The heritability of eating disorders has been estimated to range from 22% to over 62%. The aim of this study is to determine the relative influence of genetics and environment that contribute to the drive for thinness, body dissatisfaction, perfectionism, and ineffectiveness, by evaluating sex differences in a sample of adolescent twins from Valencia, Spain.
Material and methodsFive hundred eighty-four pairs of adolescent twins between 13 and 18 years of age completed the study. To determine zygosity, teachers responded to a questionnaire on physical similarity. Psychological traits of eating disorders were assessed with four sub-scales of the Eating Disorder Inventory (EDI); drive for thinness, body dissatisfaction, perfectionism, and ineffectiveness. Twin models were used to assess genetic and environmental (common and unique) factors affecting these four psychological traits.
ResultsAll four traits showed significant genetic contributions among girls, with heritability estimates of 37.7% for ineffectiveness, 42.8% for perfectionism, 56.9% for drive for thinness, and 65.5% for body dissatisfaction. Among boys, body dissatisfaction showed no additive genetic contributions, indicating significant shared and individual specific environment effects. The three other traits in boys showed significant additive genetic contributions, but were lower than in girls.
ConclusionsWith the exception of body dissatisfaction in boys, psychological traits of eating disorders show heritability patterns that differ according to sex.
La heredabilidad de los trastornos de la conducta alimentaria, como la anorexia nerviosa y la bulimia nerviosa, se ha estimado alrededor del 22% al 62%. El objetivo del presente trabajo es determinar la influencia de los factores genéticos y ambientales que contribuyen en la expresión de los factores psicológicos medidos a través del Eating Disorders Inventory, en función del sexo, en adolescentes de la Comunidad Valenciana (España).
Material y métodosQuinientas ochenta y cuatro parejas de gemelos de 13 a 18 años de edad. Para determinar la cigosidad los profesores rellenaron un cuestionario de similitud física. Se aplicaron las subescalas del Eating Disorders Inventory, impulso a la delgadez, insatisfacción corporal, perfeccionismo e ineficacia. Se ha realizado una modelización de las mismas para establecer los componentes genéticos y ambientales (comunes y específicos) de su varianza.
ResultadosEn las niñas las 4 variables mostraron un componente de heredabilidad, del 37,7% para la ineficacia, del 42,8% para el perfeccionismo, del 56,9% para el impulso a la delgadez y del 65,5% para la insatisfacción corporal. En los niños se descarta una influencia genética para la insatisfacción corporal, que aparece influenciada exclusivamente por factores ambientales. El resto de variables mostraron un componente heredable, pero en menor medida que en las niñas.
ConclusionesA excepción de la IC en niños, las actitudes y comportamientos alimentarios muestran un patrón parcialmente heredable, que varía en función del sexo.
The genetics of psychiatric disorders is complex, and it is complicated further by gene–gene and gene–environment interactions. Many genes interact, giving rise to the activation of multiple neuronal circuits resulting in the appearance of behavioural variations.1 Studies of twins are a source of information about the genetic bases of complex traits; comparing monozygotic (MZ) and dizygotic twins (DZ) makes it possible to evaluate the importance of genetic variability in the likelihood of suffering a disease.2
The heritability of eating disorders (ED), their symptoms and behavioural traits have been evaluated using multiple records of twins in North American, Australian and European populations, among others. Anorexia nervosa (AN) has been found to be heritable in from 22% to 58% of cases,3–6 while bulimia nervosa (BN) is so in from 55% to 62% of cases.3,5,7,8 Abnormal eating behaviours such as “intentional weight loss”, “over-eating” and “gorging” have also been estimated to be heritable in from 14% to 51% of cases.9–11
Less is known about the genetic factors which influence the personality traits that are associated with ED. Perfectionism has been said to be heritable in 29%–42% of cases,12–14 while for the desire to be thin (DT) this ranges from 44% to 59.4%,15,16 for bodily dissatisfaction (BD) from 49% to 60%15,17 and inefficacy from 0% to 37%.12,16 Nevertheless, no Spanish research has evaluated all of these psychological factors, which are ED risk factors, together in the same sample of twins.
The aim of our work was to evaluate the heritability of several core psychological aspects of ED measured using the “Eating Disorders Inventory” (EDI) in a sample of Spanish twins. This is the first such study to be undertaken in our country, and it analyses the influence of genetic factors in the expression of perfectionism, DT, BD and inefficacy according to sex.
Material and methodsThe sampleThe population and data of this study originate in an institutional project for the diagnosis and treatment of eating disorders which the Dirección General de Salud Pública and Consellería de Educación of the Valencian Autonomous Community (Spain) have been running for a decade (the DITCA project). Participation is voluntary and it is open to all of the schools in the Valencian Community. The overall purpose of the project is the primary prevention and early detection of ED (AN, BN or non-specific ED) in the adolescent population. The students who took part, with their parents’ consent, answered the EDI questionnaire in school hours. This project has been described in previous publications.18 The target population was aged from 13 to 18 years old. Replicating previous studies,19 based on their initials, date of birth and school, 584 pairs of twins were identified and took part in this study.
Zygosity determinationThe psychological-pedagogical departments in the schools confirmed that each pair included were brothers or sisters, and they helped to determine their zygosity. The teachers filled out a questionnaire on physical similarity for all of the pairs of twins. This questionnaire had previously been used in other studies of twins, and it makes it possible to correctly classify 96% of pairs of twins regarding their zygosity.20 The validity of the determination of zygosity by the teachers was evaluated for 108 pairs in which the questionnaire had been answered by the teacher or (telephonically) by their father/mother. Cohen's Kappa between the classification of the parents and teachers was 0.94 (P<0.001). This statistic measures the agreement between judges’ opinions when they are evaluating the same thing. A value of 1 indicates a perfect agreement. This result shows a significant degree of agreement between teachers and parents which makes it possible to consider this evaluation of zygotic status as valid.
Eating Disorder InventoryThe Eating Disorder Inventory (EDI)21 is a self-applied questionnaire designed to evaluate psychological risks and shared behaviours in AN and BN. It consists of 64 items grouped in 8 sub-scales, of which 4 are used in this study: DT, BD, inefficacy and perfectionism.
The BD sub-scale measures degree of satisfaction with specific areas of the body21 such as the waists, thighs or buttocks. Its Cronbach's α stands at 0.70. The DT sub-scale evaluates restrictive tendencies, the desire to lose weight and fear of weight gain,21 with a Cronbach's α of 0.81. The inefficacy sub-scale examines questions such as negative self-evaluation, feelings of emptiness and solitude,21 and it has a Cronbach's α of 0.60. The perfectionism sub-scale examines this trait using 6 questions such as “my goals are too high”,21 and its Cronbach's α is 0.60.
Descriptive statisticsThe descriptive statistics corresponding to the 4 variables studied were calculated using the SPSS v.17 (SPSS, 2007) computer programme. Differences between the sexes were examined using the Student's t test.
Evaluation of the presumption of environmental equalityStudies of twins make it necessary to assume what is known as the environmental equality assumption (EEA), as if this is not so it may generate errors in the estimation of heritability.22 With the aim of determining whether the EEA was correct a telephonic questionnaire was administered to the parents, based on different experiences of their children during infancy.23 This questionnaire had been used beforehand in other studies of twins.10,24,25 It includes a section on co-socialisation and another on parental treatment. The co-socialisation section evaluates whether the twins go to school together, have the same teacher or do sports or play together. Parental treatment includes questions about whether the twins dress the same, share a room or spend a lot of time together. To analyse these questions in connection with the psychological variables, we calculate Pearson's correlation coefficients (r) between co-socialisation variables and those in connection with parental treatment and the difference between the pair in terms of these variables. A significantly negative correlation (P<0.05) would indicate that the twins with the highest degree of shared experiences present more similarities in terms of the trait in question, and therefore the highest degree of non-conformity with the EEA. We also separately compare the average total values for co-socialisation and parental treatment in MZ and DZ twins. Opposite sex DZ twins were excluded from this analysis to prevent differences in sex from influencing the results.
Genetic and environmental differencesThe genetic set or genotype is the total number of genes that each individual has,26 and this remains invariable throughout their life. Heritability is an estimation of genes phenotypical expression capacity in a specific population and at a certain time.27–29 It may be defined as the proportion of differences between individuals in a population for a certain trait and at a certain time, due to the genetic differences between them. Analysis of the similarity between MZ and DZ twins was introduced by Siemens,2 who formulated the basic rule for twin pathology: a heritable disorder would be more concordant among identical twins than it is among non-identical ones, while concordance would be lower among non-siblings. Twin studies are based on the determination of causes of variation in a trait in a population, using covariation between (MZ and DZ) twins in the said trait as the source of information.30 Based on this we modelled our sample of twins using lineal structural equations, with the following suppositions as causes for variance: additive genetic effects (A), the effects of a common, shared or family environment (C) and the effects of a specific or unique environment (E). The presence of these latent variables is inferred from observed data rather than by direct measurement. Personality traits do not follow Mendalian heritability, and their expression is influenced by multiple genetic factors. The sum of their effects (additive genetics) will determine their expression. Shared environmental factors (C) contribute to similarity between both twins; they reflect the environmental influences to which both twins are exposed.31 Specific environmental factors (E) are the influences which one twin is exposed to while the other is not, leading to differences between them. Given that measurement errors may also generate differences between the twins, they have traditionally been considered to be included in E.22,31
Basing ourselves on theoretical knowledge of the subject, lineal structural equation models (SEM) were constructed that were able to represent the reality underlying the 4 traits. The contribution by the latent variables was estimated as a regression of the coefficients in a lineal regression of the observed variable with the latent variables. The MX programme makes it possible to estimate these parameters using the “normal theory of maximum similarity” and “minimum weighted squares”.2 ACE, AE, CE and E models were constructed, consisting of different combinations of A, C and E. To determine which model best fits the contribution of each one of the causal factors to the variable studied we used the x2 statistic for goodness of fit and its associated P values. A high P value (>0.05) indicates a good fit. We compare the fit of the ACE model with that of the AE, CE and E submodels by using the difference between their associated x2 statistics. When this comparison did not show any significant differences (P>0.05), we used the Akaike Information Criterion (AIC)32 as the goodness of fit indicator, so that we select the model with the lowest AIC as the one which best fits with the data, as it suggests a more parsimonious explanation.
ResultsSample characteristicsThe sample was composed of 200 MZ twins (34.2%) and 384 DZ twins (65.7%). 118 of the MZ twins were girls and 82 were boys. 189 of the DZ twins were of the same sex (102 girls and 87 boys) while 195 were of opposite sexes. Their average age was 14.01±1.04 years old, with no statistically significant differences between the MZ and DZ twins (P=0.188).
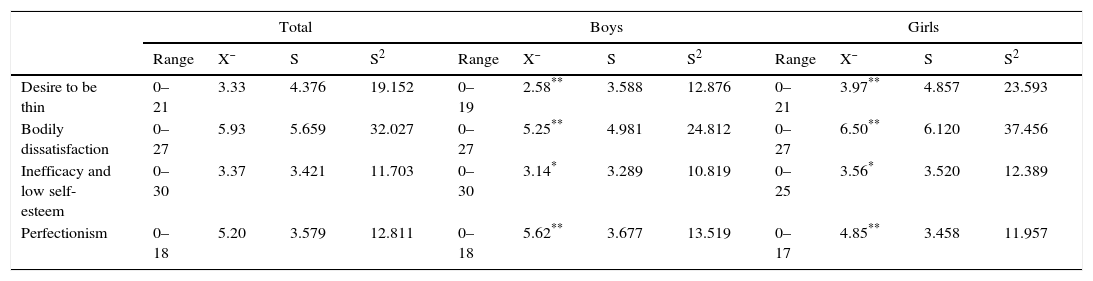
Descriptive statisticsTable 1 shows the average scores for the scales evaluated in the sample of twins. The only variable with a significantly higher average score for boys versus girls was perfectionism.
Descriptive statistics of the EDI sub-scales; the desire to be thin, bodily dissatisfaction, inefficacy plus low self-esteem and perfectionism in the total sample of twins and in boys and girls separately.
| Total | Boys | Girls | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Range | X¯ | S | S2 | Range | X¯ | S | S2 | Range | X¯ | S | S2 | |
| Desire to be thin | 0–21 | 3.33 | 4.376 | 19.152 | 0–19 | 2.58** | 3.588 | 12.876 | 0–21 | 3.97** | 4.857 | 23.593 |
| Bodily dissatisfaction | 0–27 | 5.93 | 5.659 | 32.027 | 0–27 | 5.25** | 4.981 | 24.812 | 0–27 | 6.50** | 6.120 | 37.456 |
| Inefficacy and low self-esteem | 0–30 | 3.37 | 3.421 | 11.703 | 0–30 | 3.14* | 3.289 | 10.819 | 0–25 | 3.56* | 3.520 | 12.389 |
| Perfectionism | 0–18 | 5.20 | 3.579 | 12.811 | 0–18 | 5.62** | 3.677 | 13.519 | 0–17 | 4.85** | 3.458 | 11.957 |
S: standard deviation; S2: variance; X¯: average.
No significant negative association was detected between common co-socialisation, intra-pair similarity and the traits studied. Nor were there significant differences in the degree of co-socialisation between MZ and DZ twins. No departure from the EEA was found.
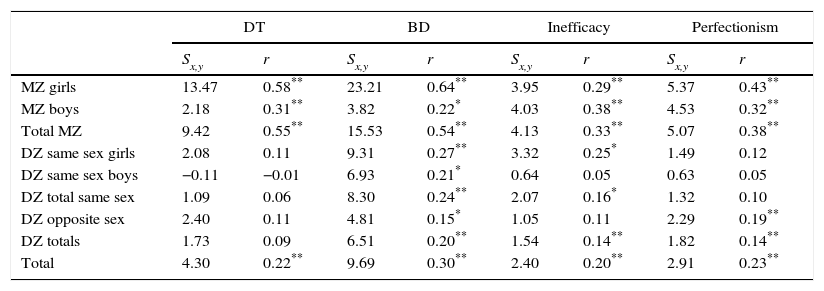
Genetic and environmental influencesTable 2 shows the intra-pair correlation for the DT, BD, inefficacy and perfectionism variables according to zygosity. Comparison of Pearson's correlation coefficients for MZ and DZ twins is the first step in evaluating the influence of genetic and environmental factors on individual differences in the expression of these traits. This gives a first intuitive idea of the proportion of the variance that is explained by genetic factors (heritability). A higher correlation in MZ twins than in DZ ones indicates that a trait is heritable to a certain extent; the greater the difference between both types of twin, the greater the degree of heritability we expect. If we make this comparison separately for boys and girls we will obtain information on the quantitative differences between the sexes in trait heritability. When the difference between the correlation in MZ and DZ twins is greater in one sex than the other, this will also be the case for the heritability of the trait.
Measurements of intra-pair correlation in the phenotypes DT, BD, inefficacy plus low self-esteem and perfectionism.
| DT | BD | Inefficacy | Perfectionism | |||||
|---|---|---|---|---|---|---|---|---|
| Sx,y | r | Sx,y | r | Sx,y | r | Sx,y | r | |
| MZ girls | 13.47 | 0.58** | 23.21 | 0.64** | 3.95 | 0.29** | 5.37 | 0.43** |
| MZ boys | 2.18 | 0.31** | 3.82 | 0.22* | 4.03 | 0.38** | 4.53 | 0.32** |
| Total MZ | 9.42 | 0.55** | 15.53 | 0.54** | 4.13 | 0.33** | 5.07 | 0.38** |
| DZ same sex girls | 2.08 | 0.11 | 9.31 | 0.27** | 3.32 | 0.25* | 1.49 | 0.12 |
| DZ same sex boys | −0.11 | −0.01 | 6.93 | 0.21* | 0.64 | 0.05 | 0.63 | 0.05 |
| DZ total same sex | 1.09 | 0.06 | 8.30 | 0.24** | 2.07 | 0.16* | 1.32 | 0.10 |
| DZ opposite sex | 2.40 | 0.11 | 4.81 | 0.15* | 1.05 | 0.11 | 2.29 | 0.19** |
| DZ totals | 1.73 | 0.09 | 6.51 | 0.20** | 1.54 | 0.14** | 1.82 | 0.14** |
| Total | 4.30 | 0.22** | 9.69 | 0.30** | 2.40 | 0.20** | 2.91 | 0.23** |
DZ: dizygotic; BD: bodily dissatisfaction; DT: desire to be thin; MZ: monozygotic; r: Pearson's intra-pair correlation coefficient; Sx,y: intra-pair covariance.
In all of the variables studied we found that MZ twins present a higher correlation than DZ ones, indicating that the trait may be heritable. When we analysed this according to sex, only for the BD variable in boys did we find very similar correlations between MZ (0.22) and DZ (0.21) twins, suggesting that this trait is less heritable in boys than it is in girls, and that possible BD is not heritable in the latter.
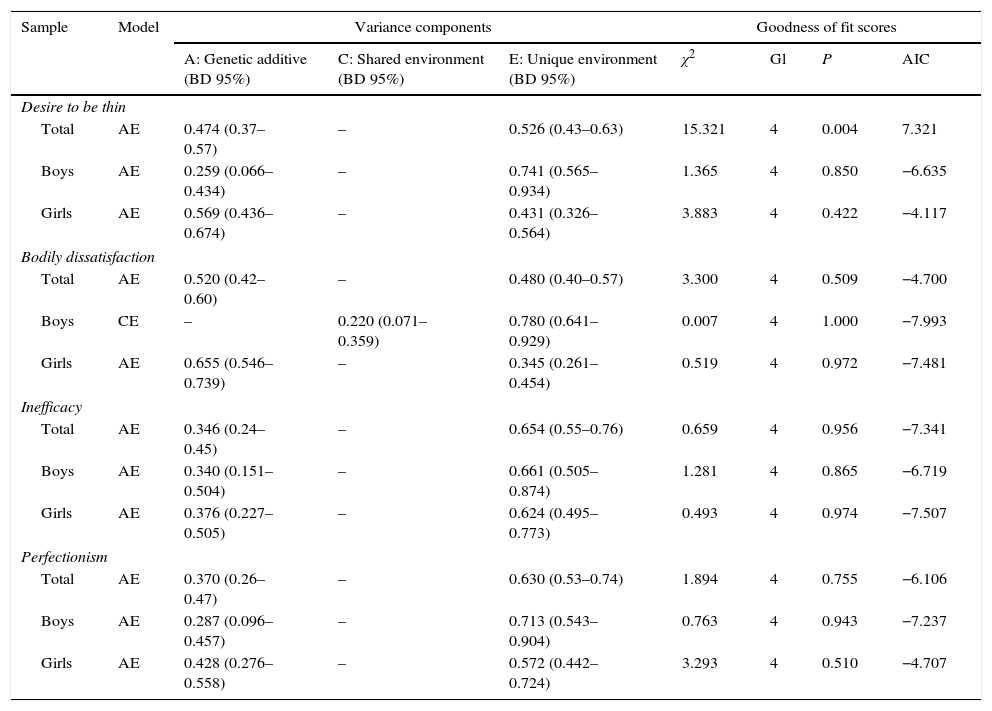
Table 3 shows the good of fit scores of the best models for the 4 traits extracted in MZ and DZ twins of the same sex (boys and girls, respectively). It also shows the proportions of variance that are explained by genetic or environmental factors. The BD 95% are also included, showing that none of them includes the value 0, so that we are able to accept that our results are valid.
Goodness of fit scores of the models which best fit the data corresponding to the phenotypes DT, BD, inefficacy plus low self-esteem and perfectionism, and proportions (percentages and BD 95%) of variance of these phenotypes explained by A, C and E for each model.
| Sample | Model | Variance components | Goodness of fit scores | |||||
|---|---|---|---|---|---|---|---|---|
| A: Genetic additive (BD 95%) | C: Shared environment (BD 95%) | E: Unique environment (BD 95%) | χ2 | Gl | P | AIC | ||
| Desire to be thin | ||||||||
| Total | AE | 0.474 (0.37–0.57) | – | 0.526 (0.43–0.63) | 15.321 | 4 | 0.004 | 7.321 |
| Boys | AE | 0.259 (0.066–0.434) | – | 0.741 (0.565–0.934) | 1.365 | 4 | 0.850 | −6.635 |
| Girls | AE | 0.569 (0.436–0.674) | – | 0.431 (0.326–0.564) | 3.883 | 4 | 0.422 | −4.117 |
| Bodily dissatisfaction | ||||||||
| Total | AE | 0.520 (0.42–0.60) | – | 0.480 (0.40–0.57) | 3.300 | 4 | 0.509 | −4.700 |
| Boys | CE | – | 0.220 (0.071–0.359) | 0.780 (0.641–0.929) | 0.007 | 4 | 1.000 | −7.993 |
| Girls | AE | 0.655 (0.546–0.739) | – | 0.345 (0.261–0.454) | 0.519 | 4 | 0.972 | −7.481 |
| Inefficacy | ||||||||
| Total | AE | 0.346 (0.24–0.45) | – | 0.654 (0.55–0.76) | 0.659 | 4 | 0.956 | −7.341 |
| Boys | AE | 0.340 (0.151–0.504) | – | 0.661 (0.505–0.874) | 1.281 | 4 | 0.865 | −6.719 |
| Girls | AE | 0.376 (0.227–0.505) | – | 0.624 (0.495–0.773) | 0.493 | 4 | 0.974 | −7.507 |
| Perfectionism | ||||||||
| Total | AE | 0.370 (0.26–0.47) | – | 0.630 (0.53–0.74) | 1.894 | 4 | 0.755 | −6.106 |
| Boys | AE | 0.287 (0.096–0.457) | – | 0.713 (0.543–0.904) | 0.763 | 4 | 0.943 | −7.237 |
| Girls | AE | 0.428 (0.276–0.558) | – | 0.572 (0.442–0.724) | 3.293 | 4 | 0.510 | −4.707 |
AIC: Akaike's information criterion; GL: degrees of freedom; BD 95%: 95% interval of confidence; P: probability associated with χ2; χ2: Chi-squared.
Except for BD in boys, for all of the variables evaluated the model that fit the best was AE. This implies that the expression of the analysed traits is basically influenced by specific genetic and environmental factors.
For BD in boys the best model is the non-genetic model CE, implying that its expression is determined by common and specific environmental factors.
DiscussionOur study reveals notable differences in heritability and environmental influences on the sex-based expression of different eating behaviours and attitudes.
The heritability of the DT and perfectionism variables was practically double in girls (at 56.9% and 42.8%, respectively) than in boys (25.9% and 28.7%, respectively). Heritability was found to be similar in both sexes for inefficacy at 34% and 37.6% in boys and girls, respectively, while the rest of the variance was due to the specific environment. Lastly, for BD the influence of genetic factors was ruled out in boys, and variance in this was attributed to environmental factors (common in 22% and specific in 78% of cases). On the other hand, for BD the girls showed 65.5% heritability, with a contribution by E of 34.5%.
Differences between the sexes for patterns of DT and BD heritability were studied by the Finnish group Keski-Rahkonen et al. (2005). In 4667 twins aged from 22 to 27 years old they found that heritability for the women was 51% for DT and 59.4% for BD, which in both cases is lower than in our population. However, in the men the expression of both traits was exclusively due to environmental factors, which in our case only occurred with the BD variable. Keski-Rahkonen et al. concluded that the heritability patterns of DT and BD are sex-specific.15 Except for BD in men their results do not agree with ours. Their results are hardly comparable with ours due to various reasons. Firstly the age difference between the samples must be taken into account, as this influences trait expression33,34 (adolescents versus adults). This was shown in a North American study of 680 twins aged 11 years old and 608 aged 17 years old. This used the reduced BD sub-scale of the EDI, and heritability was found to increase from 49% to 60% with the passing years.17 Secondly, cultural differences may also affect genetic–environmental interactions. Lastly, differences may arise due to errors in measuring the variables. As other authors have pointed out, the BD scale is more suitable for girls and young women, while in boys it may not be optimum for evaluating their worries about their appearance.35 Boys and girls may interpret the questions about BD differently, giving rise to measurement errors in males. The very high E component we found (78%) among the boys could be the expression of this.
Regarding inefficacy, a British study of 246 pairs of twins aged from 18 to 45 years old found a heritability of 37%,16 which is similar to ours. A Japanese study of 162 pairs of twins aged from 14 to 29 years old ruled out a genetic influence and showed a 47% level of common environmental factors’ influence,12 which we did not detect in our study. The differences between these studies support the hypothesis that phenotypic expression may vary with age and culture. Our results are more similar to those of the British group, which is culturally closer to us, and they differ notably from those of the Japanese group.
For perfectionism, the estimation of heritability in girls (42.8%) is somewhat higher than the level described by the aforesaid Japanese group, which found a heritable component of 37%.12 In both cases the rest of the variance in women was attributed to the specific environment.
The significant contribution of specific environmental factors in both sexes and for all of the variables studied agrees with previous studies of AN and BN, in which specific environmental factors contribute from 38% to 78%.3–8 In spite of the importance of these influences, very little research has been done into specific environmental risk factors. These specific environmental factors could explain why 2 siblings brought up in the same family show differences in eating behaviour. Examples of environmental aspects that are not shared include: differences in parental treatment (for example, parents who treat one child more severely than the other), differences in sibling interactions (such as one sibling interacting submissively with her sister, and the latter reacting with dominant behaviour), events in life (like a brother who breaks a leg) and differences in peer group characteristics (such as if the peer group of one brother smoke and drink alcohol, while the group of the other one does not).22,36
The genetics of ED is complex, and it is further complicated by gene–gene and gene–environment interactions.1 Elucidating the causes of ED requires awareness and understanding of the contribution of genetic and environmental factors. The genetic–environmental interaction implies that the genetic risk for a disease may be modified by the environment.37 3 types of genetic–environmental relationship have been described: passive, evocative and active.27,38 Passive interaction takes place when the biological parents who transmit the genes that promote the development of a psychological trait, also play a fundamental role in creating the environment in which their children are brought up. That is, the same parents who could transmit genes that predispose to an ED may model forms of anomalous forms of eating behaviour (restriction, physical exercise) and attitudes (BD, DT) in their children.39 Secondly, the genetic–environmental relationship may be evocative. For example, and individual with a genetic predisposition to an ED may ask their parents and peers for opinions about appearance in a disproportionate way. Although the resulting environment may seem to focus on appearance, in reality this emphasis is evoked by the search for reinforcement. Lastly, an active genetic–environmental relationship arises when an individual with a genetic vulnerability to suffering an ED enters environments that intensely emphasise the reinforcement about appearance, such as environments linked to fashion or gyms.38
The differences in heritability according to sex found in our study and described in the bibliography may be due in part to variations in the genetic–environmental interaction (passive, evocative and active). The predominant bodily stereotype in Western culture is slim. This standard is especially strict for women, who are subject to more pressure in the media about the ideal body as an instrument to achieve their goals and feel fulfilled.40 It is possible that this environmental pressure favours increased genetic expression of abnormal attitudes to eating.
Although we cannot directly interfere in the relationships between genes, our results show that the expression of the ED risk factors we studied is affected by genetic as well as environmental factors. That is, environmental influences are also decisive, and this makes preventive interventions possible by promoting healthy lifestyles for adolescents in their attitudes to eating and related behaviour, which may influence the genetic–environmental interaction.
This study suffers from several limitations. Firstly, evaluation is transversal, and the heritability of ED and their symptoms change with age, especially with the end of adolescence. It has been found that ageing activates the genes that predispose to the development of ED, increasing heritability from 0% to 44% through the stages of adolescence.17,33,34,41 This implies that our results should not a priori be extrapolated to other cultural environments or age ranges other than those of our population. Secondly, only the pairs of twins in schools that participated in the DITCA programme were invited to take part in this twin study. Recruitment therefore took place in two stages, which tends to lead to more losses.42 Lastly, it has to be pointed out that measuring error is confused with the specific environment effect, making it hard to interpret it with exactitude.43
ConclusionsOur results emphasise that, except for BD in boys, eating attitudes and behaviours show a heritable pattern that varies according to sex. Given that heritability is modified by environmental conditions, we can intervene in the latter to modulate the expression of these traits, especially in the girls who feel the bodily ideal of slimness more strongly.
Ethical responsibilitiesProtection of people and animalsThe authors declare that no experiments in human beings or animals were conducted for this research.
Confidentiality of dataThe authors declare that they followed the protocols of their centre of work regarding patient data publication.
Right to privacy and informed consentThe authors obtained the informed consent of the patients and/or subjects mentioned in this paper. This document is held by the corresponding author.
Conflict of interestsNone of the authors has any conflict of interests to declare.
Please cite this article as: Rojo-Moreno L, Iranzo-Tatay C, Gimeno-Clemente N, Barberá-Fons MA, Rojo-Bofill LM, Livianos-Aldana L. Influencias genéticas y ambientales en rasgos psicológicos y actitudes alimentarias en una población escolar española. Rev Psiquiatr Salud Ment (Barc). 2017;10:134–142.