To compare the pulmonary function of young adults with Down syndrome (DS) with healthy subjects.
MethodsThirty-four young adults (17 with DS and 17 apparently healthy controls), aged 20–40, participated in this study. Anthropometric variables and lung function, namely forced expiratory volume in one second (FEV1), forced vital capacity (FVC), peak expiratory flow (PEF), and the fraction of FVC expired in one second (FEV1/FVC%), were assessed in both groups.
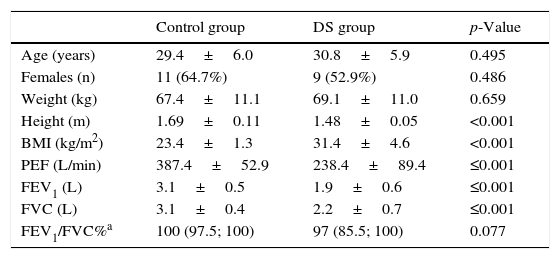
ResultsThe group of young adults with DS had a lower height and higher body mass index (31.4±4.6 vs. 23.4±1.3kg/m2, p<0.001). As regards pulmonary function, the group of participants with DS showed significantly lower values for PEF (238.4±89.4 vs. 387.4±52.9L/min, p≤0.001), FVC (2.2±0.7 vs. 3.1±0.4L, p≤0.001) and FEV1 (1.9±0.6 vs. 3.1±0.5L, p≤0.001), when compared to subjects of the control group. No changes were observed in FEV1/FVC%. An inverse correlation was observed between the body mass index and the PEF (r=−0.691, p<0.001), the FVC (r=−0.555, p=0.001), and the FEV1 (r=−0.617, p<0.001).
ConclusionYoung adults with DS showed reduced pulmonary function in comparison to age-matched controls. Additionally, the pulmonary function was inversely correlated with body mass index.
Comparar la función pulmonar entre adultos con Síndrome de Down (SD) y adultos sanos.
MétodoParticipan en el estudio 34 adultos jóvenes (17 con SD y 17 controles sanos) con edades comprendidas entre los 20 y 40 años. Se registran en ambos grupos variables antropométricas y de función pulmonar: volumen espiratorio forzado en un segundo (FEV1), capacidad vital forzada (FVC), flujo espiratorio máximo (PEF) y la relación FEV1/FVC.
ResultadosEl grupo con SD presentaron un mayor peso e índice de masa corporal (IMC) que el grupo control (31,4±4,6 vs. 23,4±1,3kg/m2, p<0,001). Se registraron valores significativamente menores en la función pulmonar de los sujetos con SD que en la del grupo control: PEF (238,4±89,4 vs. 387,4±52,9L/min, p≤0,001), FVC (2,2±0,7 vs. 3,1±0,4L, p≤0,001) and FEV1 (1,9±0,6 vs. 3,1±0,5L, p≤0,001). No se observo diferencias entre grupos en el FEV1/FVC. Se observó una correlación inversa entre el IMC y el PEF (r=-0,691, p<0,001), la FVC (r=-0,555, p=0,001) y la FEV1 (r=-0,617, p<0,001).
ConclusiónLos adultos con SD muestran una reducción de la función cuando se les compara con controles de su misma edad. La función pulmonar correlaciona inversamente con el IMC.
Down syndrome (DS) is characterised by multiple malformations, cognitive impairment and medical issues due to the presence of extra genetic material from chromosome 21.1,2 Regardless phenotype variability, there are multiple common features that contribute to establish a diagnosis of DS such as hypotonia, small brachycephalic head, epicanthal folds, flat nasal bridge, Brushfield spots, small mouth, small ears, and excessive skin at the nape of the neck, among others.1,2 Children and adolescents with DS present a combination of mental retardation, that is variable from mild to severe, with neuromuscular impairment, such as muscle hypotonicity, hypermobility of the joints or ligament laxity, light to moderate obesity, characteristic facial features, congenital heart disease, immunological dysfunction, hypothyroidism, pulmonary hypoplasia, visual and auditory problems, poor balance, perceptual difficulties, and other health problems.1,3
The pulmonary hypoplasia in children with DS results in lung growth abnormalities, such as fewer terminal lung units, acini with reduced number of alveoli, spacious and distended alveolar ducts, and smaller alveolar surface area.4 Also, lower respiratory tract infections occur more commonly in children with DS, and these combined with respiratory muscle weakness, may result in functional respiratory impairment.3,4
The assessment of pulmonary function is of great importance, as it could be used to assess the baseline respiratory function, to detect pulmonary problems, and to monitor the effectiveness of rehabilitation strategies. Additionally, it could help the definition of intervention strategies in order to improve respiratory muscles strength, thus improving lung compliance and assisting the prevention of other negative phenomena associated such as secretions retention, decreased lung volumes, recurrent lung infections and decreased effectiveness of cough. Despite being a simple, inexpensive, feasible and quantifiable measurement, few studies investigated the pulmonary function in persons with DS.5–8 Thus, the aim of the present study was to compare the pulmonary function of young adults with DS with healthy subjects.
Materials and methodsParticipantsA total of 34 young adults (20 females and 14 males) with an age range between 20 and 40 years voluntarily participated in this cross-sectional study. This convenience sample was composed by 17 apparently healthy young adults (9 females and 8 males) and 17 young adults with DS (11 females and 6 males). The group of individuals with DS was recruited at two special education and professional training centres localised in the Oporto area, Portugal, after authorisation to perform the study has been granted by the participants, their parents and/or guardians and the centres’ director. Each participant's parents/guardians were asked to complete a questionnaire detailing their relative medical history. The inclusion criteria were: adults with moderate to mild mental retardation, able to walk independently, without serious visual and/or auditory problems, and with medical clearance from the participant's physician to participate in the study. Exclusion criteria were as follows: associated congenital heart abnormalities, participating in sport activities, neuromuscular or orthopaedic disorders involving the thorax, including upper respiratory tract infection or back pain within the three weeks prior to data collection. In addition, a physiotherapist examined the participants to determine whether any of them had any spine or chest wall deformities such as scoliosis or pectus excavatum, respectively. Apparently healthy individuals (control group), complying with the same exclusion criteria, recruited in the same geographic area composed the group of adults without DS.
The study procedures were in accordance with the ethical standards on human experimentation. Written informed consent was obtained from the participants in the apparently healthy group and from the parents/guardians of the participants with DS.
ProceduresAll data were collected in the morning in a quiet room at the two centres over the course of a week, with the participants wearing comfortable clothing. Before the data collection, all participants and parents/guardians were informed about the study procedures and then were asked to sign the written informed consent.
Height and weight measurements were attained using a standard scale and stadiometer (Seca 285, Seca, Birmingham, United Kingdom). To measure weight, participants were asked to remove shoes and heavy clothing, such as sweaters, and to stand with both feet in the centre of the scale. Weight and height were recorded to the nearest decimal fraction.
Following the collection of anthropometric data, forced expiratory volume in one second (FEV1), forced vital capacity (FVC), peak expiratory flow (PEF) and the fraction of FVC expired in one second (FEV1/FVC%) were assessed using a hand-held spirometer (Micro GP, CareFusion, Basingstoke, UK), according to standard methods.9,10 This type of spirometer (turbine flow sensor) was validated to the latest American Thoracic Society/European Respiratory Society (ATS/ERS) Spirometry Standards9 and has proven accuracy, precision11,12 and repeatability.13 In comparison to a conventional spirometer, this hand-held spirometer showed an excellent correlation between FVC values (r=0.974), a very good correlation between FEV1 values (r=0.973) and a good correlation between PEF values (r=0.909).14
Immediately prior to testing, standardised instructions on the technique of performing forced expiratory manoeuvres were provided, and all participants were allowed to perform three to five practice manoeuvres prior to data collection. Following the instruction and practice period, spirometry was performed in a standing position with a nose clip. The physiotherapist supervising the spirometry evaluated whether each manoeuvre was performed according to the procedures recommended by ATS/ERS and whether each one met the criteria for acceptability.9 Participants were instructed to take a few normal breaths, inhale completely and then exhale as hard and fast and for as long as possible until their lungs were completely empty. Standardised verbal encouragement was given. Participants performed three acceptable manoeuvres; if the ATS/ERS criteria were not met in three manoeuvres, additional trials were allowed, up to eight manoeuvres.9 A rest period of 1min was allowed between each manoeuvre; the best value of three measurement trials was taken in each condition. During the lung function assessments, each participant wore a nose clip and breathed through a mouthpiece. The same investigator performed all tests.
Data analysisData was analysed using SPSS 17.0 (SPSS Inc., Chicago, IL). The normality of data distribution was tested with the Shapiro–Wilk test. The data were normally distributed with the exception of FEV1/FVC%. Baseline characteristics were compared using independent t-tests or Chi-square tests. Independent t-tests or the Mann–Whitney U test were performed to determine whether there were significant differences between groups in the outcomes. Pearson correlation or Spearman's rho tests were used to test associations between body mass index and lung function. Values are presented as means±standard deviation, with the exception of FEV1/FVC%, which are presented as median (interquartile range). Data were considered significant at p<0.05.
ResultsThe groups were comparable regarding mean age, mean weight and proportion of females. The group of young adults with DS presented a lower height and higher body mass index (Table 1).
Age, anthropometrics and pulmonary function values in both groups.
| Control group | DS group | p-Value | |
|---|---|---|---|
| Age (years) | 29.4±6.0 | 30.8±5.9 | 0.495 |
| Females (n) | 11 (64.7%) | 9 (52.9%) | 0.486 |
| Weight (kg) | 67.4±11.1 | 69.1±11.0 | 0.659 |
| Height (m) | 1.69±0.11 | 1.48±0.05 | <0.001 |
| BMI (kg/m2) | 23.4±1.3 | 31.4±4.6 | <0.001 |
| PEF (L/min) | 387.4±52.9 | 238.4±89.4 | ≤0.001 |
| FEV1 (L) | 3.1±0.5 | 1.9±0.6 | ≤0.001 |
| FVC (L) | 3.1±0.4 | 2.2±0.7 | ≤0.001 |
| FEV1/FVC%a | 100 (97.5; 100) | 97 (85.5; 100) | 0.077 |
Note: BMI, body mass index; DS, Down syndrome; FEV1, forced expiratory volume in one second; FEV1/FVC%, the fraction of FVC expired in one second; FVC, forced vital capacity; PEF, peak expiratory flow.
Regarding pulmonary function, the group of participants with DS showed significantly lower values for PEF, FVC and FEV1 (p<0.001) when compared to subjects of the control group (Table 1). No significant differences were observed between groups in FEV1/FVC% (Table 1).
An association between pulmonary function and body mass index was observed. The results showed an inverse correlation between the body mass index and the PEF (r=−0.691, p<0.001), the FVC (r=−0.555, p=0.001) and the FEV1 (r=−0.617, p<0.001).
DiscussionThe main finding of this study was that young adults with DS have reduced pulmonary function compared to age-matched healthy controls. Additionally, the pulmonary function parameters were inversely correlated with body mass index, i.e. the increase in body mass index is accompanied by a decrease in pulmonary function.
Our results showing a decreased pulmonary function in adults with DS are in agreement with those reported in previous studies enrolling children5,6,8 and adults with DS.7 Regarding, the pulmonary function values of the control group, they are similar to those reported in the literature.15
Several factors could contribute to the reduced pulmonary function observed in our study including pulmonary hypoplasia, overweight/obesity, hypotonia, and decreased respiratory muscle strength. In fact, da Silva et al.7 showed that adult male individuals with DS have reduced maximal inspiratory and expiratory pressures in comparison with healthy adults and that both hypotonia and obesity could explain the decreased respiratory muscle strength of the adults with DS. The inverse correlation observed in our study between body mass index and pulmonary function also reinforces the negative effects of overweight/obesity on lung volumes and pulmonary function observed in previous studies.16,17 Indeed, the presence of excessive adipose tissue around the rib cage, abdomen and in the visceral cavity loads the chest wall and reduces functional residual capacity and expiratory reserve volume,17 hence explaining at least partially our results.
The reduction of pulmonary function makes individuals with DS potential candidates to participate in cardiopulmonary rehabilitation or exercise training programmes. This seems especially pertinent since it was demonstrated that the performance of aerobic exercise for 30min, 5 days per week, during 8 weeks improves FEV1 and FVC in children with Down syndrome or other intellectual disabilities.18 Likewise, the participation in a 12-week programme (3 sessions per week) of aerobic exercise using a rowing ergometer improved the vital capacity, the FVC, the FEV1, and the PEF of children with DS.3 In this way, the participation in cardiopulmonary rehabilitation or exercise training programmes should be seen as a way to prevent pulmonary problems, which are a large cause of hospital admission and/or morbidity among individuals with DS.
Our study has some limitations. First, we did not assess respiratory muscle strength, cardiorespiratory capacity or physical fitness, which could have provided a wider picture of the cardiopulmonary function of young adults with DS. Also, the assessment of pulmonary function is dependent of the comprehension and cooperation of the participants. Since the individuals in the DS group have moderate to mild mental retardation, we cannot completely exclude this influence on our results. Nonetheless, they cooperated and followed all the orders and procedures, and an experienced physiotherapist validated all the tests.
In conclusion, our study showed that young adults with DS have reduced pulmonary function in comparison to age-matched controls and that the pulmonary function is inversely correlated with body mass index.
Conflict of interestsThe authors declare that they have no conflict of interests.