Transforming growth factor β3 (TGF-β3), and receptor III of transforming growth factor β (TGFβ-RIII), regulate epithelial mesenchymal interactions. The lack of TGF-β3 and TGFβ-RIII expression causes defects in palatal fusion.
ObjectiveTo observe the immunoexpression of TGF-β3 and TGFβ-RIII in pediatric patients with non-syndromic complete or incomplete cleft palate (CP).
Material and methodsThe study design was observational, descriptive, prospective and transversal. Samples were taken from 20 complete and incomplete CP patients from five to 28 months of age. Samples of mucoperiostium were removed from the cleft in the palate during palatoplasty. In order to perform immunohistochemistry, samples were processed with VENTANA BenchMark Ultra equipment, using TGF-β3 and TGFβ-RIII antibody. Immunoexpression was evaluated by means of global cell impression.
ResultsTGF-β3 immunoexpression was greater in epithelial cells than in fibroblasts of patients with complete and incomplete CP; however, TGFβ-RIII immunoexpression was greater within the fibroblasts than in epithelial cells of patients with complete cleft palate.
ConclusionsIt was concluded that the lack of TGFβ-RIII expression in the epithelium may be related to the lack of fusion of the palatal shelves. It would be of great interest to perform a more in-depth analysis of the expressions of TGF-β3 and TGFβ-RIII within different cell populations.
El factor de crecimiento transformante β3 (TGF-β3) y el receptor III del factor de crecimiento transformante β (TGFβ-RIII), regulan las interacciones epitelio-mesenquimales. La falta de expresión de TGF-β3 y del TGF-βRIII provoca defectos en la fusión palatina.
ObjetivoObservar la inmunoexpresión del TGF-β3 y del TGF-βRIII en pacientes pediátricos con paladar hendido (PH), completo o incompleto no sindrómico.
Material y métodosEl diseño de estudio fue observacional, descriptivo, prospectivo y transversal. La muestra consistió de 20 pacientes con PH completo e incompleto, de cinco a 28 meses de edad. Se tomaron muestras del mucoperiostio de la hendidura palatina durante la palatoplastia. Para realizar la inmunohistoquímica, las muestras se procesaron en un aparato VENTANA BenchMark Ultra, con el anticuerpo TGF-β3 y TGFβ-RIII. Se valoró su expresión, mediante impresión global celular.
ResultadosLa inmunoexpresión del TGF-β3 fue mayor en las células epiteliales que en los fibroblastos de pacientes con PH completo e incompleto; sin embargo, la inmunoexpresión del TGFβ-RIII fue mayor en los fibroblastos que en células epiteliales de pacientes con paladar hendido completo.
ConclusionesSe concluyó que la falta de expresión del TGFβ-RIII en epitelio, podría tener relación con la falta de fusión de las crestas palatinas. Sería de gran interés realizar un análisis más profundo de la expresión de TGF-β3 y TGFβ-RIII, en distintas poblaciones celulares.
During palatogenesis, the expression of transforming growth factor β1 (TGF-β1) and TGF-β2 accelerates the merging of the palatal processes;1,2 while TGF-β3, which is synthesized by mesenchymal cells, contributes to the dispersion of the epithelium of the medial edge (EME) during palatal fusion.3–5 It also regulates the interactions between epithelium and mesenchymal cells involved in the formation of the palate, skin, teeth, glands, among other organs,6–9 so that the failure of both of TGF-β as well as bone morphogenetic protein (BMP) leads to a variety of craniofacial deformities including cleft palate.10
On the other hand, the type III receptor, also called β-glycan, is distributed in epithelial and mesenchymal cells; it can bind to all isoforms of TGF-β,11 which can be anchored to the membrane or secreted in a soluble form.12–14 The loss of TGFβ-RIII prevents the elongation of the palatal process and its elevation, which causes the cleft.15 This is in part because of a reduction in the expression of genes TGF-β and BMP type I receptor, causing a suppression of the signaling for TGFβ-RIII, which affects the balance of such signaling pathways for adequate tissue formation.16
In some studies conducted to evaluate palotogenesis by means of immunohistochemistry in murine samples in vivo and in vitro, it was determined that the expression of TGF-β3, and TGFβ-RIII17 in the palate occurs when the crests present a vertical orientation. This immunoexpression was located in epithelial cells.
Epithelial distribution was preserved during the elevation of the palatal crests and destruction of the medial epithelium. After palatal fusion, we observed a low expression of TGF-β3,17–22 so that some previous studies showed inhibition of the signaling for activation of TGF-β3 in murine samples taken from the palate.23,24 However, mice lacking expression of TGF-β3 showed defects in palatal fusion, so that the expression of TGF-β3 is strictly limited to the epithelium of the palatal midline before fusion.25–29
With regard to the receptor, in an experimental study in mice, it was demonstrated that the expression of TGFβ-RIII during palatogenesis occurs through the epithelium, and is located specifically in the epithelium of the edge during fusion of the palatal crests.30–32 In addition, the lack of expression of TGFβ-RIII leads to an alteration in the proliferation and induction of apoptosis, as well as endothelial affection and reduction of mesenchymal cells, which affects the development of osteoblasts.33
The objective of this study was to determine the immunoexpression of TGF-β3 and TGFβ-RIII in a sample of tissue from the cleft of child patients with complete or incomplete non-syndromic cleft palate, not treated with palatoplasty.
Prior to taking the sample, the informed consent was signed by the parents or guardians of each patient and the process of obtaining the sample was approved by the Research Ethics Committee of the General Hospital «Dr. Manuel Gea González».
MATERIAL AND METHODSSample selectionThe sample consisted of 18 patients who had complete cleft palate and two patients with incomplete cleft palate; both were non-syndromic. Eight patients were female and 12 male, from five to 28 months of age, all of them scheduled for palatoplasty for the first time.
During the study we proceeded to collect a sample of tissue at the surgical procedure, which was obtained by the plastic surgeon in charge, who provided the tissue without involving an additional risk for the health of the patients.
The tissue was taken from the palatal mucosa at the site of the cleft, with a size of 0.3 to 0.5 cm. The samples were fixed with formalin in a phosphate buffer solution at 10% (MEYER) and stored at 4 oC for 8 hours. Afterwards, they were placed in a PBS solution.
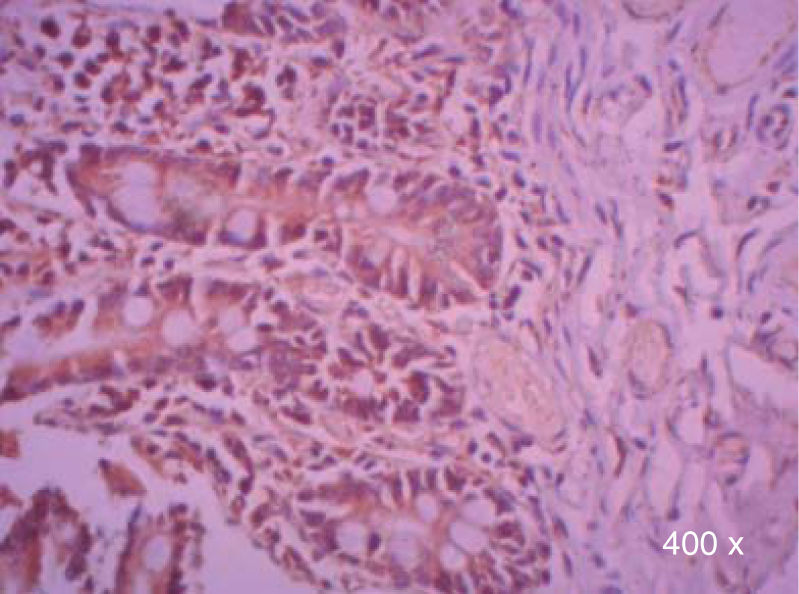
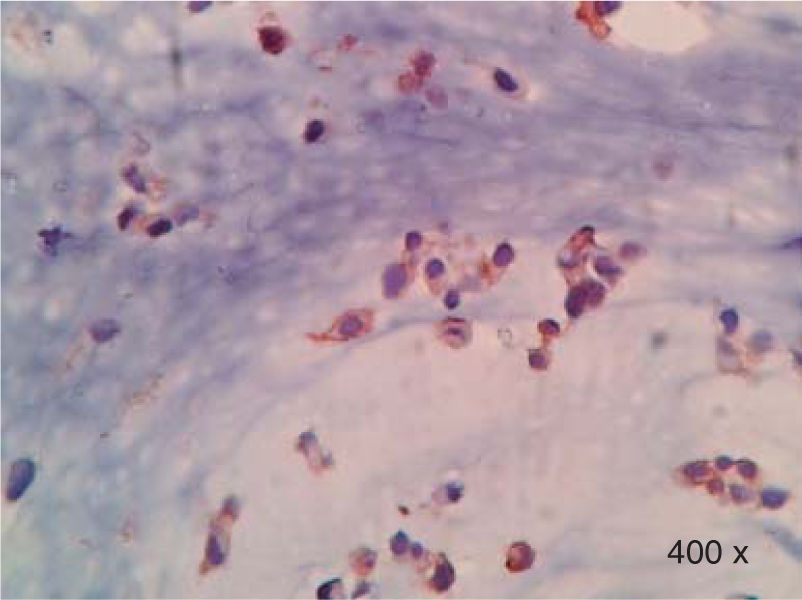
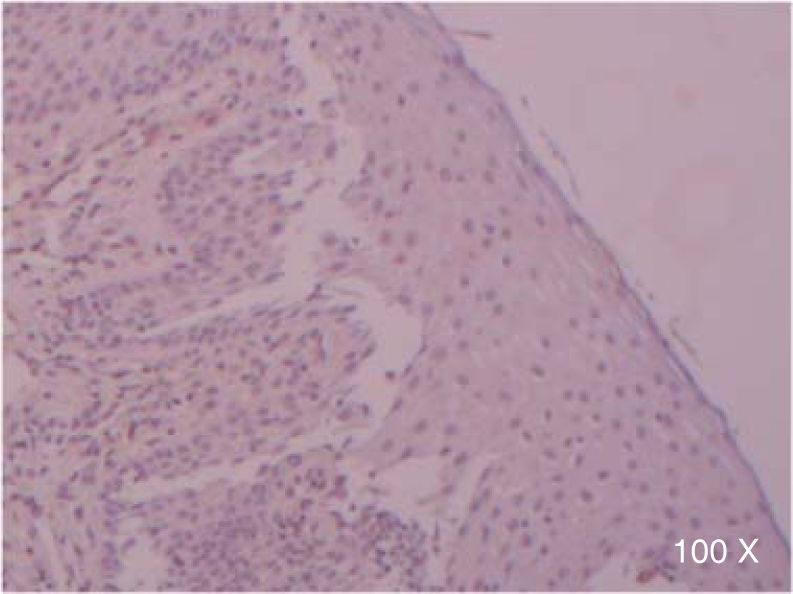
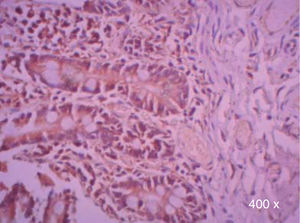
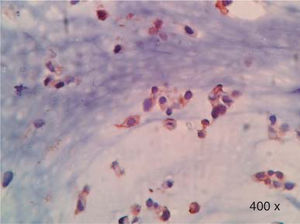
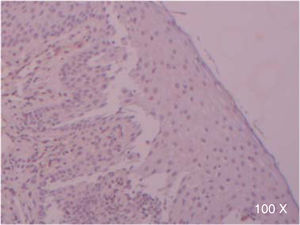
Preparation of the immunohistochemistry for TGF-β3 and TGFβ-RIIIHistological sections were performed for each sample, of 3μ each, with their respective control. The TGF-β3 antibody was previously tested in samples of small intestine (Figure 1), while the antibody for TGFβ-RIII was tested previously in samples of the HepG2 cell line (Figure 2), and human being pancreas.
The tissue samples were left to dry and de-paraffin at 60 oC overnight. Immunohistochemistry was performed in a VENTANA BenchMark Ultra machine.
For the TGFβ-RIII primary monoclonal antibody (ab78421, abcam), the UltraView detection system was used (Reference 760-500, LOT E06611, expiration date 20-09-2016). The option of de-waxing at 72 oC was selected, cellular conditioning (CC1) was conducted for 20 minutes and the primary antibody was incubated 20 minutes at 36 oC.
For the TGF-β3 polyclonal antibody (SC-82, Santa Cruz Biotechnology, Inc.), an OptiView detection system (Reference 760-700, Lot F08259, expiration date 28-10-2017) was used. The option of de-waxing at 72 oC was chosen with cell conditioning (CC1) for 16 minutes. The antibody was incubated for 12 minutes at 36 oC.
Evaluation of the expression of TGF-β3 and TGFβ-RIII by immunohistochemistryTissue observation was performed with a binocular microscope (Axio Lab. A1). The images were taken with a ZEISS Axiocam ERc 5s camera.
Monitoring and evaluation was performed by an expert pathologist who assessed the presence or absence of expression of TGF-β3 and TGFβ-RIII in the samples through the overall impression of the cells that were stained at different areas of the evaluated cleft tissue.
Statistical analysisThe information was analyzed using the SPSS v20.0 statistical package. Descriptive statistics was used. For qualitative variables, percentages were used and for quantitative variables, central tendency and dispersion measurements were used.
χ2 test was performed for nominal variables to compare the expression of TGF-β3 and TGFβ-RIII with complete or incomplete cleft palate.
The level of significance was established at p < 0.05.
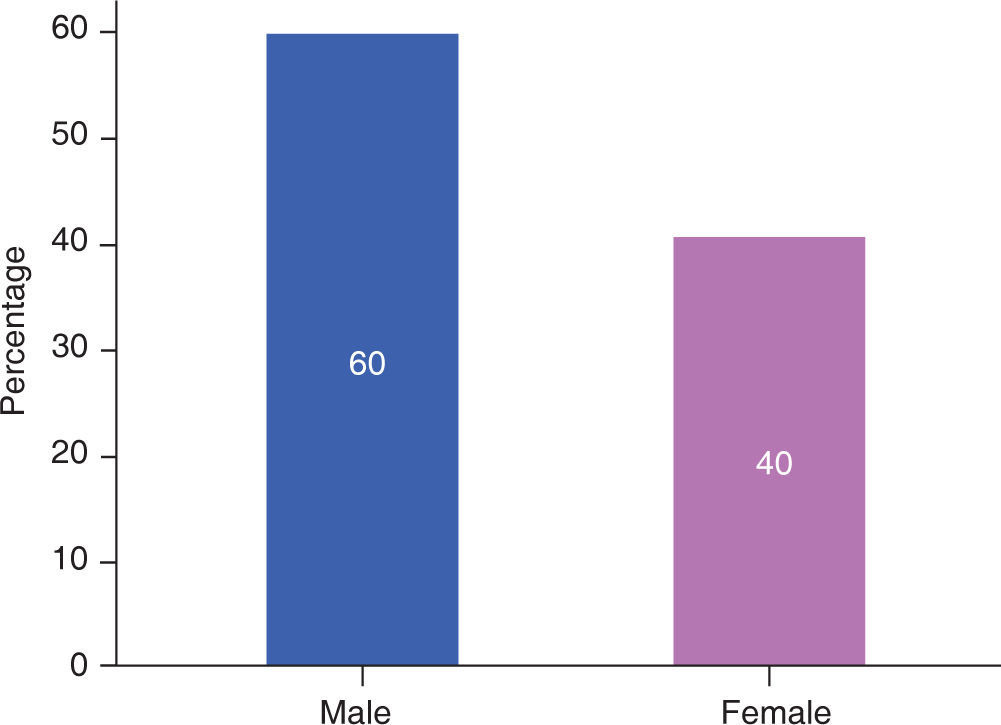
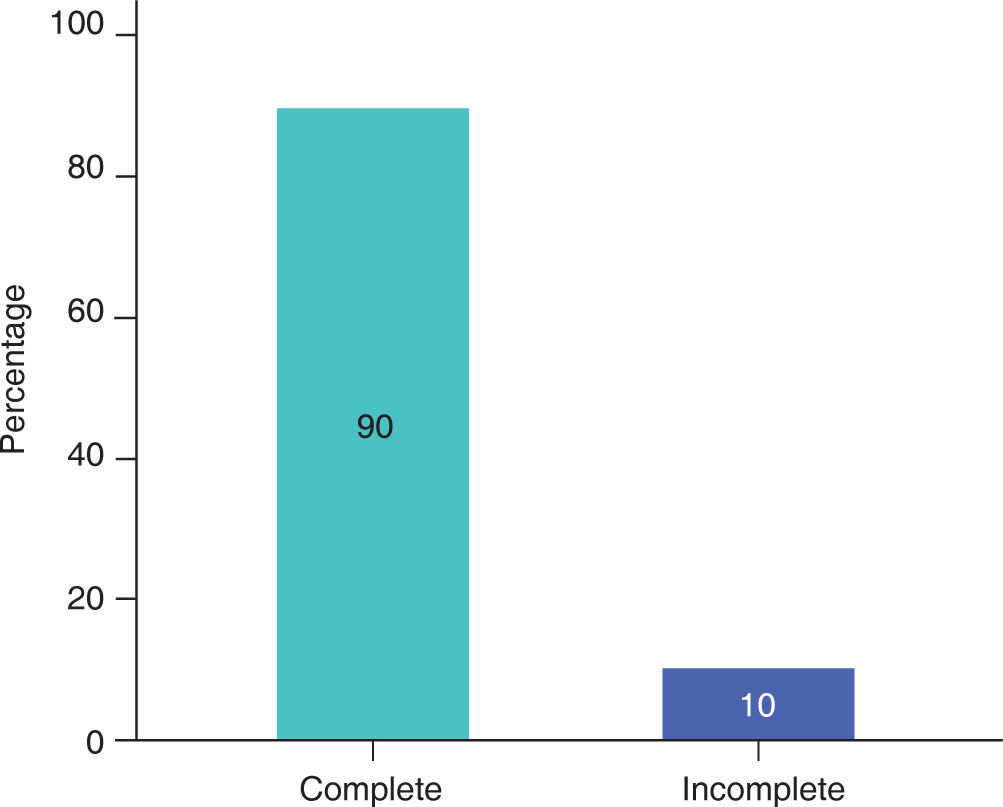
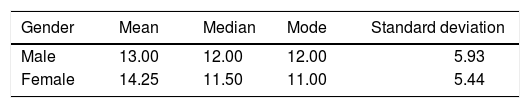
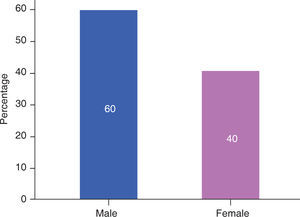
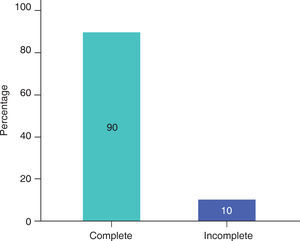
RESULTSIn this study, male patients corresponded to 60% (Figure 3) of the sample with an average of 13.00 ± 5.93 months of age (Table I); whereas the female patients corresponded to 40% (Figure 3), with an average of 14.25 ± 5.44 months of age (Table I). Ninety percent of the cases had a complete cleft palate and 10%, incomplete cleft palate (Figure 4).
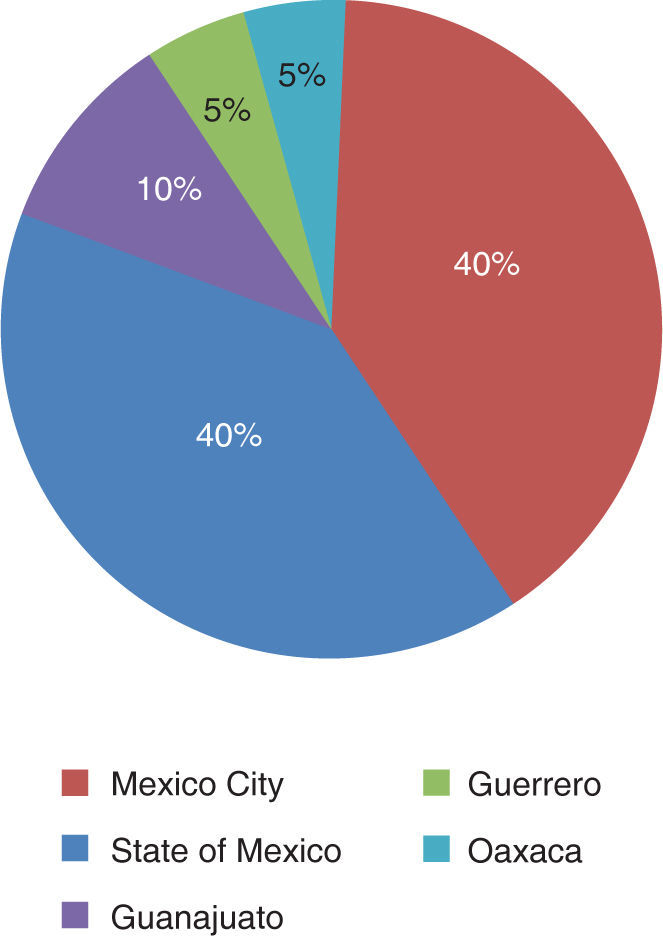
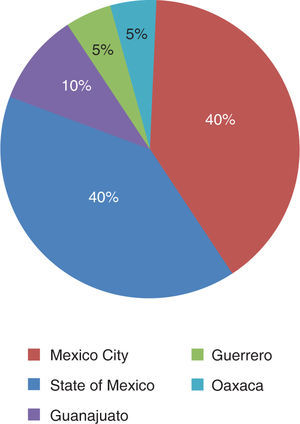
The patients came from the States of Mexico (40%), Mexico City (40%), Guerrero (10%), Guanajuato (5%) and Oaxaca (5%) (Figure 5).
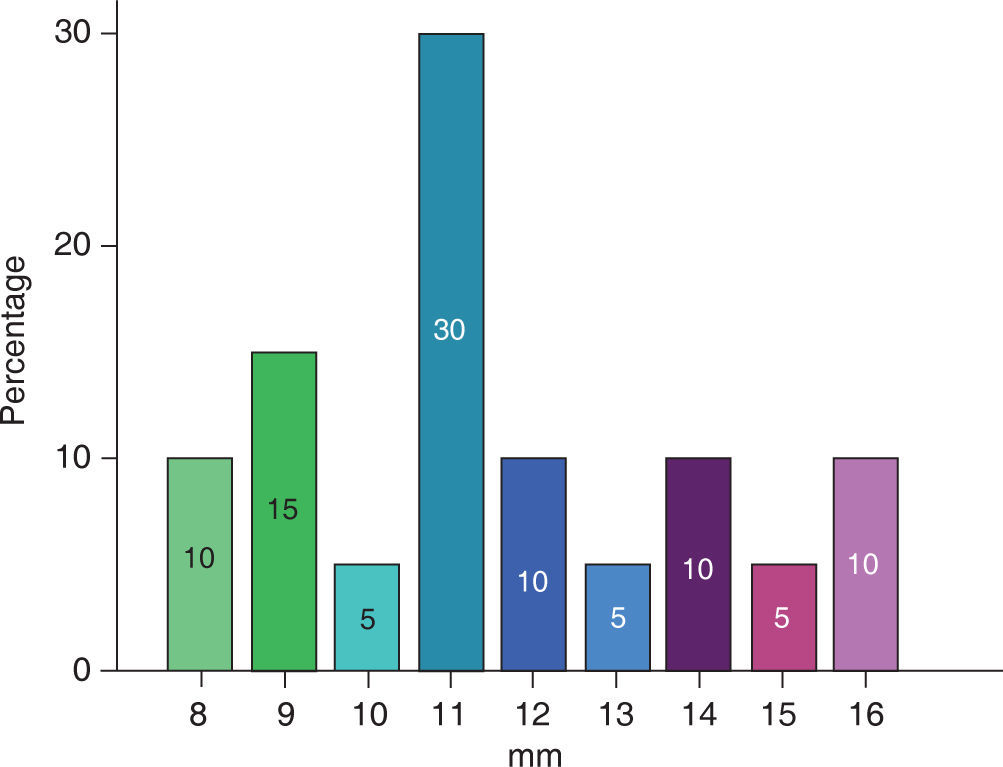
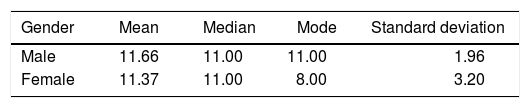
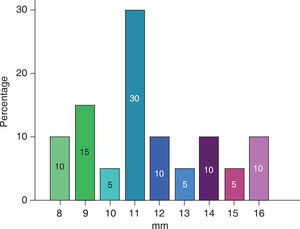
The extent of the cleft palate was 8 to 16 mm (Figure 6), the mean for male patients was 11.66 ± 1.96 mm and in female patients of 11.37 ± 3.20 mm (Table II).
A bigger cleft size was observed in the samples of complete cleft palate (11.83 ± 2.40), than in incomplete cleft palate (9.00 ± 1.41).
When comparing the frequency with which TGF-β3 and TGFβ-RIII were expressed, the following information was found:
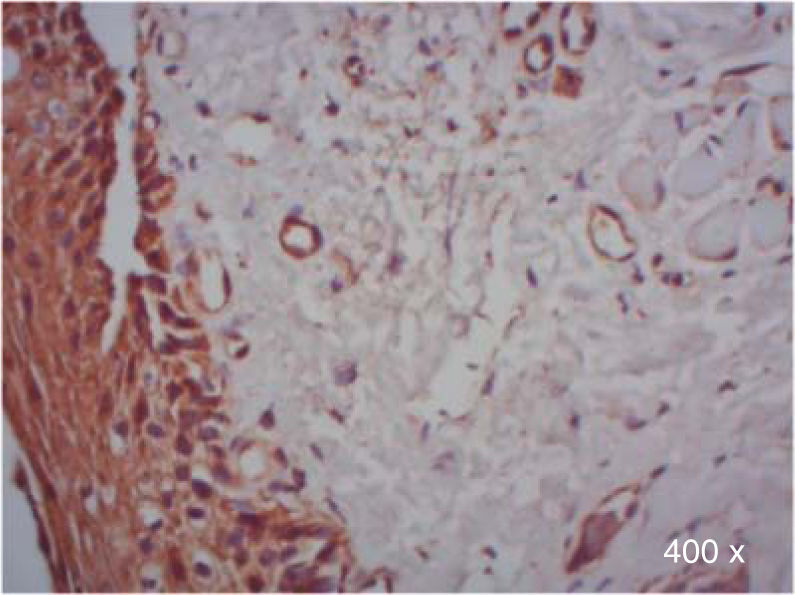
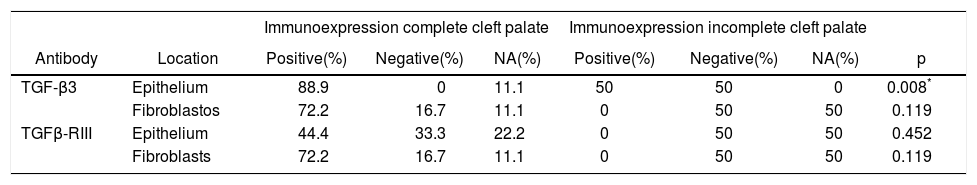
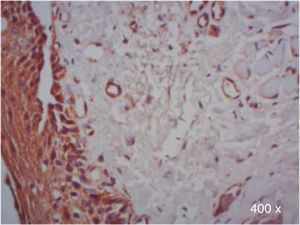
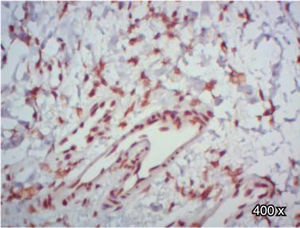
A higher positive expression of TGF-β3 was observed in the membrane and cytoplasm of epithelial cells than in the membrane and cytoplasm of fibroblasts in samples of complete and incomplete cleft palate (Table IIIandFigures 7and8).
*χ2 test. Expression of TGF-β3 and TGFβ-RIII in epithelial cells and fibroblasts of complete and incomplete cleft palate. A higher positive expression of TGF-β3, was observed in epithelium and more positive expression of TGFβ-RIII in fibroblasts.
| Antibody | Location | Immunoexpression complete cleft palate | Immunoexpression incomplete cleft palate | p | ||||
|---|---|---|---|---|---|---|---|---|
| Positive(%) | Negative(%) | NA(%) | Positive(%) | Negative(%) | NA(%) | |||
| TGF-β3 | Epithelium | 88.9 | 0 | 11.1 | 50 | 50 | 0 | 0.008* |
| Fibroblastos | 72.2 | 16.7 | 11.1 | 0 | 50 | 50 | 0.119 | |
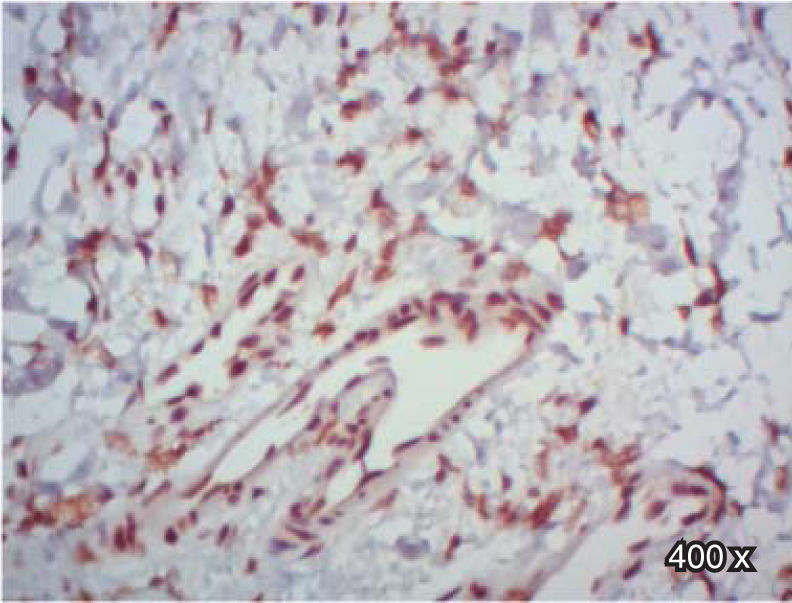
| TGFβ-RIII | Epithelium | 44.4 | 33.3 | 22.2 | 0 | 50 | 50 | 0.452 |
| Fibroblasts | 72.2 | 16.7 | 11.1 | 0 | 50 | 50 | 0.119 | |
In regard to TGFβ-RIII, we observed a higher positive expression in the membrane and cytoplasm of fibroblasts than in the membrane and cytoplasm of epithelial cells in complete cleft palate. In samples of incomplete cleft palate, there was a negative staining for TGFβ-RIII (Table IIIandFigure 9).
When performing the χ2 test, a p = 0.008* was observed in cases of complete and incomplete cleft palate, with expression of TGF-β3, which could indicate a difference between study populations in terms of type of cleft palate, and therefore, it would be important to increase the number of samples of incomplete cleft palate in subsequent studies (Table III).
DISCUSSIONThis study determined the immunoexpression of TGF-β3 and TGFβ-RIII in a tissue sample from complete and incomplete clefts of male and female patients who underwent palatoplasty at the General Hospital «Dr. Manuel Gea González». This expression was evaluated by means of immunohistochemistry in the membrane and cytoplasm of epithelial cells and fibroblasts of the cleft palate tissue in patients within the first three years of age. This study represents one of the first performed in humans after the embryonic period. When comparing age between genders, no significant differences were found, due to the selection protocol to perform the cleft palate surgery conducted by the Department of Plastic and Reconstructive Surgery.
On the other hand, more samples from patients with complete cleft were assessed in comparison with those from incomplete cleft patients. Besides, there was no significant difference in cleft width between female and male patients. This was probably due to the fact that patients received presurgical orthopaedic treatment prior to the palatoplasty.
During the evaluation of TGF-β3 samples in cleft palate, positive expression was observed in all epithelial cells, which is consistent with the data observed in murine samples.17–22
In addition to squamous epithelium corresponding to the palate, epithelium of the respiratory type was detected due to the pathological anatomy resulting from the lack of fusion of the palatine crests, which allows the connection between the oral and nasal cavity.34
It was noted that the squamous and respiratory epithelium was positive in the majority of cases with complete cleft palate, which is consistent with what was previously reported in humans, indicating that moderate expression of TGF-β3 has been detected in respiratory epithelium cells.35
The comparison of the expression between cases of complete and incomplete cleft palate was not adequate from the statistical point of view, however, when observing the expression of TGF-β3 in the cytoplasmEste documentoof completees elaboradoandporincompleteMedigraphiccleft palate epithelial cells, there was a trend toward an increased positive expression of TGF-β3 in the epithelium of complete cleft palate.
On the other hand, upon evaluation of TGFβ-RIII expression in epithelial cells of complete and incomplete cleft palate, there was a trend toward a lower positive expression in epithelium when compared with the expression of TGF-β3. This finding differs from those of previous studies,30–32 in which it was showed that the expression of TGFβ-RIII during palatogenesis occurred in the epithelium.
This difference between our data and those reported in the literature, might suggest that there is a failure in signaling during inhibition or reduction of the expression of TGFβ-RIII in epithelial cells.
However, the expression of TGF-β3 and TGFβ-RIII in fibroblasts, showed no differences.
On the other hand, this study does not coincide with those performed on murine samples, because in spite of both being mammals species,36 in humans it is observed in the majority of cases a positive inmunoexpresion; while in murine samples the absence of inmunoexpresion of both TGFβ3 and TGFβ-RIII16,23,24,37 has been detected. This could be due to the difference in pathophysiology between human and mouse.
In addition to the abovementioned, the expression of TGF-β3 and TGFβ-RIII could be related to the patient's age, so age might represent another variation in the results found in mice. It would be necessary to assess the expression of TGFβ3 and TGFβ-RIII in control samples of healthy patients, from 0 to 3 years of age.
In subsequent studies it would be interesting to explore the differences between these types of cleft palate and cells that express TGF-β3 and TGFβ-RIII therefore it would be necessary to increase the number of samples of incomplete cleft palate.
The results of the present study are all related to what has been previously reported for the placement of grafts in patients with palatal fissures because by using platelet-rich plasma, at the time of clot formation, they might release platelet-derived growth factors such as the vascular endothelial growth factor, type β transforming growth factor, epidermal growth factor, fibroblast growth factor, among others, which would induce the expression of TGFβ-RIII at epithelial level38–40 and thus strengthen the bone graft, so this is one more reason to increase research on the subject.
CONCLUSIONSAfter performing the immunohistochemistry of TGFβ3 and TGFβ-RIII, it was concluded that the absence of expression of TGFβ-RIII in the epithelium of complete and incomplete cleft palate, could be related to the lack of fusion of the palatine ridges.
It would be of great interest to continue to analyze in greater depth their expression in more and different cell populations besides fibroblasts and epithelial cells.
To all the staff of the Division of Stomatology-Orthodontics and the Division of Plastic and Reconstructive Surgery, General Hospital «Dr. Manuel Gea González» who facilitated the collection of samples. To the staff of the Department of Molecular Pathology and Immunohistopathology of the National Institute of Cancerology for all their contributions, availability, and lessons learned during the project. Mainly I would like to thank Q.F.B. Guadalupe Moncada Claudio, and Q.F.B. Maria de Lourdes Peña Torres for their great assistance for the realization of the immunohistochemistry. I also thank the Institute of Cellular Physiology, UNAM, mainly M.S. Tonatiuh Molina Villa for his contribution and support for the realization of cell cultures, which are indispensable for the achievement of the controls.
This article can be read in its full version in the following page: http://www.medigraphic.com/ortodoncia
Resident of the Division of Orthodontics and Pediatric Dentistry, General Hospital «Dr. Manuel Gea González»
Deputy Director of Pathology and in Charge of the Department of Molecular Pathology and Immunohistopathology of the National Institute of Oncology
Orthodontics visiting Professor at General Hospital «Dr. Manuel Gea González». Professor at the Metropolitan Autonomous University