms; a total number of 10 or 11 infusions during the 1 year-follow-up period.
According to the FAST scale there was a significant improvement in overall functioning during the 12-month follow-up. In the specific items for cognitive functioning, the pre-treatment score was 12.33 points (SD: 12.33) and 3.33 (SD: 2.42) after the one year follow up.
Studies published to date describing the use of ketamine have some limitations: heterogeneous definition of TRMD,5 short duration, ethical conflicts regarding the use of placebo and methodological difficulties to include patients whose severity is close to that observed in clinical practice.5,6 Our case series of 10 patients with severe depression includes patients suffering from psychotic symptoms, bipolar depression, high suicide risk and a high degree of functional disability; these features would have exclude them from clinical trials.
Following acute and serial administration of ketamine, the MADRS score for our patients decreased significantly in most cases, replicating previous studies.1–3 In some cases, a reduction in the MADRS score was sustained for a number of weeks and two patients achieved maximum improvement or remission four or ten months after last administration. This might suggest a long-term action of ketamine. Furthermore, during the follow-up phase, a total number of four patients continued in clinical remission with pharmacological maintenance treatments (fundamentally lithium7 and tricyclic antidepressants8) without requiring new ketamine infusions.
The other half of the patients followed to 12 months required occasional infusions of ketamine due to depressive relapse symptoms; mean number of administrations was less than 1 for every month and the ketamine dose used was similar to acute phase (0.5mg/kg). Our findings do not support the existence of tolerance or dependence phenomena, nor the need for continued serial administrations to sustain improvement.
In our series patients with depression-congruent psychotic symptoms achieved remission of symptomatology without requiring the use of antipsychotics and no manic switch occurred in any of our patients (unipolar or bipolar depression).
In our series of 10 patients, ketamine improved the depressive symptomatology during the acute phase and also during one year follow-up. In that period, no patterns of abuse, tolerance or cognitive related side effects have been observed. Lithium and tricyclic antidepressants may provide a useful option toward the objective of consolidating clinical improvement and preventing new episodes.