Evidence suggests the existence of cytokine disturbances in patients with schizophrenia but their association with psychopathology is still unclear. The aim of the current study was to determine if pro-inflammatory cytokine levels (tumor necrosis factor-α, interleukin (IL)-6, IL-2, IL-1β, IL-1RA) are increased in stable outpatients compared with healthy subjects, and to analyze if they could be specific biomarkers of clinical dimensions in schizophrenia.
MethodsWe studied 73 stable outpatients with schizophrenia in their first 10 years of illness and 73 age- and sex-matched healthy controls. An accurate assessment of clinical dimensions (positive, negative, depressive, cognitive) was performed in patients.
ResultsOnly IL-6 levels were significantly increased in patients after controlling for body mass index, waist circumference, smoking, and psychopharmacological treatment, compared with healthy subjects. After adjusting for several confounders, multiple linear regression models identified that Positive and Negative Syndrome Scale negative symptoms, general psychopathology, and global severity are predicted by IL-1β concentrations, while motivation and pleasure domain of Clinical Assessment Interview for Negative Symptoms and Personal and Social Performance global functioning scores are predicted by IL-2 levels. Cognitive performance, positive, and depressive symptom severity did not correlate with any cytokine.
ConclusionsOur findings suggested that IL-6 concentrations are elevated in stable patients with schizophrenia. Whereas IL-2 specifically marks severity of the motivation and pleasure domain of negative symptoms, IL-1β is not specific to this dimension as it also predicts severity of general and global symptomatology.
Hay evidencias que sugieren la existencia de alteraciones de algunas citocinas en pacientes con esquizofrenia, pero su asociación con la psicopatología aún no está clara. El objetivo de este estudio es determinar si los niveles de citocinas proinflamatorias (factor de necrosis tumoral-α, interleucina [IL]-6, IL-2, IL-1β, IL-1RA) están aumentados en pacientes ambulatorios clínicamente estables en comparación con individuos sanos, y analizar si podrían ser biomarcadores específicos de las diferentes dimensiones clínicas de la esquizofrenia.
MétodosSe evaluaron 73 pacientes con esquizofrenia en sus primeros 10 años de evolución de la enfermedad y 73 controles sanos pareados por edad y sexo. Se realizó una evaluación precisa de las dimensiones clínicas (positiva, negativa, depresiva y cognitiva) en estos pacientes.
ResultadosSolo los niveles de IL-6 están significativamente elevados en los pacientes tras controlar por índice de masa corporal, perímetro abdominal, tabaquismo y tratamiento psicofarmacológico en comparación con sus controles sanos. Tras ajustar por varios factores de confusión, los modelos de regresión lineal múltiple identificaron cómo las concentraciones de IL-1β predicen los síntomas negativos, psicopatología general y gravedad global de la Escala del Síndrome Positivo y Negativo, mientras los niveles de IL-2 predicen el dominio motivación y placer de la Entrevista de Evaluación Clínica para Síntomas Negativos y la puntuación global en la Escala de Funcionamiento Personal y Social. Sin embargo, el rendimiento cognitivo, la gravedad de los síntomas depresivos y positivos no correlacionaron con ninguna de las citocinas.
ConclusionesNuestros hallazgos sugieren que las concentraciones de IL-6 permanecen elevadas en pacientes estables con esquizofrenia. Mientras que la IL-2 marca específicamente la gravedad en el dominio motivación y placer de la sintomatología negativa, la IL-1β no es específica para esta dimensión, ya que también predice la gravedad de la sintomatología general y global.
There is evidence suggesting that inflammation plays an important role in the pathophysiology of schizophrenia.1,2 Changes in the levels of peripheral cytokines, proteins that participate in the regulation of inflammatory processes, have been described in these patients across several studies, but their utility as reliable biomarkers is still uncertain.3 A meta-analysis by Miller et al. finds that serum cytokines such as interleukin (IL)-6, IL-1β, and tumor growing factor (TGF)-β were elevated in first-episode psychosis and acute relapses in schizophrenia and normalized after treatment (state markers), while others such as tumor necrosis factor (TNF)-α, IL-12, interferon (INF)-γ, and soluble IL-2 receptor (sIL-2r) remained increased in chronic patients (trait markers).4 Also, there have been reports of elevations of IL-1β, IL-6, TNF-α, and sIL-2r in antipsychotic-naïve patients with psychosis5 and significant decreases of IL-2 and IL-6 after antipsychotic treatment.6 In contrast, studies in chronically medicated patients with schizophrenia identified higher serum IL-6 but lower or similar TNF-α levels compared with healthy controls (HC),7,8 and a recent meta-analysis found that IL-1β and IL-6 concentrations decreased following treatment but were also significantly increased in chronically ill patients.9
Discordant results have also been reported for studies intended to identify associations between cytokines and clinical dimensions.10 In several studies of patients with schizophrenia (SZ), TNF-α, IL-6, and/or IL-1 receptor antagonist (IL-1RA) showed a significant relation to positive,11,12 negative, or cognitive dimensions,13–15 while others failed to replicate these findings. IL-2 levels related to cognitive performance have been detected in some,16,17 but not all studies.15 Recently, a systematic review by Misiak et al. reported that cytokine changes may contribute to cognitive impairment, especially in the case of TNF-α, with less evidence for the involvement of IL-2 and IL-6.18
One possible explanation for these inconsistent findings may be that not all studies controlled for confounding factors as age, gender, body mass index (BMI), smoking, physical comorbidities, or stress during acute psychosis.19 Also, samples of stable SZ were not usually homogeneous as patients were in different phases of illness or in a late chronic stage (>10 years).7,20 Due to the methodological limitations noted, we conducted the current study by controlling for the said confounding factors and by selecting a homogenous population in their first 10 years of illness. Furthermore, due to the known limitations of the psychometric instruments for assessing negative and cognitive symptoms and functioning, we employ the Clinical Assessment Interview for Negative Symptoms (CAINS), MATRICS Consensus Cognitive Battery (MCCB), and Personal and Social Performance (PSP) scales.
The main objective of the present study was to determine if levels of peripheral blood pro-inflammatory cytokines (TNF-α, IL-6, IL-2, IL-1β, IL-1RA) in stable SZ during the first 10 years after onset are different from those in matched HC and to analyze if they could be specific biomarkers of clinical dimensions in these patients. Another objective was to explore potential differences between a very early phase following a first episode of psychosis (FEP) (0–5 years) and an early chronic stage (ECS) (6–10 years).
MethodsParticipantsSeventy-three outpatients with schizophrenia and 73 sex- and age-matched HC from Asturias, Spain participated in this study. Diagnoses of schizophrenia were confirmed with the Structured Clinical Interview for DSM-5 (SCID-5) administered by clinicians. Patients (ages 18–45) were in the first 10 years of illness and on stable maintenance treatment for at least 3 months, excepting a subgroup of 5 patients not using any antipsychotic medication in that period. The FEP patient subgroup (n=44) and the ECS patient subgroup (n=29) were analyzed separately. Exclusion criteria for all participants were: somatic comorbidities – both acute (infection, fever, allergic processes) and chronic (cancer, autoimmune disorder) – that could interfere with the inflammatory parameters, and treatment with immunosuppressants or vaccines during the 6 months prior to enrollment or with anti-inflammatory drugs 2 days before blood collection. HC did not have a past history of psychiatric disorders. All subjects reported no history of diabetes or cardiovascular disease. In the whole sample of both patients and control subjects, 94.5% were Caucasian while 4 patients and 4 HC were not, mostly Hispanic. All participants received information about the purposes and protocol of the study and signed informed consents before any study procedures. The study was approved by the local ethics committee, the Regional Clinical Research Ethics Committee of the Principality of Asturias (Ref. 25/2014).
Clinical assessmentPatients were assessed with the Spanish versions of the PANSS,21 the CAINS,22 Calgary Depression Scale (CDS),23 the MCCB,24 and the PSP.25 The CAINS is made up of two subscales covering “motivation/pleasure” (CAINS-MAP whose items include anticipatory pleasure and motivation related to recreational, social, work, and school activities) and “expression” (CAINS-EXP whose items include facial and vocal expression, expressive gestures, and speech).
Sociodemographic and other clinical variables were evaluated by semi-structured interviews and included: education, duration of illness, psychopharmacological treatment, substance use (tobacco, measured as cigarettes per day), and number of psychiatric hospitalizations. Each antipsychotic dose was converted to chlorpromazine equivalents in mg/day.26 The anthropometric data included weight (kg), height (m), BMI (kg/m2), and waist circumference (cm).
Collection of blood samples and cytokine assaysFasting blood samples were obtained by venipuncture in the morning between 8:00 and 10:00a.m. on the same day as the clinical assessment.
All inflammatory biomarkers were measured in duplicate from frozen plasma and serum samples using commercially available enzyme-linked immunosorbent assays according to the manufacturer's recommendations. The average of the two measurements was used in the analysis. The levels of TNF-α (Invitrogen Corp., CA, USA) were assayed in serum samples. IL-6 (Gen-Probe Diaclone SAS, Besancon Cedex, France) and IL-2, IL-1β, and IL-1RA (RayBiotech Inc., GA, USA) levels were measured in plasma samples. The sensitivities were 1.7pg/mL, 2pg/mL, 4pg/mL, 0.3pg/mL, and 0.1ng/mL, respectively.
Statistical analysisVariables in the HC and schizophrenia groups and patient subgroups were compared using a chi-square test, Student's t-test for independent samples, and the non-parametric Mann–Whitney U test for non-normally distributed continuous variables. Because cytokine levels were skewed, they were log-transformed (ln). Between-group differences were found in BMI, waist circumference, and smoking, so they were included as covariates in stepwise multiple linear regression analyses for cytokine levels as dependent variables and diagnosis of schizophrenia as a predictor. We also included psychiatric medications as covariates, binned by class as atypical antipsychotics (risperidone, clozapine, paliperidone, aripiprazole, amisulpride, olanzapine, quetiapine), antidepressants (citalopram, escitalopram, sertraline, mirtazapine, desvenlafaxine, clomipramine), benzodiazepines, or stabilizers (lithium, valproate). Only one patient was receiving a typical antipsychotic (haloperidol) in combination with an atypical antipsychotic, so this class was not independently analyzed. Differences were considered statistically significant when p<0.05.
Associations between cytokines and clinical variables in the patient group were identified through Pearson correlations with Bonferroni correction for multiple comparisons (five tests, adjusted p<0.01). Finally, stepwise multiple regression analyses were performed to explore the effect of selected cytokines and other covariates on clinical dimension scores. Covariates were selected using Pearson correlations, finding only positive associations between chlorpromazine equivalent doses and IL-1β concentrations (r=0.321, p=0.006), and between BMI and TNF-α and IL-6 (r=0.361, p=0.002; r=0.304, p=0.009). Based on literature and expert criteria we also included age, gender, education, duration of illness, smoking, waist circumference, and treatment with antidepressants, benzodiazepines, or stabilizers, as potential confounders.
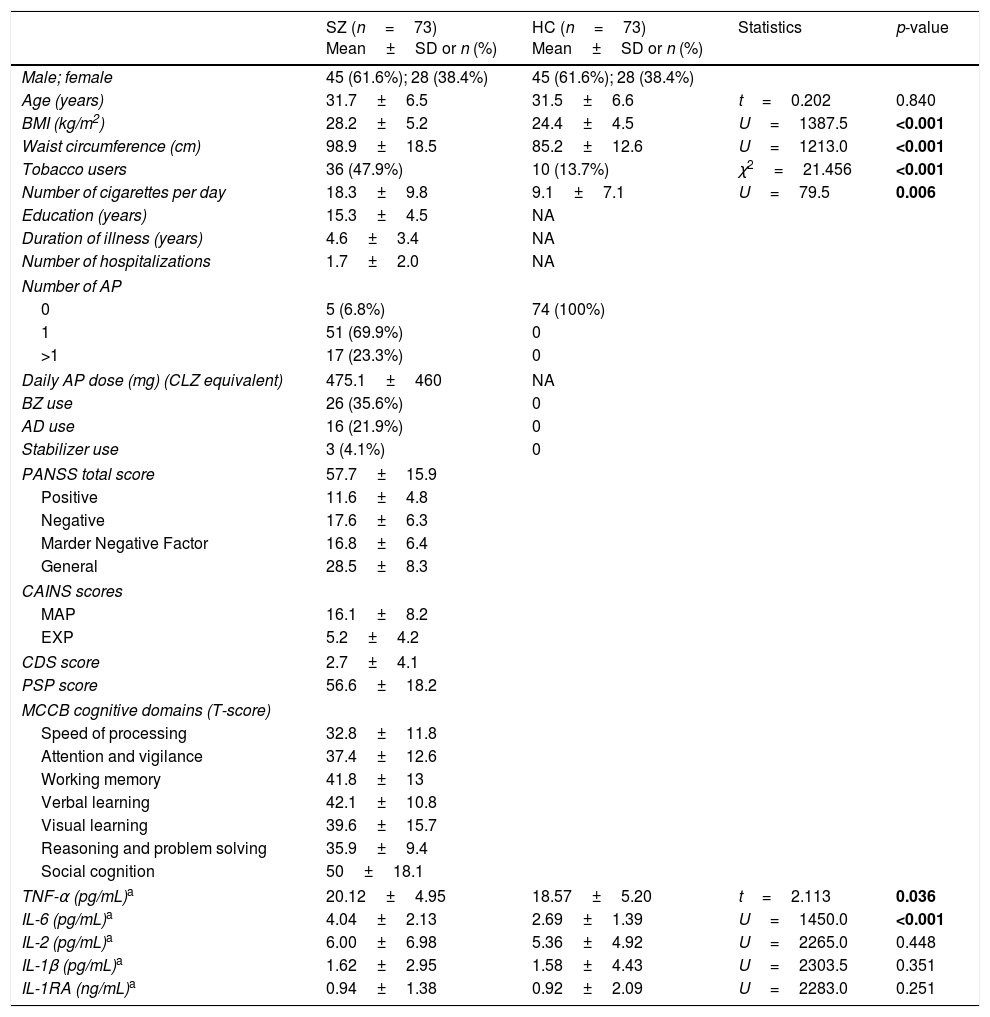
ResultsDemographic and clinical characteristicsSZ and HCDemographic and clinical characteristics of subjects are shown in Table 1. As expected, the two groups did not differ in age or sex. Nevertheless, SZ had greater BMI and waist circumference measurements, and a higher proportion of smokers than HC, as has been widely reported.
Demographic characteristics and cytokine concentrations of patients with schizophrenia (SZ) and healthy controls (HC).
| SZ (n=73) Mean±SD or n (%) | HC (n=73) Mean±SD or n (%) | Statistics | p-value | |
|---|---|---|---|---|
| Male; female | 45 (61.6%); 28 (38.4%) | 45 (61.6%); 28 (38.4%) | ||
| Age (years) | 31.7±6.5 | 31.5±6.6 | t=0.202 | 0.840 |
| BMI (kg/m2) | 28.2±5.2 | 24.4±4.5 | U=1387.5 | <0.001 |
| Waist circumference (cm) | 98.9±18.5 | 85.2±12.6 | U=1213.0 | <0.001 |
| Tobacco users | 36 (47.9%) | 10 (13.7%) | χ2=21.456 | <0.001 |
| Number of cigarettes per day | 18.3±9.8 | 9.1±7.1 | U=79.5 | 0.006 |
| Education (years) | 15.3±4.5 | NA | ||
| Duration of illness (years) | 4.6±3.4 | NA | ||
| Number of hospitalizations | 1.7±2.0 | NA | ||
| Number of AP | ||||
| 0 | 5 (6.8%) | 74 (100%) | ||
| 1 | 51 (69.9%) | 0 | ||
| >1 | 17 (23.3%) | 0 | ||
| Daily AP dose (mg) (CLZ equivalent) | 475.1±460 | NA | ||
| BZ use | 26 (35.6%) | 0 | ||
| AD use | 16 (21.9%) | 0 | ||
| Stabilizer use | 3 (4.1%) | 0 | ||
| PANSS total score | 57.7±15.9 | |||
| Positive | 11.6±4.8 | |||
| Negative | 17.6±6.3 | |||
| Marder Negative Factor | 16.8±6.4 | |||
| General | 28.5±8.3 | |||
| CAINS scores | ||||
| MAP | 16.1±8.2 | |||
| EXP | 5.2±4.2 | |||
| CDS score | 2.7±4.1 | |||
| PSP score | 56.6±18.2 | |||
| MCCB cognitive domains (T-score) | ||||
| Speed of processing | 32.8±11.8 | |||
| Attention and vigilance | 37.4±12.6 | |||
| Working memory | 41.8±13 | |||
| Verbal learning | 42.1±10.8 | |||
| Visual learning | 39.6±15.7 | |||
| Reasoning and problem solving | 35.9±9.4 | |||
| Social cognition | 50±18.1 | |||
| TNF-α (pg/mL)a | 20.12±4.95 | 18.57±5.20 | t=2.113 | 0.036 |
| IL-6 (pg/mL)a | 4.04±2.13 | 2.69±1.39 | U=1450.0 | <0.001 |
| IL-2 (pg/mL)a | 6.00±6.98 | 5.36±4.92 | U=2265.0 | 0.448 |
| IL-1β (pg/mL)a | 1.62±2.95 | 1.58±4.43 | U=2303.5 | 0.351 |
| IL-1RA (ng/mL)a | 0.94±1.38 | 0.92±2.09 | U=2283.0 | 0.251 |
Differences were assessed using a chi-square test for categorical variables, and Student's t-test for independent samples, or the non-parametric Mann–Whitney U test for continuous and non-normally distributed data. Cytokine levels were log-transformed for analyses, but in the table they are presented in the original value. SD, standard deviation; AP, antipsychotics; CLZ, chlorpromazine; BZ, benzodiazepines; AD, antidepressants; PANSS, Positive and Negative Syndrome Scale; CAINS, Clinical Assessment Interview for Negative Syndrome; MAP, Motivation and Pleasure; EXP, Expression; CDS, Calgary Depression Scale; PSP, Personal and Social Performance Scale; MCCB, MATRICS Consensus Cognitive Battery.
Patients in different stages of illness (0–5 vs. 6–10 years) did not differ in any clinical dimension or cognitive domain (all Student's t-tests with p>0.05). Also, no statistically significant differences were found in sex (63.3% vs. 58.6% males, respectively; χ2=0.186, p=0.666) or tobacco use (45.5% vs. 55.2%; χ2=.660, p=0.416). FEP patients had lower BMI and waist circumference measurements than ECS patients (27.2±4.5 vs. 29.8±5.9kg/m2, U=462.5, p=0.048; 95.1±12.6 vs. 104.8±24.1cm, U=463.5, p=0.049).
Differences in cytokine levelsSZ and HCCytokine levels in both groups are presented in Table 1. Concentrations of TNF-α and IL-6 in SZ were significantly increased compared with HC, while no differences were detected in other cytokines. The multiple linear regression model identified that both diagnosis of schizophrenia (β=0.286, p=0.001) and BMI (β=0.268, p=0.001) were factors that had a significant predictive effect on IL-6 levels and accounted for 21% of the variance (model dF=2, F=18.718, p<0.001). However, only BMI (β=0.269, p=0.001) and smoking (β=0.165, p=0.04), but not the diagnosis, were significant predictive factors in the regression model for TNF-α concentrations (model dF=2, F=8.874, p<0.001).
FEP and ECSWe found no significant differences in cytokine levels between FEP and ECS.
Associations between cytokines and clinical dimensionsBiomarkers of clinical dimensions in schizophreniaCorrelation analyses showed significant positive associations between IL-2 concentrations and PANSS-Total (r=0.345, p=0.003), PANSS-General (r=0.315, p=0.007) PANSS-Marder Negative Factor (r=0.315, p=0.007) and CAINS-MAP subscales (r=0.310, p=0.008). IL-1β levels were positively correlated with PANSS-Total (r=0.375, p=0.001), PANSS-General (r=0.314, p=0.008), PANSS-Negative (r=0.337, p=0.004) and PANSS-Marder Negative Factor subscores (r=0.386, p=0.001). Cognitive performance and positive and depressive symptom severity did not significantly correlate with any of the cytokines after Bonferroni adjustment (p≥0.029). Regarding functioning, both IL-2 and IL-1β were inversely associated with PSP scores (r=−0.448, p<0.001; r=−0.392, p=0.001, respectively). Data on these correlations segmented by stage subgroups is included in the Supplementary Material.
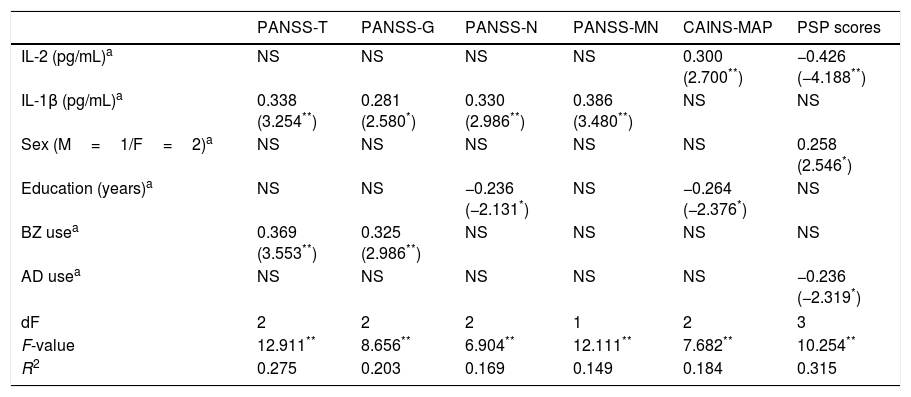
To assess the effect of IL-2 and IL-1β on clinical dimensions and functioning, adjusting for the confounding factors, we performed multiple linear regression analyses including the mentioned covariates. The final models are presented in Table 2, showing only those independent factors that explained an effect on specific clinical dimensions or functioning. While IL-2 levels specifically predict greater severity of MAP and worse PSP scores, IL-1β concentrations predict greater severity on PANSS-Total, PANSS-General, PANSS-Negative, and PANSS-Marder Negative Factor scores.
Summary of linear regression models on the association between cytokines and clinical scores in patients.
| PANSS-T | PANSS-G | PANSS-N | PANSS-MN | CAINS-MAP | PSP scores | |
|---|---|---|---|---|---|---|
| IL-2 (pg/mL)a | NS | NS | NS | NS | 0.300 (2.700**) | −0.426 (−4.188**) |
| IL-1β (pg/mL)a | 0.338 (3.254**) | 0.281 (2.580*) | 0.330 (2.986**) | 0.386 (3.480**) | NS | NS |
| Sex (M=1/F=2)a | NS | NS | NS | NS | NS | 0.258 (2.546*) |
| Education (years)a | NS | NS | −0.236 (−2.131*) | NS | −0.264 (−2.376*) | NS |
| BZ usea | 0.369 (3.553**) | 0.325 (2.986**) | NS | NS | NS | NS |
| AD usea | NS | NS | NS | NS | NS | −0.236 (−2.319*) |
| dF | 2 | 2 | 2 | 1 | 2 | 3 |
| F-value | 12.911** | 8.656** | 6.904** | 12.111** | 7.682** | 10.254** |
| R2 | 0.275 | 0.203 | 0.169 | 0.149 | 0.184 | 0.315 |
NS=variables excluded in the final model. PANSS, Positive and Negative Syndrome Scale; -T, -Total; -G, General; -N, Negative; -MN, Marder Negative Factor; CAINS, Clinical Assessment Interview for Negative Syndrome; MAP, Motivation and Pleasure; PSP, Personal and Social Performance Scale; IL, interleukin; M, male; F, female; BZ, benzodiazepines; AD, antidepressants.
We found elevated levels of IL-6 and TNF-α in stable SZ in their first 10 years of illness compared with sex- and age-matched HC. When other potential confounding factors were included in the analyses (smoking, BMI, waist circumference, medications), diagnosis of schizophrenia was a predictor for increased IL-6 concentrations but not for TNF-α. In addition, no differences in any of the cytokines between FEP and ECS patients reach statistical significance.
The initial hypothesis of IL-6 being a state-related marker4 is rejected by our results as well as by several successive studies that found that elevated levels of IL-6 persist in stable patients after relapses.7–9,27
Less numerous studies have investigated the IL-1 inflammatory pathway in schizophrenia including markers such as IL-1RA and IL-1β. Increased levels of IL-1RA have been detected in a large population of SZ28 and remained increased after one year of follow-up,29 while a previous study reported that levels are elevated in acute phases but lower after treatment.30 Inconsistent results have been found for other pro-inflammatory cytokines such as IL-27,17,31,32 and a meta-analysis by Potvin et al. reported that IL-2 is decreased in vitro but increased under in vivo conditions in schizophrenia.33 Our present study had not identified that IL-2, IL-1β, or IL-1RA levels in stable patients differed from those in HC.
On the other hand, our findings suggest that elevated levels of IL-1β have an influence on global symptom severity, and more particularly on general and negative symptomatology, measured by both PANSS-Negative and PANSS-Marder Negative Factor subscales. In addition, the specific domain of negative dimension (motivation and pleasure), measured by the CAINS-MAP, is accurately predicted by IL-2 levels. It is noteworthy that IL-1β is one of the major regulators of inflammatory response and it is closely related to IL-2, which it is induced by IL-1β.34 Also, both cytokines showed a moderate positive correlation in our study (r=0.655, p<0.001). It may be surprising that two scales measuring negative symptomatology show an independent relationship with two different cytokines. However, we believe that it is because the PANSS-Negative subscale measure different constructs from the CAINS, whereas the CAINS-MAP focuses on the nuclear factor of the negative dimension constituted by avolition and anhedonia.35
Our results contrast with the scarce studies that detected an inverse correlation between IL-2 and negative symptom severity in chronic medicated or treatment resistant SZ.17,36 In addition, negative dimension has been related to increased levels of IL-1RA, IL-6, and TNF-α,11,13,37 but other studies failed to replicate these findings. As far as we know, we are the first to report a positive association between IL-1β and PANSS-Negative score. However, it is important to point out that the negative subscale of the PANSS has several limitations of validity, as it does not incorporate relevant symptoms such as avolition and anhedonia, and it includes cognitive symptoms such as difficulty in abstract thinking,38,39 whereas the last limitation was eliminated by the Marder Negative Factor.
On the other hand, a positive correlation between the general psychopathology factor of the PANSS and mRNA expression levels of IL-1β have been already reported by Liu et al., similar to our findings.40
No other cytokines showed a significant correlation with clinical dimensions in the whole sample. Although a previous study has reported an influence of cytokines on cognitive impairment in schizophrenia,41 we could not support these findings.
Finally, we found that global function is predicted by IL-2. Few studies have described an association between cytokines and functionality in schizophrenia. For example, Hope et al. reported poorer function related to IL-1RA and TNF-R1 levels,11 but they used the Global Assessment of Functioning (GAF), which has been surpassed by the PSP with a more accurate evaluation of the functionality. It is important to highlight that avolition and anhedonia are some of the symptoms with the greatest impact on global functioning,42 which supports our results.
One of the main limitations of our study is the cross-sectional nature of the data presented. Secondly, we had one HC group but not another group of patients with different severe mental disorder, such as bipolar disorder, for comparison. Thus we cannot conclude that IL-6 is specific to schizophrenia. Another point is that most patients are on psychopharmacological treatment, which may have influenced the findings. However, whereas some studies reported an anti-inflammatory effect of antipsychotics,43 Morch et al. found no impact of psychiatric medications on inflammation in a large sample, and suggested that “the association between inflammatory markers and antipsychotics is driven by the disorder itself”.28 In our study, only IL-1β showed a positive correlation with antipsychotic dosage, which probably reflects patients with greater symptom severity. Furthermore, in our analyses, we considered psychopharmacological treatment a potential confounding factor.
One strength of this study is that adequate psychometric instruments were used for a detailed clinical assessment, especially for negative symptoms, cognition, and global functioning. To our knowledge, no previous cytokine studies in schizophrenia have employed the CAINS, MCCB, or PSP.
Taken together, cytokine disturbances in SZ have been widely described in acute phases while results are less consistent in stable phases. In addition, contradictory published data about the relation between cytokines and psychopathology suggest that this issue has not yet been clarified. In this sense, we believe the present study offers novel results on possible inflammatory disturbances specific to the nuclear factor of the negative dimension (motivation and pleasure) associated with IL-2. The potential involvement of IL-2 in the pathophysiology of symptoms such as avolition and anhedonia is presented as a possible target for the development of new therapeutic molecules.
FundingThis study is supported by a grant from the Ministry of Economy and Competitiveness, Instituto de Salud Carlos III (PI13/02263), and the European Regional Development Fund (ERDF).
Conflict of interestLGB was supported by a grant from the Spanish Foundation of Psychiatry and Mental Health. Also, this author has received speaker fees and travel expenses for attending conferences from Janssen-Cilag, Otsuka, Lundbeck, and Pfizer.
MPGPG has been a consultant to and/or has received honoraria/grants from Alianza Otsuka-Lundbeck, CIBERSAM, European Commission, Instituto de Salud Carlos III, Janssen-Cilag, Lilly, Lundbeck, Otsuka, Pfizer, Servier, Roche, and Rovi.
LGA has received honoraria from the 7th Framework Program European Union.
PAMS has been a consultant to or has received honoraria or grants from Adamed, AstraZeneca, Brainpharma, Bristol-Myers Squibb, CIBERSAM, Esteve, European Commission, Ferrer inCode, GlaxoSmithKline, Instituto de Salud Carlos III, Janssen-Cilag, Lilly, Lundbeck, Otsuka, Pfizer, Plan Nacional Sobre Drogas, Rovi, and Servier.
JBG has received research grants and served as consultant, advisor or speaker within de last 5 years for: AB-Biotics, Adamed, Almirall, AstraZeneca, Bristol-Myers Squibb, Ferrer, Glaxo-Smith-Kline, Hoffman La Roche, Janssen-Cilag, Indivior, Lilly, Lundbeck, Merck, Novartis, Organon, Otsuka, Pfizer, Pierre-Fabre, Reckitt-Benckiser, Sanofi-Aventis, Servier, Shering-Plough and Shire, research funding from the Spanish Ministry of Economy and Competiveness – Centro de Investigacion Biomedica en Red area de Salud Mental (CIBERSAM) and Instituto de Salud Carlos III, Spanish Ministry of Health, Social Services and Equality – Plan Nacional sobre Drogas – and the 7th Framework Program of the European Union.
LFT, CIG, SRG, and ACM have no conflicts of interest to disclose.
Please cite this article as: González-Blanco L, García-Portilla MP, García-Álvarez L, de la Fuente-Tomás L, Iglesias García C, Sáiz PA, et al. ¿Pueden ser la interleucina-2 y la interleucina-1β biomarcadores específicos de la sintomatología negativa en la esquizofrenia? Rev Psiquiatr Salud Ment (Barc). 2019;12:9–16.