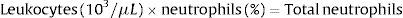Cyclic neutropenia occurs due to the fluctuation of cellular production levels of bone marrow stem cells. This is to say, they change during the cycle, and although numbers are recurrently low, function is normal. Cyclic neutropenia was first described by Leale in 1910 with dominant autosomal character. It manifests approximately every 21 days, with range of 14 to 36 days, lasting 3-6 days per episode. During the time when there are few circulating neutrophils, the patient is susceptible to infections. The clinical picture of this process includes: susceptibility to infection, feverish conditions, oral ulcers, impetigo, increased lymph nodes, periodontitis and stomatitis. It is important to stomatologically handle these patients; after inter-consultation with treating physician, prevent infection in a prophylactic scheme based on amoxicillin 50mg/per kg weight or clindamycin 20mg/per kg weight, review recent blood count results (maximum ten days before treatment). In emergency treatments pain and infection will be handled conservatively, with use of 0.12% chlorhexidine mouthwashes and Philadelphia solution when necessary.
La neutropenia cíclica ocurre debido a que los niveles de producción celular por parte de las células madre de la médula ósea fluctúan, es decir, cambian durante el ciclo; aunque el número es recurrentemente bajo, su función es normal. Fue descrita por primera vez por Leale en 1910, con carácter autosómico dominante. Se presenta aproximadamente cada 21 días con un rango de 14 a 36 días durando un total de 3 a 6. Durante el periodo en el que existen pocos neutrófilos circulantes, el paciente es susceptible a las infecciones. Dentro del cuadro clínico se presentan: susceptibilidad a infecciones, cuadros febriles, fatiga, úlceras orales, impétigo, aumento de ganglios linfáticos, periodontitis, estomatitis. Es importante manejar estomatológicamente a estos pacientes, previa interconsulta con médico tratante, prevenir cuadros infecciosos bajo un esquema profiláctico a base de amoxicilina 50mg/kg peso o clindamicina 20mg/kg peso, revisar biometría hemática reciente (máximo 10 días previos al tratamiento); en tratamientos de urgencia se manejará de forma conservadora el dolor e infección, uso de enjuagues con clorhexidina al 0.12% y solución Philadelphia en caso de ser necesario.
Patients afflicted with cyclic neutropenia suffer periodic decreases of neutrophil numbers; this is an infrequent disorder and is characterized by habitual fever episodes.
Cyclic neutropenia occurs due to the fluctuation of bone marrow stem cells’ cellular production, that is to say, a change during the cycle. Although the number is recurrently low, their function is normal.
Neutrophils are polymorphonuclear granulocytes, they approximately measure 8 to 12 micrometers, they are the most abundant leukocytes present in the blood, they live for 72 hours after having exited the bone marrow, and thus can be considered cells with the following characteristics: they are phagocytic, mobile cells, with gelatinous consistency; they can easily cross blood vessels as well as respond to inflammation and infection stimuli five hours after initiation of process.
Therefore, neutropenia can be considered a significant decrease in the number of polymorphonuclear neutrophils per mm3 in peripheral blood. It is caused by alterations in cell production, excessive destruction of peripheral tissues, cell non-maturation or a disrupted cell distribution.1
These patients exhibit certain susceptibility to infections; therefore, neutropenia can be classified in the following manner:
- a)
Length: acute, chronic.
- b)
State of medullar reserve: preserved or low.
- c)
Severity: mild, moderate or severe.
- d)
Nature or origin:
1) Acquired: medication:
- •
Anticonvulsant.
- •
Antihistaminic.
- •
Barbiturates.
- •
Chemo-therapeutic drugs.
- •
Diuretics.
- •
Sulfonamides/antibiotics.
2) Congenital:
- •
Kostmann disease.
- •
Shwachman syndrome.
- •
Cyclic neutropenia.
- •
Mielokatesis.
Cyclic neutropenia was first described by Leale in 1910. He depicted it as a condition with autosomal dominant character, a result of the mutation of EIA2 gene, position 13.3 of the short arm of chromosome 19 which codifies neutrophil elastase. Its time span is occurrence within a rank of 14 to 36 days, normally around day 21, with duration of 3 to 6 days. It is more common for infections to appear during the period when there are few circulating neutrophils.2
There is an interruption in cell production of bone marrow stem cells. This can cause from normal production up to severe stagnation of neutrophil maturation. Pharmacological treatment of granulocyte colonies stimulating factor (G-CSF) promotes maturation of neutrophil-precursor cells.
Under normal circumstances, G-CSF is mainly produced in the bone marrow. When there is bacterial infection, G-CSF production increases due to the stimuli produced by certain components of the infectious agent on immune system cells. Final result is increase of neutrophil maturation in the bone marrow, greater release of said neutrophils into the bloodstream as well as activation of said cells functions, that is to say, their ability to destroy pathogen agents.
With respect to incidence it can be said that frequency is 2 cases per million subjects.3
The clinical picture might include the following manifestations: susceptibility to infections, fever, fatigue, impetigo, increase in the size of lymph nodes, periodontitis, cheilitis, stomatitis.
Diagnosis can be emitted when the following can be established: cyclic episodes of 200 total neutrophils per mm3, every three weeks for a period of 3 to 6 days. To achieve confirmation, blood count must be performed 2 to 3 times a week, during 8 weeks.
Treatment is IV administration of «granulocyte colonies stimulation factor» (G-CSF), whose function, as indicated by its name, is to stimulate neutrophil production and shorten neutropenia duration, which brings along symptom's decrease. In cases when the patient does not react favorably, bone marrow transplant or corticosteroids can be used.
When patients reach adolescence, they experiment symptom decrease and cycles become less noticeable.4
PROTOCOL FOR STOMATOGNATIC ASSESSMENT OF PATIENT WITH CYCLIC NEUTROPENIA CONDUCTED AT THE MEXICO'S CHILDREN'S HOSPITAL «FEDERICO GÓMEZ»1) Condition identification, determination and detection of signs and symptoms characteristic of the condition.
2) Inter-consultation with treating pediatric hematologist concerning:
- a)
Actual status of the patient.
- b)
Recent blood counts (total neutrophil count).
- c)
Phase of neutropenic cycle which the patient is presently undergoing.
- d)
Type of pharmacological treatment used by the patient to control his neutropenic cycle.
3) Potential problems that can be encountered:
- a)
Formation of infectious processes.
- b)
Periodontitis.
- c)
Herpetic stomatitis.
- d)
Presence of acute pain.
- e)
Thrombocytopenia data when they are acquired by chemo-therapeutic drugs.
4) In order to avoid complications it is important to follow:
- a)
Anti-bacterial prophylactic scheme based on amoxicillin 50 mg/kg weight or clindamycin 20/mg/kg weight in case of allergy so as to avoid infectious processes. It can be used as therapeutic scheme (every 8hours for 7 days) whenever appointments are consecutive, or as a prophylactic scheme (1 hour before initiating dental treatment, single doses) whenever it is an emergency treatment or isolated appointments.
- b)
Revision of total number or neutrophils in blood counts conducted before and after dental treatment.
5) Modifications to treatment plan:
- a)
When leukocyte count suddenly drops, antibiotics must be used in order to avoid post-operative infection.
6) Emergency treatments:
a) Avoid use of non steroid anti-inflammatory drugs, especially acetylsalicylic acid when there are thrombocytopenia data, nevertheless, paracetamol can be used.
b) Use of Philadelphia solution when there is presence of stomatitis:
- •
Bismuth sub-salicylate 20mL.
- •
Dyphenhydramine hydrochloride 10mL.
- •
Sucralfate ½ tablet.
- •
Xylocaine 2mL.
In 5mL mouthwash rinses every 6hours.
c) Use of palliative techniques for oral hygiene.
d) Use of.12% chlorhexidine rinses.
PERIODONTAL TREATMENTImmuno-suppression is defined as the inhibition of one or more components of the immune system. It is the result of an underlying disease or it can be intentional through the use of drugs or treatments such as radiation. These patients are under greater risk of contracting infections, therefore, even minor periodontal infections can become life-threatening in cases when immune suppression is severe. Fungal, viral and bacterial intraoral infections might appear.
Treatment of these patients targets prevention of life-threatening oral complications. The greatest risk of infection occurs during extreme immune-suppression periods, therefore, treatment must be conservative and palliative.
Teeth with unfavorable diagnosis are extracted. Deep debridement of remnants is conducted so as to minimize bacterial load. Dental professionals must stress the importance of oral hygiene.7
In order to avoid secondary infections, 8 mouthwashes with antimicrobial agents such as.12% chlorhexidine are recommended, especially in patients showing mucositis.
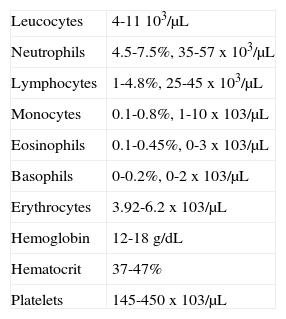
BLOOD COUNTWhen handling hematologic diseases, blood count is essential; it represents a diagnostic aid. Blood counts include: platelet, leukocyte, erythrocyte, hemoglobin and hematocrit counts as well as determining number, variety percentage concentration and quality of blood cells. It is therefore essential to know the normal values of each cell group so as to ascertain at which moment a safe and complication-free oral rehabilitation can be undertaken.
Data considered normal at the Mexico's Children s Hospital «Federico Gómez» are the following:
When dealing with patients suffering from hematologic disorders it is compulsory to know how NAN (neutrophil absolute number) can be obtained.
Therefore:
> Than 2,000 NAN x mm3 ---- patient can be treated without need to administer prophylactic antibiotics.
1,000 to 2,000 NAN x mm3 ---- prophylactic antibiotics must be administered for any type of dental procedure.
< 1,000 NAN x mm3 patients with severe immunesuppression, must only be treated with palliative care.
In cases of thrombocytopenia, platelet replacement must be considered in cases when platelet count is lesser than 80.000 per mm3.5
CLINICAL CASE PRESENTATIONFemale patient, 15 years old, weighing 30.200kg and measuring 134cm. Post-natal history revealed that patient suffered fetal distress, was in incubator for the first month of her life, was not breast-fed, and suffered speech problems as well a multiple respiratory infections during childhood.
Familial history revealed the following: 35 year old mother, apparently healthy, 6 year old brother, 34 year old father with history of cyclic neutropenia, maternal grandmother deceased due to dropsy.
The patient reported that she was admitted for the first time at the Mexico's Children's Hospital «Federico Gómez» in August 1994, due to a two-month evolution, persisting cough. A series of possible diagnoses were presented to the family. The list presented was the following:
- •
At 6 years of age, the patient was diagnosed with toxic neutropenia and speech disorders.
- •
At 7 years of age, a physiological murmur was detected, with presence of peri-anal abscesses and mild anemia.
- •
At 8 years of age, chronic neutropenia data were observed.
- •
At 9 years, alongside the possible diagnosis of chronic neutropenia, signs of mild, non decompensated anemia were observed.
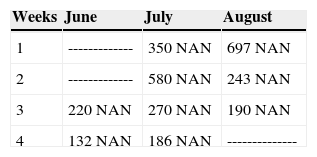
- •
At 10 years of age, real diagnosis is verified with the help of laboratory studies: 10 blood counts were taken every week taking special care of assessing total neutrophil numbers. In all executed blood counts, the patient exhibited an amount of neutrophil absolute numbers (NAN) which was below normal indexes (Table 1). Clinically, it was additionally found that the patient suffered from urinary incontinence and 2nd degree malnutrition. From that point onwards, the patient has been treated at the Hospital's Hematology Department with «granulocyte colonies stimulating factor» (G-CSF), approximately every month to the present date, as long as her financial circumstances would allow it.
- •
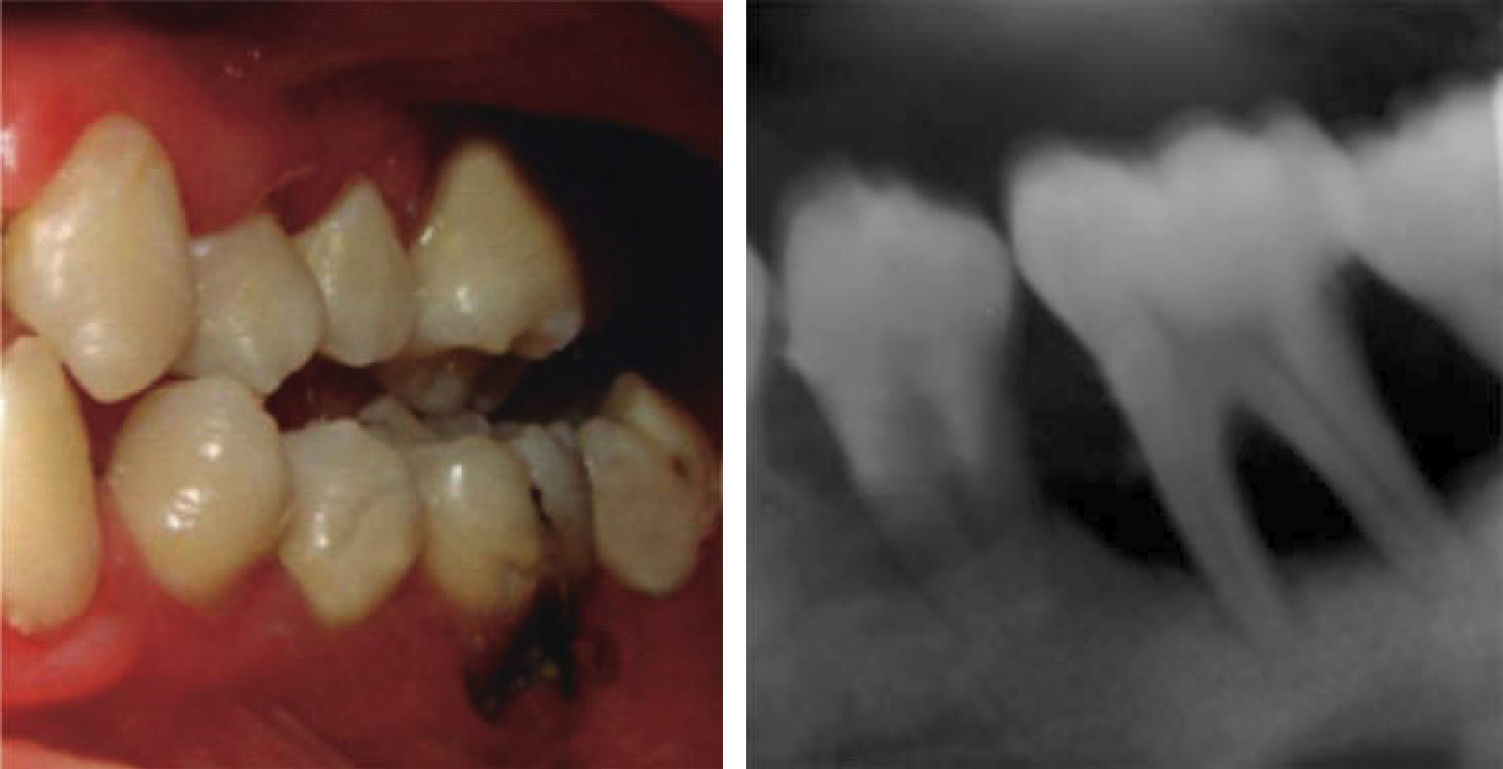
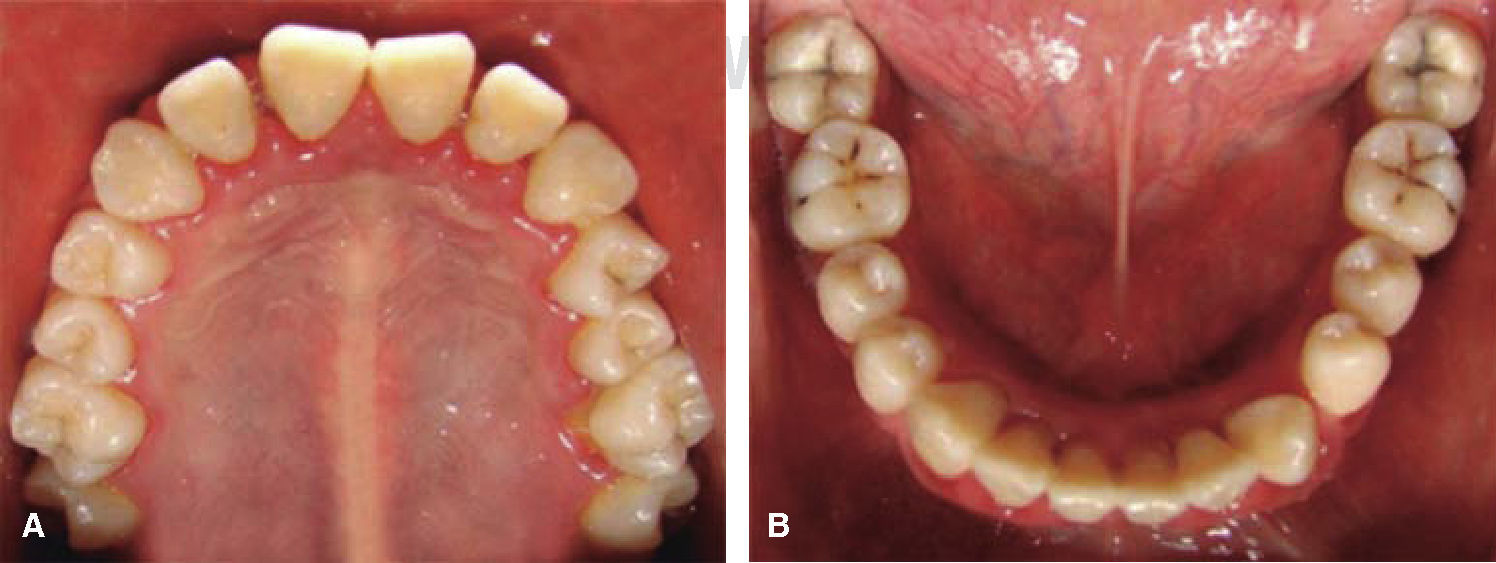
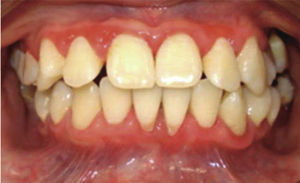
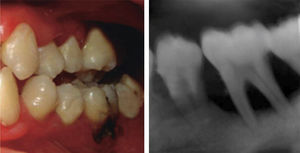
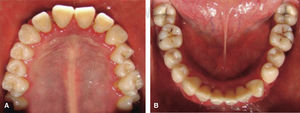
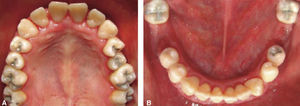
At 15 years of age, the patient attended the Pediatric Stomatology Department having been referred by the Hematology Department of the Mexico's Children's Hospital «Federico Gómez». The patient's mouth was clinically and radiographically examined, said examination revealed the following: generalized gingivitis associated to dental biofilm, periodontitis, soft tissues with suitable hydration, color and vascularization, appropriate frena implantation, symmetrical, continuous and whole alveolar processes, permanent dentition with dental malposition, presence of inter-dental spaces (Figure 1), bone resorption (Figure 2) different degree caries in teeth number 17, 16, 15, 14, 24, 25, 26, 27, 37, 36, 35, 46 and 47 (Figure 3), angular cheilitis, whitish lesions in progression, recording high caries risk index according to Tinanoff's6 assessment criteria.
Inter-consultation with the Hematology Department was undertaken. Due to the presence of pain experienced by the patient, it was decided to simultaneously conduct hematological and stomatological treatments.
Therefore, a series of 7 blood counts (hematic biometry) was programmed, they should be taken from one day before rehabilitation initiation up to two days after completing it, so as to assess total neutrophil counts.
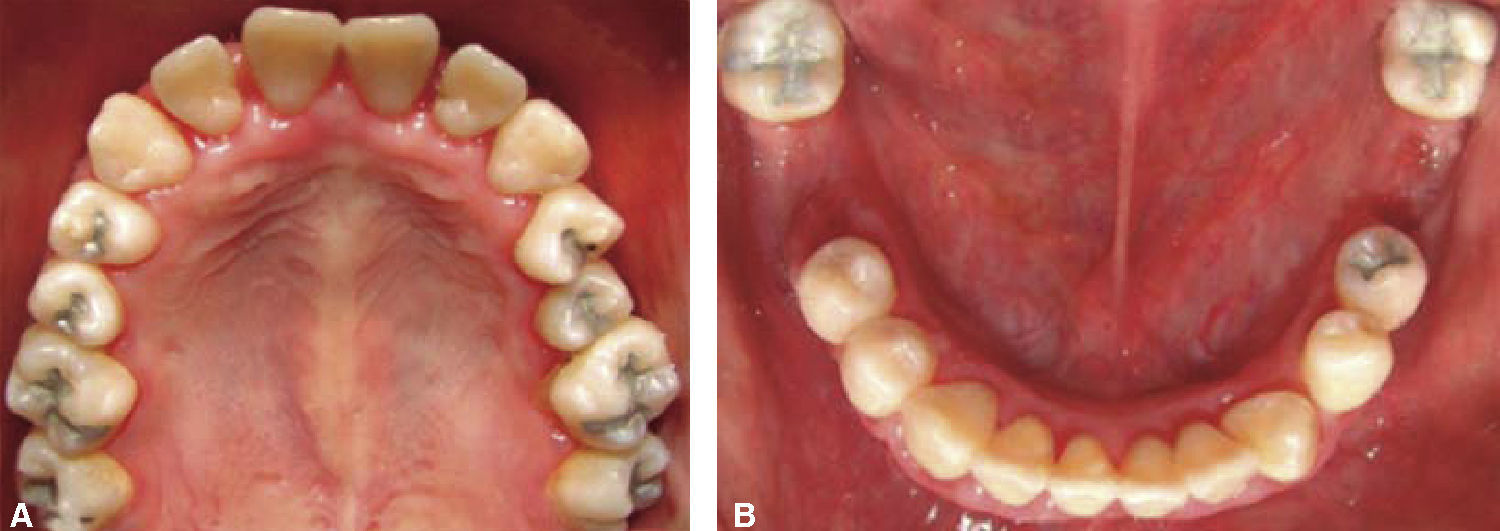
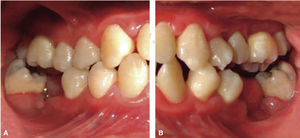
Drug selected by the Hematology Department was administered 48hours before oral treatment (Figure 4). The first blood count revealed neutropenia data (460 total neutrophil). For this reason it was decided to initiate oral rehabilitation on the next day.
The next day a new blood count was taken which revealed 4200 total neutrophil, therefore, it was decided to initiate dental treatment. This treatment was divided into three phases:
- •
Phase I: hygiene preventive program: preventive hygiene measures were taken such as plaque control, brushing after all meals, with toothpaste containing 1,000 to 1,500ppm fluoride, topical applications every 3 months since the patient was considered at high risk to suffer caries (modified Stillman technique). Use of dental floss was implemented as well as scheduled meals, decrease of carbohydrates and refi ned sugars ingestion, as well as avoiding snacks between meals.
- •
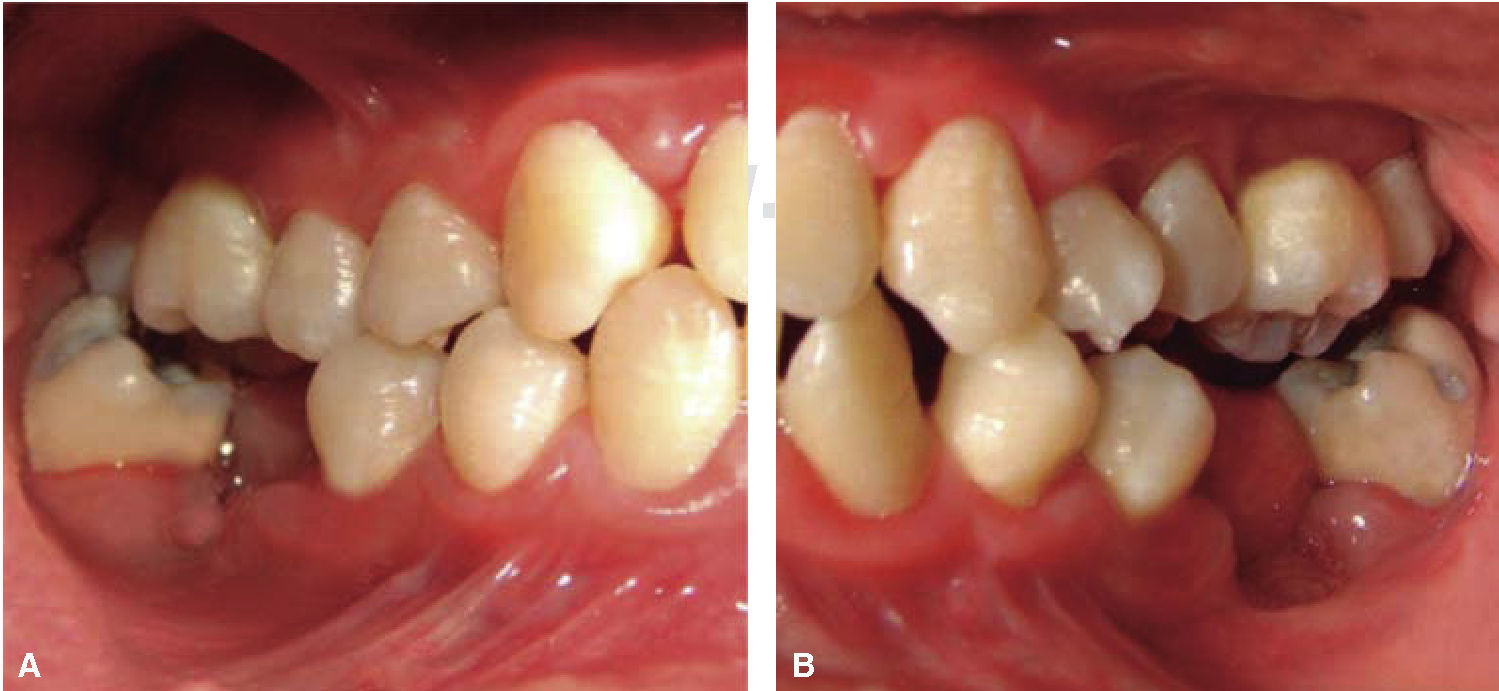
Phase II: restorative therapy: white lesions were monitored, cavity and progress lesions were restored. An aggressive treatment was followed to minimize caries progression, using 2% lidocaine with epinephrine as the dose recommended by Dr. Stanley Malamed's in «Clinical action of specific agents. Handbook of local anesthesia» (4.4 mg/kg) administering total dose of 36mg at every appointment.6 Thus, oral rehabilitation consisted on amalgam fillings in teeth 17, 16, 15, 14, 24, 25, 26, 27, 37, 35, 47, as well as extraction of teeth 46 and 36. With behavior handling techniques, the most recent notes of the Hematology Department were consulted, personally inter-consulting with the treating pediatric hematologist. Review of daily blood count results (total neutrophil count), assessment of «granulocyte colonies stimulation factor (G-CSF)» applied at the Hematologic Department, as well as oral hygiene care and use of chlohexidine mouthwash. On extraction day antibacterial prophylaxis was prescribed, amoxicillin, 50mg/kg one hour before treatment initiation so as to avoid formation of infectious processes or any other post-operative complication. The full restorative procedure was completed in three short appointments (Figures 5 and 6).
- •
Phase III: multi-disciplinary care: additionally to being in constant communication with the Hematology Department, the case was also treated within the Periodontics Department so as to control infectious processes and implement suitable oral hygiene techniques.
The Hospital's Nutrition Department contributed with dietary advice.
CONCLUSIONSHematologic disorders represent one of the most interesting problems to be considered by the dentist in his everyday practice. Susceptibility to infectious processes makes them a special group to be carefully considered in order to avoid post-operative complications. Research of an hematologic disorder requires careful laboratory and clinical studies.
According to scientific literature, there are two types of leukocyte disorders: leukocytosis and leukopenia, which compromise the body's defense system.
There are additionally many other causes for the decrease of leukocytes (4-11 mm3) in the blood. A patient afflicted with severe leukopenia is susceptible to infections and periodontal disease. These potential problems can become severe when related to dental treatment. In order to avoid these complications, it is important to possess a serial count of white blood cells, and thus, select the appropriate moment in the cycle when the count is very close to normal. It is additionally important to know how to prescribe antibiotic prophylaxis in necessary cases.
Pediatric stomatologists, as members of the multi-disciplinary team in the treatment of medically compromised patients, must understand the protocol care for children with hematological alterations, in this case, white blood cells:
- 1)
Identify the disease and its signs and symptoms.
- 2)
Inter-consult with treating hematologist to ascertain the patient's comprehensive current circumstances.
- 3)
Identify potential problems which might occur before, during or after treatment.
- 4)
Determine antibacterial prophylactic regime.
- 5)
Review total neutrophil numbers in blood counts taken before, during and after dental treatment.
- 6)
If there are emergency treatments to be undertaken, when the patient is at the lowest stage of total neutrophil count in his cycle, use of acetylsalicylic acid and non steroid anti-inflammatory drugs must be avoided, since the patient will probably exhibit evidence of thrombocytopenia. Nevertheless, paracetamol (with or without codein) can be used palliatively as well as Philadelphia solution or chlorhexidine rinses during the patient's oral hygiene technique until circumstances improve.
When following step-by-step the stomatological handling of patients with hematologic disorders, we decided to treat with all confidence the 15 year old female patient. It is worth mentioning that, in spite of counting with guides in literature such as assessing the patient's present circumstances, possessing a recent blood count as well as prophylactic antibiotic scheme, in the present case there were a series of hematological alterations, which caused delay in the oral rehabilitation process. Once the patient's total neutrophil count was controlled, it was decided to continue treatment. Satisfactory results were thus obtained, and, what is more important, the patient's life was not compromised.
A comprehensive, safe, and complication-free rehabilitation will be achieved when clinicians can identify emergency oral treatments and are able to solve them as part of a multi-disciplinary team, especially with a hematologic specialist.
It is important to bear in mind that these patients have received poor oral and dental care, since there are no specific prevention guides or protection programs. Post-operative control as well as follow-up appointments are extremely important to maintain the patient under control.
Mexico Children's Hospital «Federico Gómez»