Myelopathy is a condition that significantly impacts a person's mobility and independence. In people with intellectual disabilities, such as Down syndrome, the negative impact of myelopathy is magnified. Myelopathy in Down syndrome may be related to atlanto-axial instability or degenerative pathology. Our experience with these patients has led us to hypothesize that their myelopathy is commonly undiagnosed until very severe. In this study we seek to determine whether patients with Down syndrome present with more severe myelopathy than those without Down syndrome.
MethodsWe performed a retrospective medical record review of patients with Down syndrome who were treated for myelopathy by the Tufts Neurosurgical Practice. Eight patients met the criteria and were graded for severity of myelopathy on the Nurick Scale. We compared the patients with cervical spondylotic myelopathy and Down syndrome to patients who were treated for cervical spondylotic myelopathy as reported in Furlan et al. and Fehlings et al.
ResultsThe average Nurick grade for patients with Down syndrome was 4.2 (SD 0.84, n=5). The average Nurick grade as reported by Furlan et al. was 2.8 (SD 0.68, n=81) and by Fehlings et al. was 3.14 (SD 0.97, n=278). The independent samples t-test resulted in a P value<0.000 and 0.016 with Furlan et al. and Fehlings et al. respectively.
ConclusionsThe patients with Down syndrome in our study presented to neurosurgery with more severe myelopathy than patients without Down syndrome. It is important for physicians caring for people with Down syndrome to be aware of the presentation of myelopathy and consider the condition in the differential diagnosis of a Down syndrome patient with functional decline.
La mielopatía es una enfermedad que afecta de manera significativa la movilidad y la independencia del paciente. En personas con discapacidades intelectuales como el síndrome de Down, el efecto negativo de la mielopatía se magnifica. La mielopatía en el síndrome de Down puede estar relacionada con inestabilidad atlantoaxial o una enfermedad degenerativa. Nuestra experiencia con estos pacientes nos ha llevado a formular la hipótesis de que su mielopatía no se suele diagnosticar hasta que es muy grave. En este estudio nos proponemos determinar si los pacientes con síndrome de Down presentan mielopatía más grave que los que no tienen síndrome de Down.
MétodosLlevamos a cabo una revisión retrospectiva de historiales médicos de pacientes con síndrome de Down que recibieron tratamiento para la mielopatía por parte del Tufts Neurosurgical Practice. Ocho pacientes cumplían los criterios y se les evaluó la gravedad de la mielopatía según la escala de Nurick. Comparamos a los pacientes con mielopatía cervical espondilótica con los pacientes con síndrome de Down que recibieron tratamiento para la mielopatía cervical espondilótica, según la información en Furlan et al. y Fehlings et al.
ResultadosLa media del grado de Nurick para pacientes con síndrome de Down fue de 4,2 (DE 0,84, n=5). La media del grado de Nurick según la información en Furlan et al. fue de 2,8 (DE 0,68, n=81) y según Fehlings et al. fue de 3,14 (DE 0,97, n=278). Las pruebas t independientes de las muestras arrojaron un valor p<0,000 y 0,016 con Furlan et al. y Fehlings et al., respectivamente.
ConclusionesLos pacientes con síndrome de Down de nuestro estudio acudieron a Neurocirugía con una mielopatía más grave que la de los pacientes sin síndrome de Down. Para los médicos es importante atender a personas con síndrome de Down para darse cuenta de la presentación de mielopatía y considerar la afección en el diagnóstico diferencial de un paciente con síndrome de Down y con deterioro funcional.
Myelopathy is a common spine condition in which the spinal cord is compressed and damaged.1 It can be caused by acute injury, progressive degeneration, or vertebral instability. It is most often caused by disk degeneration and stenosis of the spinal canal. If untreated, narrowing of the spinal canal and compression of the cord can cause demyelination and necrosis, which are irreversible.2 It can affect the cervical, thoracic, and lumbar spine. Cervical spondylotic myelopathy (CSM) predominates and worldwide is the most commonly treated pathology of the spinal cord.2,3 Neurosurgical intervention is indicated when myelopathy is symptomatic and progressive.1 A favorable outcome correlates strongly with early treatment: it is important for people with myelopathy to be identified quickly because people whose disability is severe are less likely to improve from treatment.2
There are several well-studied conditions in people with Down syndrome (DS) which can cause myelopathy. One is atlanto-axial instability caused by laxity of the transverse ligament. It can lead to compression of the cervical spine.4 Atlanto-axial instability affects 10–20% of people with DS but is asymptomatic in 98–99% of cases.5 The danger posed by atlanto-axial instability to pediatric patients with DS has led to specific recommendations for cervical spine X-rays by treating physicians whenever there is a change in neurological function.6
CSM of the sub-axial cervical spine is common in people with DS, even if it is not commonly diagnosed. In people with DS some studies have found a 45% prevalence of moderate or severe CSM.7 Clumsiness and gait change are typical onset symptoms in the natural history of CSM, but symptoms of CSM can be as severe as incontinence and quadriplegia.8 The high prevalence of CSM in people with DS is due primarily to degenerative changes that result in stenosis of the spinal canal.9
Clumsiness and gait abnormality are the most common symptoms people notice at the onset of CSM.8 Insomuch as pain is the most motivating symptom for a person to seek treatment, myelopathy may be insidious because it is often painless. When there is pain caused by CSM, many people complain of shoulder pain or referred pain.8
Many people with CSM experience a decline-plateau of symptoms.10 In a typical scenario, a person might notice a new symptom, such as decreased dexterity of an extremity, which will then remain unchanged for some time. Symptoms can remain static for years; then suddenly existing symptoms become worse or a new symptom of the developing myelopathy will emerge. This step-wise feature of myelopathy can alter a person's pursuit of treatment. If an initial symptom is problematic but not severe, and has plateaued, the person may resist seeking treatment or accepting any non-conservative treatment. Upon decline in function or worsening disability, the person may then pursue treatment including surgical intervention.
Over the last several decades there has been a large increase in the rate of diagnosis and treatment of myelopathy.11 Associated with this increase has been improvement in how rapidly people seek neurosurgical evaluation for suspected CSM. Diagnosis is made through a detailed examination and patient history combined with radiological findings. Although X-ray and CT can be useful radiographic modalities to aid in the diagnosis of myelopathy, MRI is the most appropriate and definitive for almost all etiologies.3
Treatments for myelopathy range from conservative options such as physical therapy to neurosurgical treatments such as decompression and fusion. The severity of the symptoms is the foremost consideration for the advised treatment. Another important consideration is the severity of the radiological findings.12 A person with mild symptoms but with severe radiological findings may also be advised to receive neurosurgical treatment to avoid sudden decline in function.
The likelihood of a favorable outcome depends on the severity of the symptoms and how early treatment is administered. If a patient suffers only mild symptoms from cervical spondylosis and there is minimal evidence that it has progressed to myelopathy, conservative treatment may be recommended. Conservative treatment may include exercises, immobilization, or a combination. Immobilization is achieved through the use of a cervical collar or a more rigid device such as a Minerva body jacket. The goal of these treatments is to strengthen the muscles of the neck and decrease movement of the cervical spine. This is intended to reduce impingement on the spinal nerve and reduce the advancement of myelopathy. For people for which myelopathy is radiographically and symptomatically demonstrable but is not yet severe, non-surgical treatment with long-term physical therapy and therapeutic injections might be advised.
If surgical intervention is indicated, there are a variety of procedures employed to decompress the spinal nerve and immobilize the vertebrae in the affected area. A full accounting of the procedures and their indications is beyond the scope of this paper.
Surgical outcomes have improved steadily in the treatment of myelopathy, and in many cases surgery results in a satisfactory outcome for the patient. Fehlings et al. performed a comprehensive analysis of outcomes for surgical treatment of myelopathy to which we compare our cohort of patients with DS. The researchers conclude surgical treatment of CSM is safe and substantially relieves symptoms for most people.13
There are several scoring systems used to quantify the severity of a person's neurological dysfunction as it relates to myelopathy. The two most common and well studied are the mJOA Scale and the Nurick scale. The Nurick scale for CSM was selected for this study because it has been used widely in the literature as a scale of disability and is broad enough to assess people in a retrospective medical record review. Furthermore, it has been carefully evaluated against a variety of scales and found to be accurate and valid.14 In this study we include consideration of myelopathy in the cervical, thoracic, and lumbar spine. Though the Nurick scale is designed to rate disability in cervical myelopathy, it also works well as a grade of overall disability in thoracic and lumbar myelopathy.
The Nurick scale is a 6 point system in which the least severe disability is assigned a 0 (radiculopathy only), and the most severe disability a 5 (non-ambulatory). The scale is as follows:
Grade 0: Signs or symptoms of root involvement but without evidence of spinal cord disease.
Grade 1: Signs of spinal cord disease but no difficulty in walking.
Grade 2: Slight difficulty in walking which did not prevent full-time employment.
Grade 3: Difficulty in walking which prevented full-time employment or the ability to do all housework, but which was not so severe as to require someone else's help to walk.
Grade 4: Able to walk only with someone else's help or with the aid of a frame.
Grade 5: Chairbound or bedridden.15
Subjects and methodsThe institutional review board of Tufts Medical Center approved this retrospective medical record review. Patients in the Tufts Neurosurgical Practice database were assessed to find those who have DS and were treated for myelopathy. We excluded people who received emergency treatment for a traumatic injury or whose treatment was specific to recent traumatic injury.
Each patient with DS and myelopathy was then reviewed independently by the researchers and ranked on the Nurick scale. When there was disagreement between researchers in the grade for a patient, the lower grade (which indicates less severe disability) was used in order to reduce the risk of a Type 1 error in our analysis.
In addition to rating on the Nurick scale, each included patient record was reviewed for relevant radiographic findings and patient history information.
The Nurick grade for the patients with DS was compared to patients in Furlan et al. and Fehlings et al.13,16 These are large, contemporaneous studies of degree of myelopathy in the overall neurosurgical patient population. These studies also used the Nurick scale. Statistical analyses of the patient group with DS compared to the overall patient population were completed using IBM's SPSS statistical software.
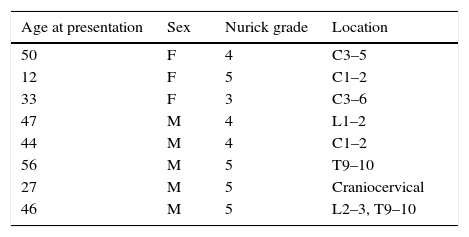
Results8 patients were found to have DS and myelopathy for which they were treated by the Tufts Neurosurgical Practice between 2007 and 2015. These 8 were included in our study. Of these 8 patients 4 had cervical myelopathy, 1 had craniocervical, 1 had thoracic, 1 had lumbar, and 1 had both thoracic and lumbar myelopathy (Table 1).
Neurosurgical patients with Down syndrome at Tufts Medical Center between 2007 and 2015 with Nurick Grade and spine level of myelopathy.
| Age at presentation | Sex | Nurick grade | Location |
|---|---|---|---|
| 50 | F | 4 | C3–5 |
| 12 | F | 5 | C1–2 |
| 33 | F | 3 | C3–6 |
| 47 | M | 4 | L1–2 |
| 44 | M | 4 | C1–2 |
| 56 | M | 5 | T9–10 |
| 27 | M | 5 | Craniocervical |
| 46 | M | 5 | L2–3, T9–10 |
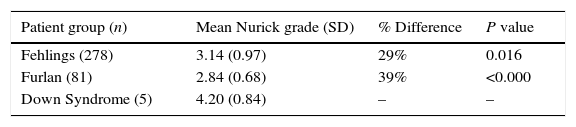
In our initial analysis, we considered only the 5 patients with CSM compared to the patient samples presented in Furlan et al. and Fehlings et al.13,16 (Table 2).
Mean Nurick Grade comparison of patients with Down syndrome and CSM only to Fehlings et al. (2013) and Furlan et al. (2011).
| Patient group (n) | Mean Nurick grade (SD) | % Difference | P value |
|---|---|---|---|
| Fehlings (278) | 3.14 (0.97) | 29% | 0.016 |
| Furlan (81) | 2.84 (0.68) | 39% | <0.000 |
| Down Syndrome (5) | 4.20 (0.84) | – | – |
We found that the 5 patients with CSM and DS had a statistically significant difference between the mean Nurick grade from the patients in Furlan et al. and Fehlings et al.13,16 The difference in mean Nurick grade for patients with DS compared to Fehlings et al. is 29% and Furlan et al. is 39%. We used independent samples t-tests in IBM SPSS Statistics version 24 to compare the DS group with the groups from Fehlings et al. and Furlan et al.
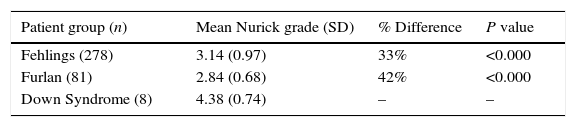
The patients with thoracolumbar myelopathy were then included as part of our analysis (Table 3).
Mean Nurick Grade comparison of patients with Down syndrome and both cervical and thoracolumbar myelopathy to Fehlings et al. (2013) and Furlan et al. (2011).
| Patient group (n) | Mean Nurick grade (SD) | % Difference | P value |
|---|---|---|---|
| Fehlings (278) | 3.14 (0.97) | 33% | <0.000 |
| Furlan (81) | 2.84 (0.68) | 42% | <0.000 |
| Down Syndrome (8) | 4.38 (0.74) | – | – |
We found that the 8 patients with myelopathy and DS had a statistically significant difference between the mean Nurick grade compared to Fehlings et al. and Furlan et al. of 33% and 42% respectively.13,16
The percent difference is greater when all areas of the spine are included in our analysis. However, for both groups the difference is statistically significant in comparison to Fehlings et al. and Furlan et al.13,16
DiscussionWe conclude that patients with DS present with more severe disability from CSM than the overall patient population with CSM. Furthermore, when patients with DS and compressive myelopathy from any spinal region are compared to other patients, the patients with DS have more severe disability.
We chose Furlan et al. and Fehling et al. for comparison to the patients in this study because they are contemporaneous and because they have a large, varied body of patients. Furthermore, these studies use the Nurick grade, which we determined to be the most useful for this study because it focuses on disability. As a gross measure of the progression of myelopathy, severity of a patient's disability is a helpful shorthand. The other typical rating of severity of CSM is the mJOA. This scale is much more in depth, but we were concerned that as a measure it might not have the specificity we need. In other words, some elements of the mJOA might indicate that our patients had advanced myelopathy due more to symptoms of DS than to symptoms of myelopathy.
A critic might argue that the use of the Nurick grade among people with lumbar and thoracic myelopathy is invalid. It was originally designed as a measure of disability in cervical spondylotic myelopathy, not lumbar or thoracic myelopathy. However, as a measure, it is only concerned with disability. It does not, for example, measure arm strength or muscle tone. If it combined the measure of specific motor function in the arms as does the mJOA, it would not present a comparable degree of disability between people with lumbar myelopathy and cervical myelopathy. As an overall measure of disability, Nurick grading allows us to make a fair comparison between patients with various foci of myelopathy.The patients with DS in our study had severe disability as a result of myelopathy. Out of the 8 overall patients, half had a Nurick grade of 5 which is a chairbound or bedridden patient. 3 of the patients had urine or stool incontinence secondary to myelopathy. The average age of the patients with a Nurick grade of 5 is 35 years old, and includes a patient who was 12 years old when the patient presented to Tufts Neurosurgical Practice. The average age of patients with all degrees of myelopathy in Fehlings et al. is 56 years and in Furlan et al. is 57 years, nearly 20 years older than patients in this study. The severe disability is amongst DS patients who are meaningfully younger than typical patients with myelopathy.
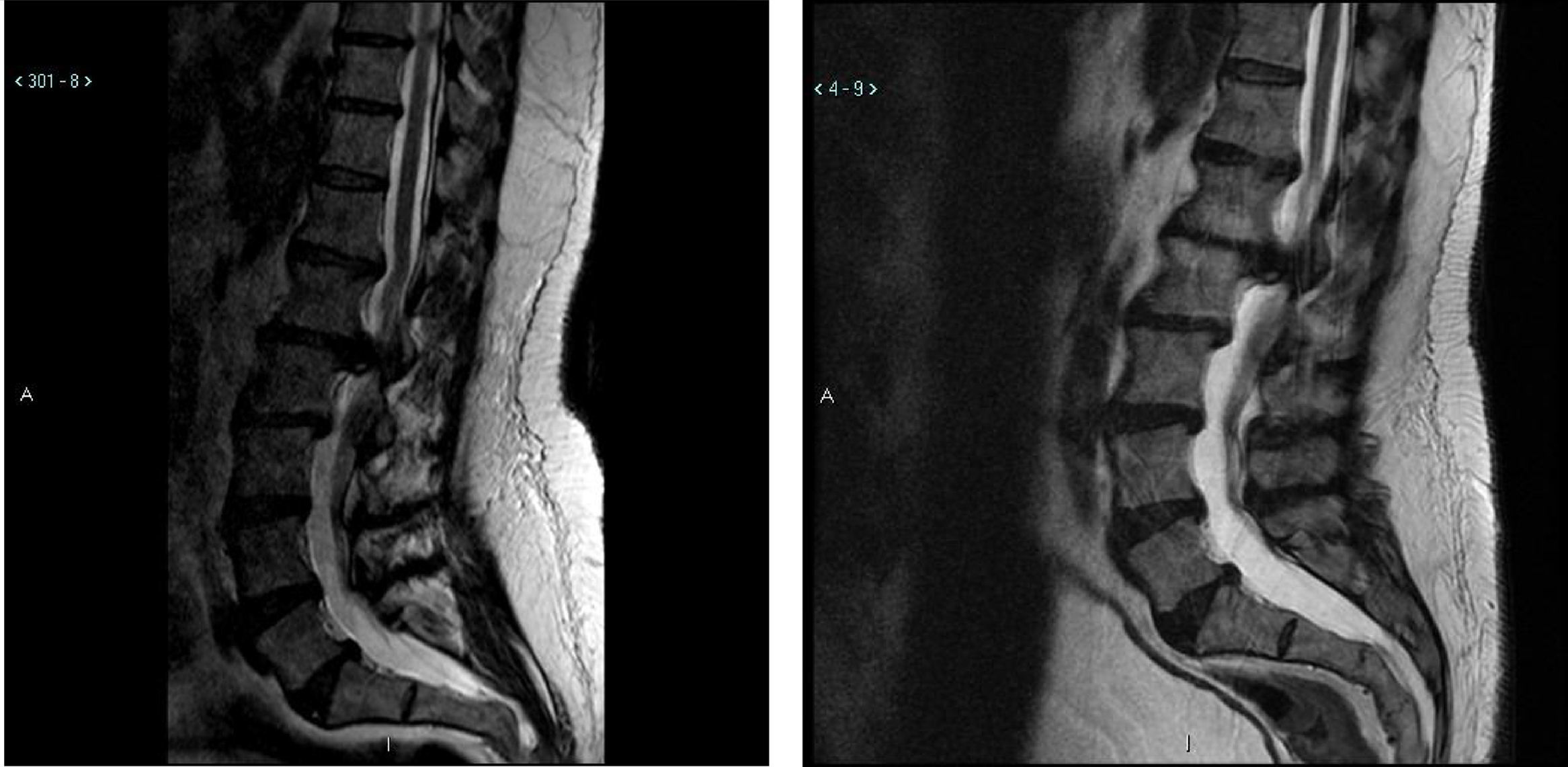
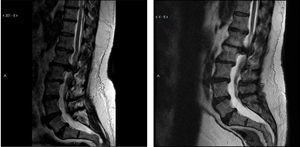
Illustrative caseThis 47 year old male with Down syndrome presented to Tufts Neurosurgical Practice with incipient fecal incontinence and about a four year history of urinary incontinence. He had been very active in organized athletics before his symptoms started. He had bilateral foot drop for the past ten years and had worsening balance. His parents and trainers noticed a very significant reduction in his sport capability over the past 3 years, and he could no longer even jump. These were severe disabilities for which he required braces and daily assistance. In the workup for the new incontinence, the patient received an MRI of the lumbar spine which revealed a large L1–L2 disk herniation causing significant compression of the thecal sac and conus medularis (Fig. 1).
Of note is that the patient had received a lumbar MRI almost 5 years prior which shows the same impressive disk herniation. The patient underwent decompressive surgery by microdiscectomy and at followup the patient's caregivers noted significant improvement in his walking and foot strength. Improvement is not always seen after decompressive surgery for longstanding and severe myelopathy. In short, just over half of the patients in this study had meaningful and substantial improvement in their disability from neurosurgical treatment.
It is important to explore the possible reasons why a person with DS might experience more severe myelopathy. One hypothesis is that people with DS develop severe symptoms faster than people without DS. This seems unlikely and is not borne out in this study's cohort of patients. Importantly, such a mechanism for more aggressive myelopathy progression in DS has not been described. While there is a greater prevalence of spinal stenosis in people with DS, and it develops at an earlier age, this does not seem to affect the speed of the development of symptoms. Furthermore, many people with DS have asymptomatic cervical stenosis. This indicates that CSM does not develop more commonly in people with DS and stenosis than does myelopathy develop among neurologically normal people who have stenosis.
Another reason a person with DS might experience more severe myelopathy is that people with DS may have a lower functional reserve. A person with DS might have a sole method of accomplishing a task that works within the constraints of the intellectual disability. If something upsets that particular method, the person with DS may not have the flexibility to accommodate the new constraints. For example, a person with DS may walk with a less stable gait strategy.17 Atypical gait strategies can require more complex neuromuscular coordination. An incipient neurological deficit brought about by spinal stenosis could eliminate the coordination necessary to maintain the less stable kinematics. A person with a stable gait strategy might be able to accommodate the new constraint of reduced neuromuscular coordination secondary to myelopathy. The person with DS may not be able to accommodate the reduced coordination and this could lead to reduced mobility and increased disability.
Delayed diagnosis and/or treatment is a likely factor as evidenced by the case example. Greater attention to other potential diagnoses is contributary. Bosma et al. hypothesizes that “the attention of physicians in patients with DS with walking difficulties and bladder dysfunction may be mainly focused on brain disorders or atlanto-axial subluxation”.4 The problem would simply be one of inattention to non-psychological causes, or excessive focus on atlanto-axial compression as the cause of myelopathy without attending carefully to the rest of the spine.
Another possible reason that people with DS have more severe myelopathy than other people is reduced self-report of symptoms. Clumsiness and gait change are typical early symptoms of CSM. It is likely that a neurologically typical person would quickly seek medical care upon any new clumsiness or gait change. People with DS seem to have as robust proprioception as neurologically typical people, so it is likely that a person with DS would indeed notice incipient clumsiness.18 However, for all people there are hurdles to overcome in order to seek medical care and for a person with DS there are likely to be additional hurdles. Whether the special hurdle for the person with DS is communication, cognition, or memory, the burden is likely to fall to the caregiver to notice the incipient deficit and seek care for it. In our research, there were examples of subtle early extremity weakness such as a change in arm movement while dancing that were not addressed. If an early symptom of incipient myelopathy in a person with DS can be as subtle as a change in dancing pattern, physicians must be highly attentive to the reports of caregivers.
What are the current guidelines in the workup of a person with DS who is experiencing neurological decline or new deficit in the Activities of Daily Living (ADLs)? There is a dearth of clear guidelines for how to proceed with a patient who experiences symptoms which might be related to myelopathy. As mentioned previously, the danger posed by atlanto-axial instability to pediatric patients with DS has led to specific recommendations for cervical spine X-rays by treating physicians whenever there is a change in neurological function.6 The Special Olympics has made a clear recommendation and requirement to screen for atlanto-axial instability before a participant with DS can compete.19 It seems that a significant amount of the focus on atlanto-axial instability in DS relates to the success of the Special Olympics in promoting their decades old guideline. As for the rest of the spine, there is lack of clear guidelines for physicians. In Bosma et al. the recommendation is made that “in patients with DS presenting with ataxia, progressive gait disorder, weakness of arms or legs, or bladder dysfunction, one should consider lower cervical spondylarthrotic myelopathy in the differential diagnosis since this is an underestimated disorder with serious consequences”.4 In this same paper, MRI study is recommended where “clinical suspicion of cervical myelopathy” exists.4 This is a good foundation for a clinical guideline in the workup of a patient who experiences neurological decline, but it is important for clinicians to consider the whole spine. It is likely that a focus on atlanto-axial instability, while important, may overlook myelopathy that results from the subaxial spine. Although the cervical spine is the most common location for myelopathy, we believe that it is also important for clinicians to give full attention to the entire spine upon the discovery of a neurological deficit.
Our findings suggest that physicians and caregivers together should increase their awareness of myelopathy in DS. We recommend that myelopathy be considered when there is an unexplained neurological deficit, change in ADLs, or new functional impairment in a person with DS. This should elicit a physical examination with attention paid to potential myelopathy and then MRI of the cervical, thoracic, and lumbar spine if appropriate. Myelopathy is a common problem in DS, and the symptoms can be subtle and insidious, therefore the index of suspicion should be low. Though the symptoms may be subtle at onset, they can lead to severe and potentially irreversible disability. The outcome tends to be favorable for people who receive treatment for myelopathy early in the disease.
Originality of the material: The above authors declare that the article is original and has not, wholly or any of its parts, been published or submitted previously, and is not subject to consideration by any other publication.
Conflict of interestThe above authors declare that there is no funding or any other aspect that could lead to a conflict of interest.
A previous revision of the abstract for this research was presented to the assembled attendees at the New England Neurosurgical Society Annual Meeting on June 24, 2016.