Mortality rate of invasive Candida infections is raising mainly amongst immunocompromised patients. These infections are hard-to-treat mainly due to the increasing incidence of resistance. The overexpression of ATP-binding cassette and major facilitator superfamily transporters is the main responsible for the failure of antifungal therapies. In a Saccharomyces cerevisiae model, β-lapachone inhibited Pdr5p, a transporter homologous to those found in Candida albicans.
AimsTo determine whether β-lapachone reverses the resistance phenotype mediated by efflux transporters in C. albicans clinical isolates.
MethodsThe antifungal activity of β-lapachone combined with fluconazole was measured by agarose chemosensitization and microdilution assays. CaCdr2p and CaMdr1p activities were evaluated through fluorescent dyes accumulation. ATPase activity was assessed using transporter-enriched plasma membranes.
Resultsβ-lapachone reverted antifungal resistance of S. cerevisiae and C. albicans strains overexpressing CaCdr2p and CaMdr1p transporters by inhibiting these proteins activities. CaCdr2p ATPase activity was not impaired by the compound.
Conclusionsβ-lapachone is a promising drug candidate to be used as an adjuvant in the treatment of candidiasis caused by fluconazole-resistant C. albicans strains.
Las tasas de mortalidad de infecciones invasivas causadas por Candida están en aumento, principalmente entre los pacientes inmunocomprometidos. Estas infecciones son difíciles de tratar debido a la creciente incidencia de resistencia a los antifúngicos. La sobreexpresión de los transportadores dependientes de ATP y los de la superfamilia de facilitadores principales es el mayor responsable del fracaso de las terapias antimicóticas. En un modelo de Saccharomyces cerevisiae, la β-lapachona inhibió Pdr5p, un transportador homólogo a los encontrados en Candida albicans.
ObjetivosDeterminar si la β-lapachona revierte el fenotipo de resistencia mediado por transportadores de eflujo en aislamientos clínicos de C. albicans.
MétodosSe midió la actividad antifúngica de la β-lapachona combinada con fluconazol mediante ensayos de quimiosensibilización con agarosa y microdilución. Las actividades CaCdr2p y CaMdr1p se evaluaron mediante la acumulación de colorantes fluorescentes, y la actividad de ATPasa se evaluó usando membranas plasmáticas enriquecidas con transportador.
ResultadosLa β-lapachona revirtió la resistencia antifúngica de las cepas de S. cerevisiae y C. albicans que sobreexpresaban los transportadores CaCdr2p y CaMdr1p al inhibir sus actividades. El compuesto no afectó la actividad ATPasa de CaCdr2p.
ConclusionesLa β-lapachona es una candidata prometedora para ser utilizada como adyuvante en el tratamiento de la candidiasis causada por cepas de C. albicans resistentes al fluconazol.
Candida species are the major fungal pathogens that threaten human beings, especially those under immunosuppressive conditions, leading to potentially fatal infections.8 Fluconazole is the first-choice substance to treat Candida infections. However, the overuse of azole drugs during the last decades have induced the appearance of resistant strains. Furthermore, there are few antifungal agents available to treat candidiasis. These circumstances point to an urgent need of discovering new therapeutic strategies to overcome Candida infections.14
The development of antifungal resistance is related to the multidrug resistance (MDR) phenotype, which consists in cross resistance between structurally unrelated substances. Concerning Candida, its pivotal mechanism relies on the overexpression of ATP-binding cassette (ABC) and major facilitator superfamily (MFS) transporters within plasma membrane.12,13 Impairing the activity of these proteins would allow fluconazole to reach the intracellular concentrations required to exert its antifungal activity.1
In a previous study,2 we observed that β-lapachone, a natural naphthoquinone that displays several pharmacological activities, inhibits Saccharomyces cerevisiae MDR protein Pdr5p, which is homologous to Candida albicans efflux transporters. Therefore, the aim of the present study was to assess whether β-lapachone may inhibit efflux proteins related to MDR in C. albicans.
Material and methodsFour mutant strains of S. cerevisiae were used; AD/1234567 was deleted from all genes related to MDR transporters, while CaCdr1p+, CaCdr2p+ and CaMdr1p+ strains were derived from AD/1234567 but heterologously overexpress CaCdr1p, CaCdr2p and CaMdr1p, transporters naturally found in C. albicans.7 Moreover, three C. albicans strains were used. Strain 95-142 overexpresses both CaCdr1p and CaCdr2p,15 while PRI overexpresses CaMdr1p.11 ATCC 10231™ strain does not overexpress MDR transporters and was used as control. The S. cerevisiae strains were kindly gifted by Drs. Richard Cannon and Brian Monk (University of Otago), and the strain 95-142 by Theodore White (University of Missouri). PRI strain is part of our yeast collection, and was collected from a subgingival secretion of an HIV-patient.11 CaCdr2p+ strains plasma membranes were obtained and stored at liquid nitrogen.3
Before each procedure, cells were incubated in yeast-peptone-dextrose medium (YPD) during 17h at 30°C (S. cerevisiae) or 37°C (C. albicans) at 100rpm. β-lapachone was synthesized from lapachol.5 Agarose, RPMI-1640, rhodamine 6G (R6G), and Nile red were purchased from Sigma Aldrich (St. Louis, USA), and fluconazole was purchased from Farmacopa (Rio de Janeiro, Brazil). Antifungal activity of β-lapachone, alone or combined with fluconazole, was firstly evaluated in S. cerevisiae mutant strains through the agarose diffusion chemosensitization assay.9 Cell suspensions were incorporated into molten YPD agar medium in the presence or absence of fluconazole. Then, 50μg of β-lapachone were applied onto 6-milimeter Whatman 3MM® paper disks (Sigma–Aldrich®) and placed on the surface of the plate. Plates were incubated at 30°C for 48h. A microdilution method was performed in order to evaluate the minimal inhibitory concentration (MIC) of β-lapachone and to assess the interaction between this substance and fluconazole.4 Four strains, namely CaCdr2p+, CaMdr1p+, 95-142, and PRI were used in this procedure.
To verify if β-lapachone and fluconazole have a synergic activity due to efflux transporters inhibition, an assay with fluorescent probes was carried on. Efflux assays were performed using the R6G and Nile red fluorescent probes to evaluate the inhibition of CaCdr2p12 and CaMdr1p,6 respectively. AD/1234567 and ATCC 10231 were used as control. CaCdr2p is an ABC transporter and then it hydrolyses ATP to promote the efflux of substances. Blocking its ATPase activity could hamper efflux process and sensitize the yeasts to fluconazole. CaCdr2p ATPase activity was measured through incubation of enriched-plasma membranes in presence or absence of β-lapachone at 100μg/ml.2
ResultsIn agarose diffusion chemosensitization assay, halo diameters larger than 6mm were considered as growth inhibition. In absence of fluconazole, β-lapachone inhibited the growth of CaCdr2p+ (11 mm-diameter) and CaMdr1p+ (11mm-diameter) strains. Furthermore, it was observed that β-lapachone improved the antifungal activity of fluconazole against the same strains (15mm-diameter). Since these microorganisms overexpress CaCdr2p or CaMdr1p, fluconazole resistant C. albicans strains, namely 95-142 and PRI, that possess the same efflux proteins, were used in the following experiments.
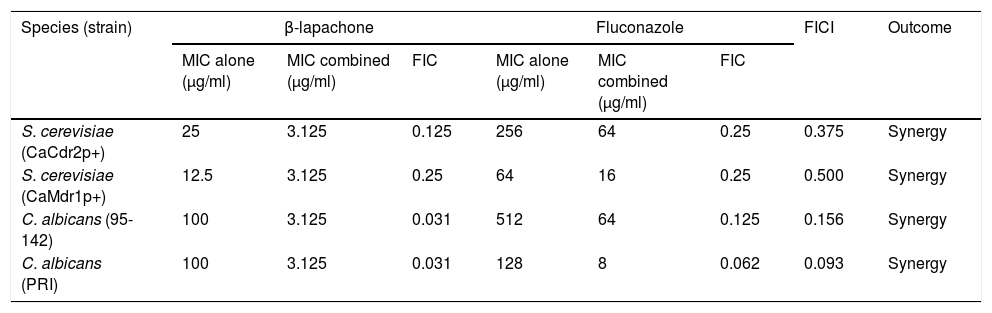
MIC of β-lapachone was assessed since it showed antifungal activity in the previous experiment. β-lapachone impaired the growth of S. cerevisiae strains and C. albicans isolates, with MIC values of 25μg/ml for CaCdr2p+ strain, 12.5μg/ml for CaMdr1p+ strain, and 100μg/ml for both C. albicans strains. Combinations were evaluated using fractional inhibition concentration index (FICI). FICI≤0.5 points to a synergic interaction, whereas 0.5<FICI<4.0 indicates synergism, and FICI>4 indicates antagonistic interaction.10 The FICI calculated for all the tested strains were less than or equal to 0.5, implying that the combination of β-lapachone and fluconazole was synergic (Table 1). The data obtained show that β-lapachone impaired the efflux mechanism in CaCdr2p+, CaMdr1p+, 95-142 and PRI strains at 74.9%, 49%, 28.1% and 43%, respectively. Neither AD/1234567 nor ATCC 10231 were able to extrude the probes, being used as negative controls. Results show that β-lapachone does not inhibit efflux through decreasing CaCdr2p ATPase activity.
Checkerboard assay of S. cerevisiae and C. albicans strains overexpressing multidrug efflux pumps CaCDR2p and CaMDR1p.
| Species (strain) | β-lapachone | Fluconazole | FICI | Outcome | ||||
|---|---|---|---|---|---|---|---|---|
| MIC alone (μg/ml) | MIC combined (μg/ml) | FIC | MIC alone (μg/ml) | MIC combined (μg/ml) | FIC | |||
| S. cerevisiae (CaCdr2p+) | 25 | 3.125 | 0.125 | 256 | 64 | 0.25 | 0.375 | Synergy |
| S. cerevisiae (CaMdr1p+) | 12.5 | 3.125 | 0.25 | 64 | 16 | 0.25 | 0.500 | Synergy |
| C. albicans (95-142) | 100 | 3.125 | 0.031 | 512 | 64 | 0.125 | 0.156 | Synergy |
| C. albicans (PRI) | 100 | 3.125 | 0.031 | 128 | 8 | 0.062 | 0.093 | Synergy |
MIC: minimal inhibitory concentration; FIC: ratio between MIC of a compound combined with a second compound and its MIC alone; FICI: sum of each FIC value.
Our results show that β-lapachone inhibit CaCdr2p and CaMdr1p, two of the three main transporters involved in MDR phenotype within C. albicans, thereby allowing fluconazole to reach intracellular concentrations required to its antifungal activity. Interestingly, despite the homology between CaCdr1p and CaCdr2p, only the latter was inhibited by β-lapachone. Moreover, β-lapachone did not inhibit CaCdr2p ATPase activity; it was unexpected, considering the results obtained on our previous study, where β-lapachone inhibited Pdr5p ATPase activity.2 This data shows that, although CaCdr2p and Pdr5p are homologous proteins, substances may act differently at each transporter. Therefore, our results corroborate that the ability of inhibiting ABC efflux transporters is not mandatorily related to ATPase activity impairment.
In summary, it can be concluded that β-lapachone is a promising drug candidate to be used as an adjuvant in the treatment of candidiasis caused by fluconazole-resistant strains. Further in vivo studies need to be conducted to clarify the applicability of this combination on infections caused by Candida.
Funding sourcesThis work was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brazil (CAPES) – Finance Code 001.
Conflict of interestNone.
We thank Brayan Bayona Pacheco (UNINORTE - Colombia) for correcting the Spanish version of the abstract, and Ms. Geralda Rodrigues Almeida for her technical support.