In recent years, there has been an increase in studies of the implications of the gut microbiota (GM) in children with ASD. There is a hypothesis which propose a relationship between the emotional state and the abundance of intestinal microbes through the so-called microbiota-intestine-brain axis. In this sense, dysbiotic GM could be a contributing factor to the appearance of ASD. This systematic review article analyses the results of the intervention using prebiotics (Ascidians, Aspergillus niger, vitamin A, myeloperoxidase, etc.), probiotics (mainly: Lactobacillus, Bifidobacterium, etc.) and Transplantation of Fecal Microbiota in ASD children. In conclusion, the results of the initial studies suggest changes in ASD symptoms, gastro-intestinal symptoms and GM composition after the interventions. However, the results should be taken with caution because there are very few studies that analyse the efficacy of long-term treatments and the different combinations of them.
En los últimos años, ha habido un aumento en los estudios sobre las implicaciones de la microbiota intestinal (MI) en niños con TEA. La hipótesis es que existe una posible relación entre el estado emocional, la abundancia y/o la proporción de diferentes colonias bacterianas intestinales, aunque no haya cambios en la cantidad total, a través del llamado eje microbiota-intestino-cerebro. En este sentido, la MI disbiótica podría ser un factor que contribuye a la aparición de TEA. Este artículo de revisión sistemática se analizan los resultados de la intervención mediante prebióticos (Ascidians, Aspergillus niger, vitamin A, myeloperoxidase, etc.), probióticos (fundamentalmente: Lactobacillus, Bifidobacterium, etc.) y Trasplante de Microbiota Fecal en los niños TEA. En general, los resultados de los estudios iniciales sugieren cambios en los síntomas TEA, síntomas gastro-intestinales y composición de GI tras las intervenciones. Sin embargo, los resultados deben tomarse con cautela dado que son muy pocos los estudios que analizan la eficacia de los tratamientos a largo plazo y las diferentes combinaciones de los mismos.
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterised by deficits in social communication and the presence of restricted interests and repetitive behaviours.1
Several studies indicate that children with ASD may have problems with food. In particular, they may be more sensitive to certain foods2 and their lower calcium and protein intake can be significantly lower as a result.3 Furthermore, there is a higher prevalence of gastrointestinal symptoms such as diarrhoea, constipation and abdominal pain in children with ASD compared to other healthy children.4 One of the explanations for these gastrointestinal symptoms and the aetiology of ASD itself is proposed by the microbiota-intestine-brain axis hypothesis. This hypothesis is based on an explanatory model that attempts to relate the ASD symptomology, findings in neuroscience and bacteriology. The microbiota-gut-brain axis is defined as a two-way communication system between neuronal, immune, endocrine and metabolic pathways. Recent scientific literature has attempted to determine whether there is a group of bacteria directly involved in ASD or whether there is general dysbiosis in the gut microbiota (GM) of children with ASD.5 Dysbiosis is a disturbance of the microbial balance of normal GM that may be due to quantitative or qualitative changes in its composition and/or changes in metabolic activities and distribution.6 The abundance of bacteria in the gastrointestinal (GI) tract varies from approximately 108 colony-forming units (CFU) per gram measured in ileum to 1011 CFU/g measured in faeces,7 with Bacteroidetes, Firmicutes, Proteobacteria, Actinobacteria and Fusobacteria being the main bacterial phyla found in the GI tract of mammals.8 Currently, GM is largely charactarised through culture-independent techniques such as massive sequencing of 16S ribosomal RNA genes through polymerase chain reaction (PCR), as this enables easy identification of a large proportion and diversity of bacteria, and provides rapid results.9,10
However, despite several recently published studies on the subject, the aetiology of nutritional and gastrointestinal problems in children with ASD is still unknown,4,11 and everything seems to indicate that it is a combination of various associated factors.5,12,13
Although the study of GM in ASD is relatively recent, intervention studies have been published based on the use of prebiotics, probiotics and Microbiota Transfer Therapy (MTT). Prebiotics are indigestible food ingredients such as resistant starch, non-starch polysaccharides, oligosaccharides, galactooligosaccharides and xyloligosaccharides, which are used by the GM. These are, therefore, functional foods that stimulate the growth of one or more bacterial strains that inhabit the GI tract, modifying their composition and activity, thus achieving improved health and well-being of the host.14 Probiotics are living non-pathogenic microorganisms that beneficially affect human health when administered in appropriate amounts as a food ingredient or supplement. Commonly used probiotics are Lactobacillus and Bifidobacterium species, Saccharomyces cerevisiae and some E. coli and Bacillus species.15 MTT is a technique where GM is transferred from a healthy donor to a recipient who has gastrointestinal symptoms and can be performed by colonoscopy, enema, orogastric tube or orally in capsule form.16
There is great interest within the scientific and professional community about the implications of these types of interventions on ASD symptoms. In this sense, we intend to look at whether this type of intervention would help improve the quality of life of children with ASD. Specifically, we aim to determine the scope of this type of intervention in 1) stabilising dysbiosis in certain bacteria; 2) reducing gastrointestinal symptoms, and 3) reducing symptoms of emotional distress in children with ASD.
ObjectiveThe aim of the present study is to conduct a systematic review of the psychobiological effects of probiotics, prebiotics and MTT in children with ASD.
MethodThe search methodology was carried out following the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) protocol.17 The terms selected to carry out the bibliographic searches were (autism* OR asd* OR "autism spectrum disorder" OR autistic*) AND (probiotic* OR "probiotic* therapy" OR prebiotic* OR "prebiotic* therapy" OR "fecal microbiota transplantation" OR "microbiota transfer therapy"). A literature search was performed in several databases and search engines (Scopus, Web of Science, Pubmed and PsycINFO) for articles published until January 2020. The searches performed in the 4 databases included all terms in the widest possible field. The results from the databases were cross-checked with EndNote X7 software to detect possible duplicates. The inclusion criteria were: 1) studies with human population with ASD; 2) articles published in journals with impact index, and 3) studies relating GM and the effect of probiotics, prebiotics and faecal microbiota transplantation in children with ASD. Exclusion criteria were: 1) descriptive reviews and systematic reviews; 2) articles published in languages other than English; 3) book chapters or books; 4) editorial material, letters to the editor, and abstracts without significant data; 5) small surveys; 6) cases n = 1; 7) effects of probiotics, prebiotics, and faecal microbiota transplantation in children with other mental disorders; and 8) articles on patents.
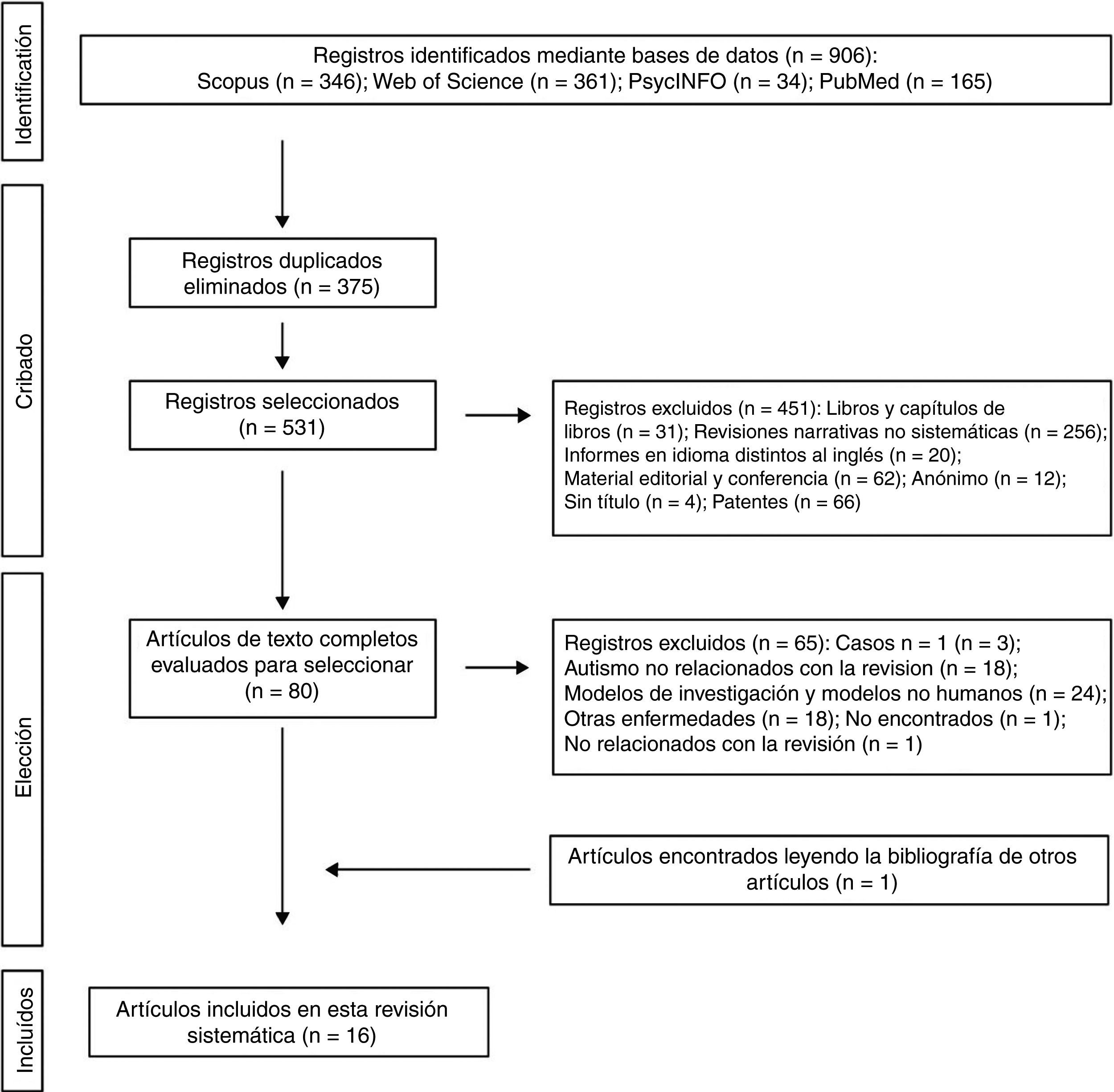
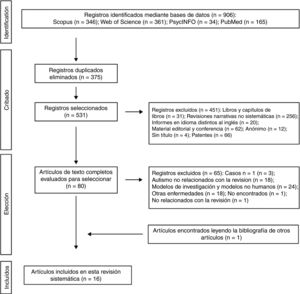
The 2 authors of this systematic review formed the review team to implement measures to minimise random errors and bias at all stages of the review. They also independently selected titles, abstracts and full texts of articles for possible inclusion. Disagreements about whether or not an article should be included were resolved through discussion. The 4-level flowchart for the PRISMA method used in this systematic review is shown in Fig. 1. First, the references found through the database search were identified. Secondly, papers retrieved in duplicate were eliminated and the 4 databases were refined by selecting article-type publications. Thirdly, after reviewing the abstracts of each of the remaining articles, those that related to the subject matter of the study were chosen. The full papers were downloaded from different pages that allow downloads. The papers that could not be obtained by this means were requested by e-mail from the authors. In the design of the present systematic review, we also followed the process for eliminating non-relevant documents according to the PRISMA17 guidelines, and other authors who propose descriptive tabulation of the results of the studies5,13,18 (Fig. 1). It should be noted that some of the rejected publications may belong to more than one elimination group, but the final decision was made by the authors by consensus. Once the number of studies included for this review was obtained, quality assessment of these studies started, which consisted of assessment of the risks of bias of each of the studies, following the model published in previous studies5,13 (Tables 1 and 2).
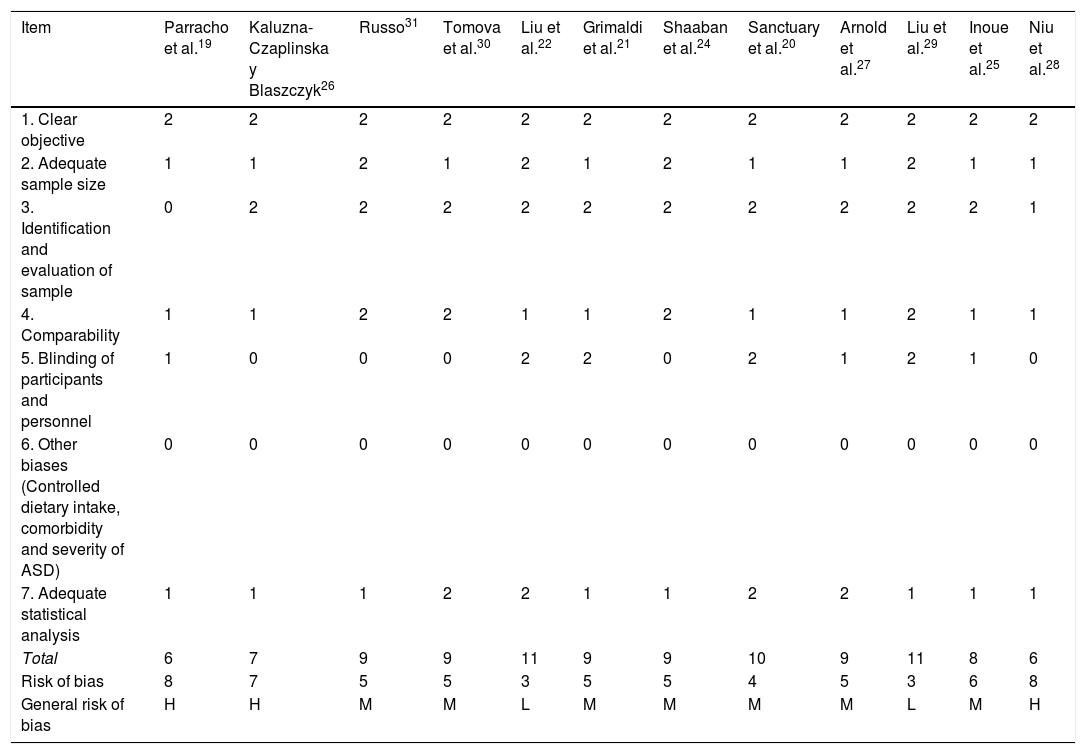
Risk of bias for the methodological quality of studies on prebiotics and probiotics in ASD.
| Item | Parracho et al.19 | Kaluzna-Czaplinska y Blaszczyk26 | Russo31 | Tomova et al.30 | Liu et al.22 | Grimaldi et al.21 | Shaaban et al.24 | Sanctuary et al.20 | Arnold et al.27 | Liu et al.29 | Inoue et al.25 | Niu et al.28 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Clear objective | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| 2. Adequate sample size | 1 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 1 | 2 | 1 | 1 |
| 3. Identification and evaluation of sample | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 |
| 4. Comparability | 1 | 1 | 2 | 2 | 1 | 1 | 2 | 1 | 1 | 2 | 1 | 1 |
| 5. Blinding of participants and personnel | 1 | 0 | 0 | 0 | 2 | 2 | 0 | 2 | 1 | 2 | 1 | 0 |
| 6. Other biases (Controlled dietary intake, comorbidity and severity of ASD) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7. Adequate statistical analysis | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 2 | 2 | 1 | 1 | 1 |
| Total | 6 | 7 | 9 | 9 | 11 | 9 | 9 | 10 | 9 | 11 | 8 | 6 |
| Risk of bias | 8 | 7 | 5 | 5 | 3 | 5 | 5 | 4 | 5 | 3 | 6 | 8 |
| General risk of bias | H | H | M | M | L | M | M | M | M | L | M | H |
ASD: Autistic Spectrum Disorder.
Note: 0: not reported; 1: not appropriately or clearly evaluated; 2: appropriately evaluated.
M: medium (8–10); L: low (11–14); H: high (7−1).
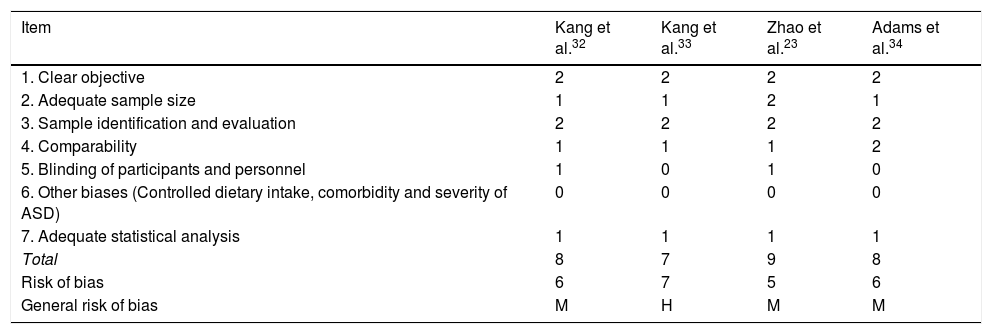
Risk of bias for the methodological quality of the studies on MMT in ASD.
| Item | Kang et al.32 | Kang et al.33 | Zhao et al.23 | Adams et al.34 |
|---|---|---|---|---|
| 1. Clear objective | 2 | 2 | 2 | 2 |
| 2. Adequate sample size | 1 | 1 | 2 | 1 |
| 3. Sample identification and evaluation | 2 | 2 | 2 | 2 |
| 4. Comparability | 1 | 1 | 1 | 2 |
| 5. Blinding of participants and personnel | 1 | 0 | 1 | 0 |
| 6. Other biases (Controlled dietary intake, comorbidity and severity of ASD) | 0 | 0 | 0 | 0 |
| 7. Adequate statistical analysis | 1 | 1 | 1 | 1 |
| Total | 8 | 7 | 9 | 8 |
| Risk of bias | 6 | 7 | 5 | 6 |
| General risk of bias | M | H | M | M |
ASD: Autistic Spectrum Disorder.
Note: 0: not reported; 1: not appropriately or clearly evaluated; 2: appropriately evaluated.
M: medium (8–10); L: low (11–14); H: high (7−1).
We found a total of 16 studies on intervention with prebiotics (4 studies), probiotics (8 studies) and MTT (4 studies) in children with ASD. No studies were found in adults. The age range of the total studies in the ASD sample was between 2 and 17 years, while the age range for the neurotypical sample was between 2 and 16 years. The n range of studies for the ASD sample was between 9 and 64 subjects, while the sample range for neurotypical children was between 10 and 40 subjects.
Twelve articles using prebiotics and probiotics in children with ASD were found (3 from USA, 2 from UK, 2 from China, one from Poland, one from Egypt, one from Taiwan, one from Japan and one from Slovakia). Only 18.75% (3/16) studies included a healthy control group, with two for probiotic intervention and one for MTT. Similarly, only 37.5% (6/16) of the selected studies included a placebo group in their study. None of the studies reported intellectual disability, or the level of severity of ASD in the sample description.
Of the studies with probiotics and prebiotics, 33.33% had a randomised, double-blind, placebo-controlled study design.19–22 On the other hand, 100% of the studies with MTT had an open clinical trial design and only one study included placebo.23
The selected studies and the results of the intervention with prebiotics, probiotics and faecal microbiota transplantation in children with ASD are shown in Table 3. The following are the findings:
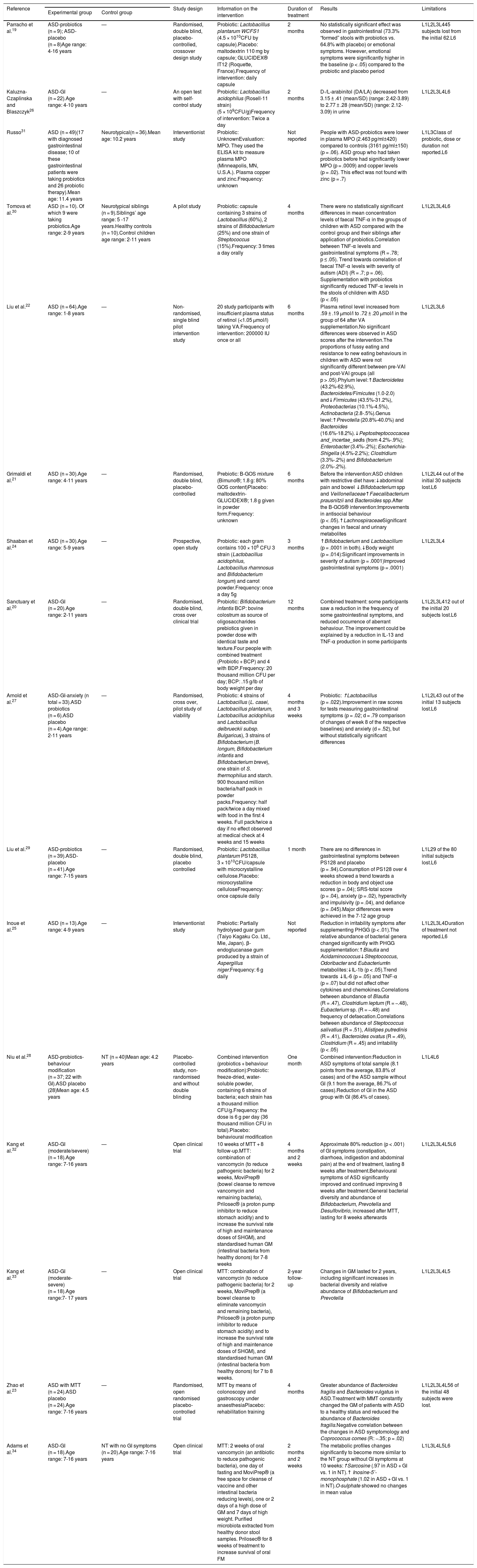
Principal characteristics of the studies included in the systematic review.
| Reference | Study design | Information on the intervention | Duration of treatment | Results | Limitations | ||
|---|---|---|---|---|---|---|---|
| Experimental group | Control group | ||||||
| Parracho et al.19 | ASD-probiotics (n = 9); ASD-placebo (n = 8)Age range: 4-16 years | — | Randomised, double blind, placebo-controlled, crossover design study | Probiotic: Lactobacillus plantarum WCFS1 (4.5 × 1010CFU by capsule).Placebo: maltodextrin 110 mg by capsule; GLUCIDEX® IT12 (Roquette, France).Frequency of intervention: daily capsule | 2 months | No statistically significant effect was observed in gastrointestinal (73.3% “formed” stools with probiotics vs. 64.8% with placebo) or emotional symptoms. However, emotional symptoms were significantly higher in the baseline (p < .05) compared to the probiotic and placebo period | L1L2L3L445 subjects lost from the initial 62.L6 |
| Kaluzna-Czaplinska and Blaszczyk26 | ASD-GI (n = 22).Age range: 4-10 years | — | An open test with self-control study | Probiotic: Lactobacillus acidophilus (Rosell-11 strain) (5 × 109CFU/g)Frequency of intervention: Twice a day | 2 months | D-/L-arabinitol (DA/LA) decreased from 3.15 ± .41 (mean/SD) (range: 2.42-3.89) to 2.77 ± .28 (mean/SD) (range: 2.12-3.09) in urine | L1L2L3L4L6 |
| Russo31 | ASD (n = 49)(17 with diagnosed gastrointestinal disease; 10 of these gastrointestinal patients were taking probiotics and 26 probiotic therapy).Mean age: 11.4 years | Neurotypical(n = 36).Mean age: 10.2 years | Interventionist study | Probiotic: UnknownEvaluation: MPO. They used the ELISA kit to measure plasma MPO (Minneapolis, MN, U.S.A.). Plasma copper and zinc.Frequency: unknown | Not reported | People with ASD-probiotics were lower in plasma MPO (2.463 pg/ml±420) compared to controls (3161 pg/ml±150) (p = .06). ASD group who had taken probiotics before had significantly lower MPO (p = .0009) and copper levels (p = .02). This effect was not found with zinc (p = .7) | L1L3Class of probiotic, dose or duration not reported.L6 |
| Tomova et al.30 | ASD (n = 10). Of which 9 were taking probiotics.Age range: 2-9 years | Neurotypical siblings (n = 9).Siblings’ age range: 5 -17 years.Healthy controls (n = 10).Control children age range: 2-11 years | A pilot study | Probiotic: capsule containing 3 strains of Lactobacillus (60%), 2 strains of Bifidobacterium (25%) and one strain of Streptococcus (15%).Frequency: 3 times a day orally | 4 months | There were no statistically significant differences in mean concentration levels of faecal TNF-α in the groups of children with ASD compared with the control group and their siblings after application of probiotics.Correlation between TNF-α levels and gastrointestinal symptoms (R = .78; p ≤ .05). Trend towards correlation of faecal TNF-α levels with severity of autism (ADI) (R = .7; p = .06). Supplementation with probiotics significantly reduced TNF-α levels in the stools of children with ASD (p < .05) | L1L2L3L4L6 |
| Liu et al.22 | ASD (n = 64).Age range: 1-8 years | — | Non-randomised, single blind pilot intervention study | 20 study participants with insufficient plasma status of retinol (<1.05 μmol/l) taking VA.Frequency of intervention: 200000 IU once or all | 6 months | Plasma retinol level increased from .59 ± .19 μmol/l to .72 ± .20 μmol/l in the group of 64 after VA supplementation.No significant differences were observed in ASD scores after the intervention.The proportions of fussy eating and resistance to new eating behaviours in children with ASD were not significantly different between pre-VAI and post-VAI groups (all p > .05).Phylum level:↑Bacteroidetes (43.2%-62.9%), Bacteroidetes/Fimicutes (1.0-2.0) and↓Firmicutes (43.5%-31.2%), Proteobacterias (10.1%-4.5%), Actinobacteria (2.8-.5%).Genus level:↑Prevotella (20.8%-40.0%) and Bacteroides (16.6%-18.2%).↓Peptostreptococcacea and_incertae_sedis (from 4.2%-.9%); Enterobacter (3.4%-.2%); Escherichia-Shigella (4.5%-2.2%); Clostridium (3.3%-.2%) and Bifidobacterium (2.0%-.2%). | L1L2L3L6 |
| Grimaldi et al.21 | ASD (n = 30).Age range: 4-11 years | — | Randomised, double blind, placebo-controlled | Prebiotic: B-GOS mixture (Bimuno®; 1.8 g: 80% GOS content)Placebo: maltodextrin-GLUCIDEX®; 1.8 g given in powder form.Frequency: unknown | 6 months | Before the intervention:ASD children with restrictive diet have:↓abdominal pain and bowel ↓Bifidobacterium spp and Veillonellaceae↑Faecalibacterium prausnitzii and Bacteroides spp.After the B-GOS® intervention:Improvements in antisocial behaviour (p < .05).↑LachnospiraceaeSignificant changes in faecal and urinary metabolites | L1L2L44 out of the initial 30 subjects lost.L6 |
| Shaaban et al.24 | ASD (n = 30).Age range: 5-9 years | — | Prospective, open study | Probiotic: each gram contains 100 × 106 CFU 3 strain (Lactobacillus acidophilus, Lactobacillus rhamnosus and Bifidobacterium longum) and carrot powder.Frequency: once a day 5g | 3 months | ↑Bifidobacterium and Lactobacillum (p = .0001 in both).↓Body weight (p = .014):Significant improvements in severity of autism (p = .0001)Improved gastrointestinal symptoms (p = .0001) | L1L2L3L4 |
| Sanctuary et al.20 | ASD-GI (n = 20).Age range: 2-11 years | — | Randomised, double blind, cross over clinical trial | Probiotic: Bifidobacterium infantis BCP: bovine colostrum as source of oligosaccharides prebiotics given in powder dose with identical taste and texture.Four people with combined treatment (Probiotic + BCP) and 4 with BDP.Frequency: 20 thousand million CFU per day; BCP: .15 g/lb of body weight per day | 12 months | Combined treatment: some participants saw a reduction in the frequency of some gastrointestinal symptoms, and reduced occurrence of aberrant behaviour. The improvement could be explained by a reduction in IL-13 and TNF-α production in some participants | L1L2L3L412 out of the initial 20 subjects lost.L6 |
| Arnold et al.27 | ASD-GI-anxiety (n total = 33).ASD probiotics (n = 6).ASD placebo (n = 4).Age range: 2-11 years | — | Randomised, cross over, pilot study of viability | Probiotic: 4 strains of Lactobacillus (L. casei, Lactobacillus plantarum, Lactobacillus acidophilus and Lactobacillus delbrueckii subsp. Bulgaricus), 3 strains of Bifidobacterium (B. longum, Bifidobacterium infantis and Bifidobacterium breve), one strain of S. thermophilus and starch. 900 thousand million bacteria/half pack in powder packs.Frequency: half pack/twice a day mixed with food in the first 4 weeks. Full pack/twice a day if no effect observed at medical check at 4 weeks and 15 weeks | 4 months and 3 weeks | Probiotic: ↑Lactobacillus (p = .022).Improvement in raw scores for tests measuring gastrointestinal symptoms (p = .02; d = .79 comparison of changes of week 8 of the respective baselines) and anxiety (d = .52), but without statistically significant differences | L1L2L43 out of the initial 13 subjects lost.L6 |
| Liu et al.29 | ASD-probiotics (n = 39).ASD-placebo (n = 41).Age range: 7-15 years | — | Randomised, double blind, placebo controlled | Probiotic: Lactobacillus plantarum PS128, 3 × 1010CFU/capsule with microcrystalline cellulose.Placebo: microcrystalline celluloseFrequency: once capsule daily | 1 month | There are no differences in gastrointestinal symptoms between PS128 and placebo (p = .94).Consumption of PS128 over 4 weeks showed a trend towards a reduction in body and object use scores (p = .04); SRS-total score (p = .04), anxiety (p = .02), hyperactivity and impulsivity (p = .04), and defiance (p = .045).Major differences were achieved in the 7-12 age group | L1L29 of the 80 initial subjects lost.L6 |
| Inoue et al.25 | ASD (n = 13).Age range: 4-9 years | — | Interventionist study | Prebiotic: Partially hydrolysed guar gum (Taiyo Kagaku Co. Ltd., Mie, Japan). β-endoglucanase gum produced by a strain of Aspergillus niger.Frequency: 6 g daily | Not reported | Reduction in irritability symptoms after supplementing PHGG (p < .01).The relative abundance of bacterial genera changed significantly with PHGG supplementation:↑Blautia and Acidaminococcus↓Streptococcus, Odoribacter and EubacteriumIn metabolites:↓IL-1b (p < .05).Trend towards ↓IL-6 (p = .05) and TNF-α (p = .07) but did not affect other cytokines and chemokines.Correlations between abundance of Blautia (R = .47), Clostridium leptum (R = −.48), Eubacterium sp. (R = −.48) and frequency of defaecation.Correlations between abundance of Steptococcus salivatius (R = .51), Alistipes putredinis (R = .41), Bacteroides ovatus (R = .49), Clostridium (R = .45) and irritability (p < .05) | L1L2L3L4Duration of treatment not reported.L6 |
| Niu et al.28 | ASD-probiotics-behaviour modification (n = 37; 22 with GI).ASD placebo (28)Mean age: 4.5 years | NT (n = 40)Mean age: 4.2 years | Placebo-controlled study, non-randomised and without double blinding | Combined intervention (probiotics + behaviour modification):Probiotic: freeze-dried, water-soluble powder, containing 6 strains of bacteria; each strain has a thousand million CFU/g.Frequency: the dose is 6 g per day (36 thousand million CFU in total).Placebo: behavioural modification | One month | Combined intervention:Reduction in ASD symptoms of total sample (8.1 points from the average, 83.8% of cases) and of the ASD sample without GI (9.1 from the average, 86.7% of cases).Reduction of GI in the ASD group with GI (86.4% of cases). | L1L4L6 |
| Kang et al.32 | ASD-GI (moderate/severe) (n = 18).Age range: 7-16 years | — | Open clinical trial | 10 weeks of MTT + 8 follow-up.MTT: combination of vancomycin (to reduce pathogenic bacteria) for 2 weeks, MoviPrep® (bowel cleanse to remove vancomycin and remaining bacteria), Prilosec® (a proton pump inhibitor to reduce stomach acidity) and to increase the survival rate of high and maintenance doses of SHGM), and standardised human GM (intestinal bacteria from healthy donors) for 7-8 weeks | 4 months and 2 weeks | Approximate 80% reduction (p < .001) of GI symptoms (constipation, diarrhoea, indigestion and abdominal pain) at the end of treatment, lasting 8 weeks after treatment.Behavioural symptoms of ASD significantly improved and continued improving 8 weeks after treatment.General bacterial diversity and abundance of Bifidobacterium, Prevotella and Desulfovibrio, increased after MTT, lasting for 8 weeks afterwards | L1L2L3L4L5L6 |
| Kang et al.33 | ASD-GI (moderate- severe) (n = 18).Age range:7- 17 years | — | Open clinical trial | MTT: combination of vancomycin (to reduce pathogenic bacteria) for 2 weeks, MoviPrep® (a bowel cleanse to eliminate vancomycin and remaining bacteria), Prilosec® (a proton pump inhibitor to reduce stomach acidity) and to increase the survival rate of high and maintenance doses of SHGM), and standardised human GM (intestinal bacteria from healthy donors) for 7 to 8 weeks. | 2-year follow-up | Changes in GM lasted for 2 years, including significant increases in bacterial diversity and relative abundance of Bifidobacterium and Prevotella | L1L2L3L4L5 |
| Zhao et al.23 | ASD with MTT (n = 24).ASD placebo (n = 24).Age range: 7-16 years | — | Randomised, open randomised placebo-controlled trial | MTT by means of colonoscopy and gastroscopy under anaesthesiaPlacebo: rehabilitation training | 4 months | Greater abundance of Bacteroides fragilis and Bacteroides vulgatus in ASD.Treatment with MMT constantly changed the GM of patients with ASD to a healthy status and reduced the abundance of Bacteroides fragilis.Negative correlation between the changes in ASD symptomology and Coprococcus comes (R: −.35; p = .02) | L1L2L3L4L56 of the initial 48 subjects were lost. |
| Adams et al.34 | ASD-GI (n = 18).Age range: 7-16 years | NT with no GI symptoms (n = 20).Age range: 7-16 years | Open clinical trial | MTT: 2 weeks of oral vancomycin (an antibiotic to reduce pathogenic bacteria), one day of fasting and MoviPrep® (a free space for cleanse of vaccine and other intestinal bacteria reducing levels), one or 2 days of a high dose of GM and 7 days of high weight. Purified microbiota extracted from healthy donor stool samples. Prilosec® for 8 weeks of treatment to increase survival of oral FM | 2 months and 2 weeks | The metabolic profiles changes significantly to become more similar to the NT group without GI symptoms at 10 weeks:↑Sarcosine (.97 in ASD + GI vs. 1 in NT).↑ Inosine-5’-monophosphate (1.02 in ASD + GI vs. 1 in NT).O-sulphate showed no changes in mean value | L1L3L4L5L6 |
ASD: autistic spectrum disorder; FCU: colony forming units; GI: with gastrointestinal symptoms; GM: gut microbiota; L1: severity of ASD is not described; L2: healthy control group not included; L3: placebo group not included; L4: little statistical value due to low n; L5: group with prebiotics or probiotics not included; L6: not mentioned whether the person has an intellectual disability; MPO: myeloperoxidase; MTT: microbiota transfer therapy; NT: neurotypical; VA: vitamin A; —: control group not included.
Few studies were found with the use of prebiotics in ASD. Specifically, there are four studies, which use different types of prebiotic compounds. From carrot powder,24 partially hydrolysed guar gum25 or vitamin A,22 to galactooligosasarides.21
With regard to emotional symptoms and ASD symptoms, while some studies found a statistically significant improvement in ASD symptomatology after the application of prebiotics21,25 other studies found no such effect.22
The results found are disparate since the variables studied to test the effect of prebiotics are different from each other. Thus, the study by Inoue et al.25 found a significant decrease in microbial diversity, and some cytokines and chemokines (IL-1β, IL-6 and TNF-α) after application of prebiotic diet based on guar gum and β-endoglucanase produced by a strain of Aspergillus niger. On the other hand, Grimaldi et al.21 found a significant increase in the Lachnospiraceae family, as well as significant changes in faecal and urinary metabolites, and in the antisocial behaviour of children with ASD after prebiotic intervention based on B-GOS® (Bimuno®; 1.8 g: 80% GOS content). Another study also found a significant increase in the Bacteroidetes/Firmicutes ratio after a vitamin A supplement.22
Probiotics in autism spectrum disorderThe probiotic strains used in the different trials are based mainly on the bacterial genera Lactobacillus and Bifidobacterium: Lactobacillus plantarum,19,22Lactobacillus acidophilus,26 a mixture of Lactobacillus acidophilus, Lactobacillus rhamnosus and Bifidobacteria longum,24 and a mixture of Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus delbruecki, Bifidobacterium longum and Bifidobacterium bifidum.27
The results of the intervention studies with probiotics in ASD are grouped below in relation to their effects on symptomatology in ASD and emotional state, gastrointestinal symptoms, bacterial abundance in the GM and the immune system.
Studies that have analysed the effects of probiotic administration on ASD symptoms and emotional symptoms are very limited. Some studies find significant improvements in the severity of ASD using Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus rhamnosus and Bifidobacterium longum.22,24,26,28,29 However, most of the studies found do not analyse these variables or highlight that the use of Bifidobacterium infantis and bovine colostrum as a source of prebiotic oligosaccharides can help reduce repetitive behaviour and emotional symptoms compared to the combination of prebiotics and probiotics.22 Liu et al.29 used Lactobacillus plantarum (PS128, 3 × 1010 CFU/capsule) as a probiotic with microcrystalline cellulose. These results showed improvements in emotional symptoms and behaviour after the probiotic intervention. However, this study did not analyse statistical differences between the probiotic and placebo groups. Thus, while some studies find statistical differences after the application of probiotics in emotional symptoms and symptoms in ASD20,24,28,29 others find no such differences.19,27
Studies measuring the effects of probiotics on gastrointestinal symptoms in ASD are few, but appear to show improvement in gastrointestinal symptoms.19,20,24,28 Specifically, improvements in gastrointestinal symptoms such as constipation, stool consistency, flatulence, and abdominal pain were found,20,24 which correlated strongly with improvements in ASD severity after treatment with probiotics.24 However, some studies find a decrease in gastrointestinal symptoms after the combination of probiotics and a behavioural training programme28 and other studies do not analyse these differences.27,30,31
With regard to the effects of probiotic diet on GM, probiotic supplements (primarily Lactobacillus acidophilus, Lactobacillus rhamnosus and Bifidobacterium longum) vary. While some studies find a significant increase in Bifidobacterium and Lactobacillus colony counts in the stools of children with ASD,24 others find a decrease in the amount of Firmicutes, Bifidobacterium, Desulfovibrio and Lactobacillus.30
With regard to the effects of probiotics on the immune system, some studies find a decrease in the frequency of certain immune cells: TNF-α30 D-arabinitol and a proportion of D-/L-arabinitol26 with probiotic intervention, CD4+/IL-13+ T cells using a combination of prebiotics and probiotics20 and CD8+/TNF-α+ T cells after prebiotic treatment in children with ASD.20 Russo31 found that plasma myeloperoxidase and copper levels were significantly lower in children with ASD after probiotic intervention. However, this study does not describe the specific characteristics of the probiotics used. Another recent study using Lactobacillus, Bifidobacterium and S. thermophilus as probiotics, found no statistically significant difference after the intervention.27
Microbiota transfer therapyWe found 4 papers on MTT in ASD, 75% of which were produced in the USA. A clinical trial evaluated the impact of MTT on GM composition and gastrointestinal symptoms in ASD. The MMT included treatment beforehand with antibiotics for 2 weeks and bowel cleanse. The gastrointestinal symptom rating scale revealed a reduction of approximately 80% in gastrointestinal symptoms at the end of treatment, including significant improvements in constipation, diarrhoea, indigestion and abdominal pain. Similarly, clinical evaluations showed that behavioural symptoms of ASD improved significantly and continued to improve 8 weeks after treatment had ended. In addition, the overall bacterial diversity and abundance of Bifidobacterium, Prevotella and Desulfovibrio also increased and these changes persisted after treatment.32 A study conducted with the same participants found similar results, with a significant increase in bacterial diversity and the relative abundance of Bifidobacterium and Prevotella with the changes persisted 2 years later.33 Another study found a decrease in the abundance of Bacteroides fragilis and symptoms in ASD (10.8%) after 4 months of MMT intervention.23 Finally, a recent study found significant improvement in metabolic profiles, gastrointestinal symptoms, ASD symptoms and in the indices of some metabolites (sarcosine and inosine-5′-monophosphate) after MMT treatment.34
ConclusionsGiven the heterogeneity of the results and the variables included in the studies, it is not possible to perform a meta-analysis study to calculate the effect size or analysis of moderators. Thus, for example, at an observational level there are no differences in the variables age group, type of bacteria used, duration of treatment, dose, etc., between the studies that found statistical differences after the application of probiotics in emotional symptoms and symptoms in ASD,20,24,28,29 and those that found no differences.19,27
According to the research design, only 33.33% of studies with probiotics19,20,22 and prebiotics21 have a randomised, double-blind, placebo-controlled study design. Similarly, only one study with MTT has this type of research design.23 Therefore, the results of these studies should be interpreted with great caution. To be specific, studies with this type of design using probiotics have a very small sample, fewer than 9 subjects,19,20 and lack homogeneity in the bacterial strain used, 2 of the 3 studies use the Lactobacillus plantarum as a probiotic.19,29 Although, both studies find an improvement in the emotional symptoms of children with ASD, one of the conclusions reached by this review study is that there is a general crisis of replicability in the studies.35 Future studies should follow the line of the study by Liu et al.29 With regard to studies with prebiotics and MTT there is still a long way to go before we can conduct studies with greater methodological rigour.
In summary, the studies on probiotics, prebiotics and MMT are promising for the time being, but there is insufficient empirical evidence. Analysis of the risk of bias indicates that 58.33% of the studies on probiotics and prebiotics have a medium bias, 25% high and 16.66% low, while in the studies performed with MMT medium bias is present in 75%, and high bias in 25%. These results reflect, to some extent, the reliability of the data at the global level, and therefore should be taken with caution. The risk of bias in these studies can be explained by a number of major limitations that prevent the replication of the studies at the international level: 1) the lack of homogeneity of the sample characteristics among the studies (e.g., severity of ASD, whether or not intellectual disability is present, etc.); 2) the small sample size or lack of homogeneity of the sample sizes being compared; 3) the heterogeneity of the interventions. For example, different types and doses of prebiotics and probiotics are used. In addition, other authors propose natural compounds that have not yet been tested such as the use of ascidians or sea potatoes36; 4) methodological heterogeneity (e.g., whether to include a control group in the waiting list, whether to include a placebo, length of follow up, etc.); 4) the absence in most of the studies of data analyses that include mean and standard deviation, as well as the magnitude of differences; and 5) the lack of inclusion in test evaluation protocols of tests that comprehensively measure ASD (e.g., severity of repetitive behaviour, level of anxiety, etc.). Future studies should take into consideration previously found studies on possible dysbiosis in ASD5,12,13,18 as a starting point for the design of intervention protocols including prebiotics, probiotics and MMT. Similarly, studies are needed in countries with cultures other than Asian and Anglo-Saxon, and that present different nutritional patterns such as in Mediterranean countries (Spain, Italy, Greece, etc.) and in Latin America.
FundingThis paper had no source of funding.
Conflicts of interestThe authors have no conflict of interests to declare
Please cite this article as: Martínez-González AE, Andreo-Martínez P. Prebióticos, probióticos y trasplante de microbiota fecal en el autismo: una revision sistemática. Rev Psiquiatr Salud Ment (Barc). 2020;13:150–164.