Odontogenic keratocysts (OKCs) and orthokeratinized odontogenic Cysts (OOCs) are distinct clinicopathological entities. OKC appears to behave in a way more similar to that of a neoplasm, such as ameloblastoma (AB). The aim of this study is to compare the influence of Ki-67, Cyclin D1 and COX-2 in the diagnosis and pathogenesis of OKC, OOC and AB.
Materials and methodsA cross-sectional observational study of 41 samples was organized into 3 groups: (1) OKC n=22; (2) AB n=13 and (3) OOC n=6. Paraffin blocks were sectioned and stained with hematoxylin and eosin (H&E). Immunohistochemical study using Bond Polymer Refine Red Detection Kit, Leica, Wetzlar, Germany, was performed for the following antibodies: Ki-67, Cyclin D1 and COX-2. Double blind immunostaining was quantified subjectively. Staining: nuclear or cytoplasmic; nuclear (Ki-67 and Cyclin D1>5% positive) and cytoplasmic (COX-2; 1; 1–30 cytoplasm: 2; 31–60 cytoplasm; 3; 61–100 cytoplasm). Considering positive stained 61-100 cytoplasms.
ResultsThe expression of Ki-67 was higher in the OKC group than in the AB group (p<0.05). Cyclin D1 showed a higher expression in OKC vs. OOC and OKC vs. AB (p<0.05). Finally, expression of COX-2 was higher in OKC vs AB (p<0.05).
ConclusionsCOX-2, Ki-67 and Cyclin D1 show statistically significant differences between the groups, suggesting that they could be useful tools in the differential diagnosis between OKCs and OOC and a predictive indicator for their biologic behaviour. The higher expressions of these 3 markers of OKC vs AB highlight once more the aggressive behaviour of this now re-considered cystic lesion. These markers could prove useful in the choice of more aggressive surgical treatment in OKCs as their behaviour appears to be similar to that of a neoplasm.
Los queratoquistes odontogénicos (QQO) y los quistes odontogénicos ortoqueratinizantes (QOO) son entidades clínico-patológicas diferentes y el QQO parece estar más próximo al comportamiento de una neoplasia, como el ameloblastoma (AB).
El objetivo de este estudio es comparar la influencia de Ki-67, ciclina D1, COX-2, en el diagnóstico y patogenia de QQO, QOO y AB.
Materiales y métodosSe ha diseñado un estudio observacional transversal con 41 muestras, en 3 grupos de estudio: (1) QQO: n=22, (2) AB n=13, y (3) QOO n=6. Se han seccionado bloques de parafina y teñido con hematoxilina y eosina y sometidas a una incubación con anticuerpos monoclonales (Kit Bond Polymer Refine Detection, Leica, Wezlar, Alemania) para la realización de un estudio inmunohistoquímico. Se ha cuantificado la inmunotinción, (nuclear o citoplasmática); nuclear (Ki-67 y ciclina D1; >5% positiva) y citoplasmática (COX-2; 1; 1-30 citoplasmas; 2; 31-60 citoplasmas; 3; 61-100 citoplasmas). Considerando positivo tinción a partir de 61-100 citoplasmas.
ResultadosLa expresión de Ki-67 ha sido mayor en el grupo del QQO con respecto a la de AB (p<0,05). Respecto a ciclina D1, mostró una expresión mayor en QQO vs. QOO (p<0,05); En el caso del marcador COX-2, la expresión ha sido mayor en QQO vs. AB (p<0,05).
ConclusionesLa mayor expresión de estos 3 marcadores en QQO vs. AB resaltan una vez más el comportamiento agresivo de esta lesión ahora reconsiderada como «quística». Estos marcadores pueden ayudar a decidir tratamientos quirúrgicos más agresivos en las lesiones de QQO ya que parece comportarse como una «neoplasia».
Odontogenic cysts account for 14.4% of oral biopsies of the maxillofacial area.1 They derive from the dental lamina due to genetic dysregulation in the odontogenesis process due to genetic mutations, the most important being the Patched 1 gene, (PTCH 1). Up to 40% are also derived from the reduced epithelium of the dental follicle, explaining the dentigerous origin of these lesions.2–6
In January 2017 the WHO updated its classification on head and neck tumours with regard to odontogenic and bone tumours of the maxillofacial area. Odontogenic cysts that had been eliminated from the 3rd Edition in 20057 were included again8–10 and the keratocystic odontogenic tumour (KCOT) renamed as OKC.8,11
Despite the new classification and the fact that the OKC is no longer considered by experts to be a tumour, but a cyst, the OKC and OOC are still being considered separate entities.8
AB is the most common odontogenic tumour after odontoma and has been used in the study as a control, as it has a biological pattern very similar to that of OKC.9,12,13
The role of immunohistochemistry (IHC) in OKC, OOC and AB has been intensively studied. Cell markers are proteins expressed in cells at different times of the cell cycle and help to assess the aggressive and invasive potential of lesions, mainly by measuring cell proliferation. Cell proliferation index is a prognostic marker in many types of tumours and is evaluated using histochemistry, counting the cells with a positive expression per stained field of each sample obtained. This expression is detected by IHC.14–16 These markers are essential for the diagnosis, prognosis, management and prediction of a possible recurrence or even the possibility of a malignant transformation.17–20
The Ki-67 marker is a nuclear and nucleolar protein that determines cell proliferation and is found in all active parts of the cell cycle and therefore is related to abnormal cell growth. COX-2 is an enzyme that induces the production of cytokines; it increases in OKCs and is marker for the behaviour and nature of these lesions.
Finally, Cyclin D1 is a nuclear protein that plays a crucial role in the cell cycle, allowing the progress of the DNA synthesis phase and therefore evaluates cell proliferation.14,17,21
The main objective of this study is to compare the influence of Ki-67, Cyclin D1 and COX-2 on the diagnosis and pathogenesis of OKC, OOC and AB.
Materials and methodsForty-one blocks embedded in paraffin and fixed in formaldehyde from 1970 to 2007 inclusive, were collected by non-random sampling from the Hospital Universitario 12 de Octubre (Madrid).
Cases were reconsidered using double-blind evaluation by two specialists in pathomorphology (B.D) of H&E stained sections, according to the 2017 WHO classification, confirming: 22 OKC, 6 OOC and 13 AB of solid type.
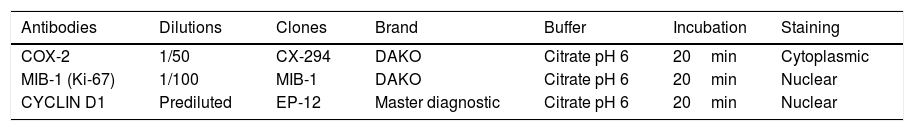
The samples were deparaffinized and hydrated and the hydroxymethylene bridges formed between the amino-acid sequence were broken by heat treatment to expose tissue antigens using a citrate buffer (pH 6.0). The primary antibody used to recognize the desired antigen was then incubated for 20min. Table 1 summarizes the primary antibodies, dilutions, clones, antibody brand, buffer used and incubation time.
Immunohistochemistry: primary antibodies, dilutions, clones, antibody label, buffer used and incubation time.
| Antibodies | Dilutions | Clones | Brand | Buffer | Incubation | Staining |
|---|---|---|---|---|---|---|
| COX-2 | 1/50 | CX-294 | DAKO | Citrate pH 6 | 20min | Cytoplasmic |
| MIB-1 (Ki-67) | 1/100 | MIB-1 | DAKO | Citrate pH 6 | 20min | Nuclear |
| CYCLIN D1 | Prediluted | EP-12 | Master diagnostic | Citrate pH 6 | 20min | Nuclear |
Subsequently, incubation was performed with the Bond Polymer Refine Detection Kit from Leica, Wetzlar, Germany, of the secondary antibody directed to the primary antibody (peroxidase-labeled polymer) and developed with Diamenobenzidine (DAB). DAB is a chromogenic chemical compound that binds to the antibody and reacts with peroxidase creating a dark brown stain in the deposits formed by the antigen-antibody complex. Finally, a brief incubation with H&E was realized to visualize the cell structure using a conventional light microscope Olympus® (Model BX43F, U-LHLEDC, Tokyo, Japón INC) at magnification 20×.
The quantification of the immunostaining was evaluated manually, the number of positive staining cells per 1000 epithelial cells was expressed as a percentage and depended on whether the staining was cytoplasmic or nuclear. For nuclear staining, such as Ki-67 and Cyclin D1, more than 5% positive nuclei was considered positive and cytoplasmic staining for COX-2 greater than 61% of cells was considered positive.
Statistic analysisData was analysed using SPPS version 23 and descriptive statistics were used. Pearson's Xi square and ANOVA test were performed and statistical significance was quantified with a p value<0.05.
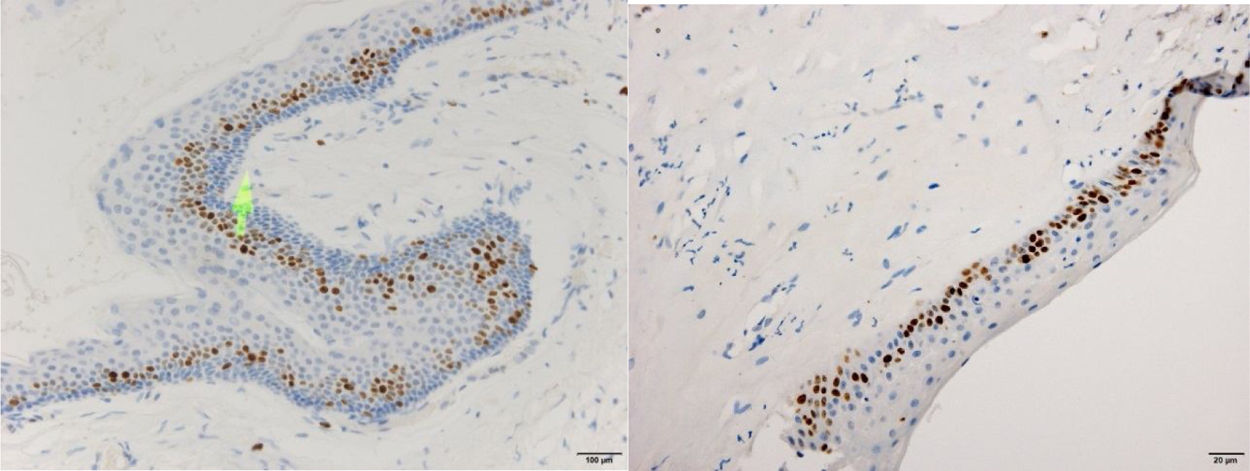
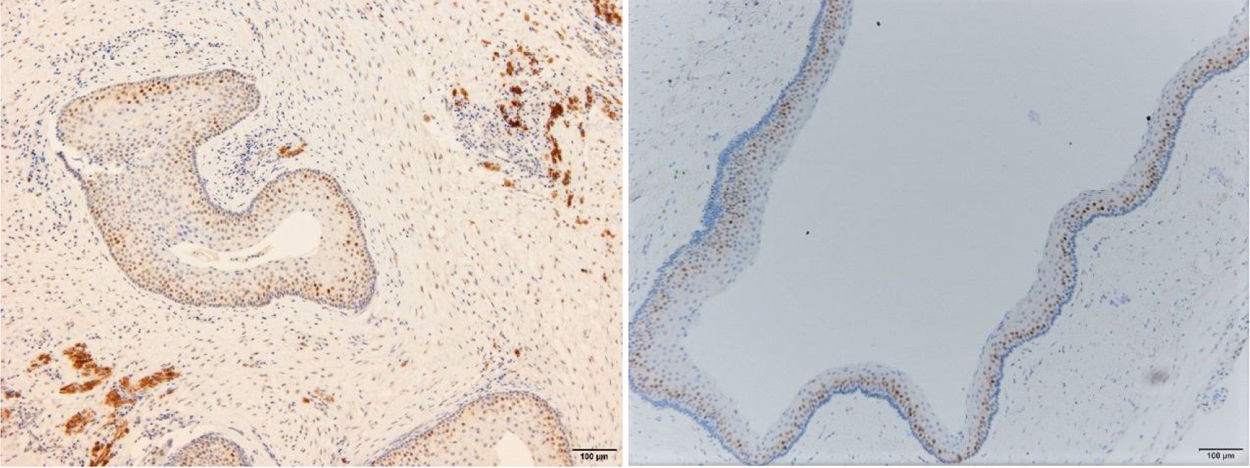
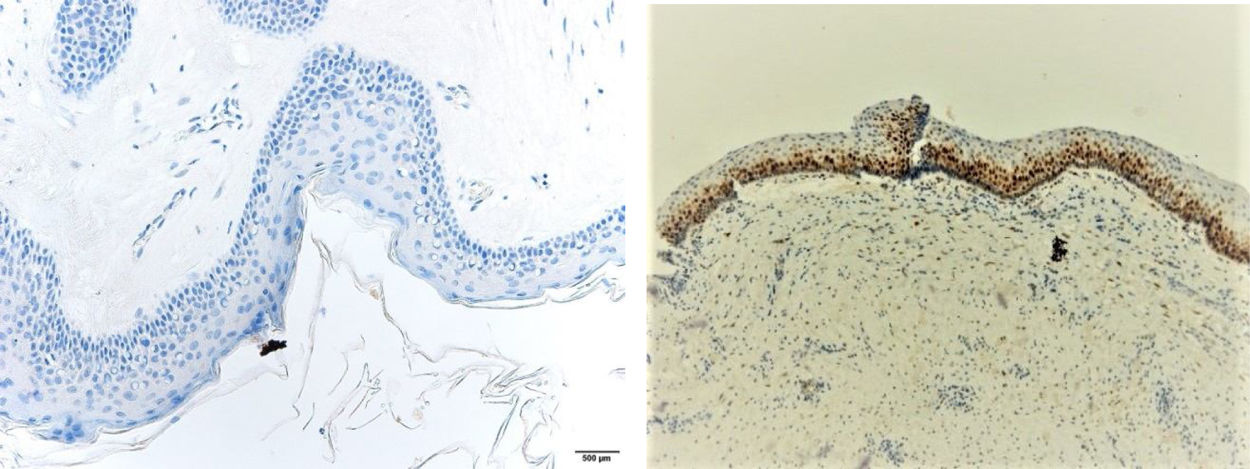
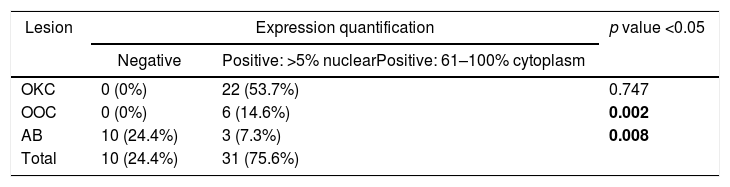
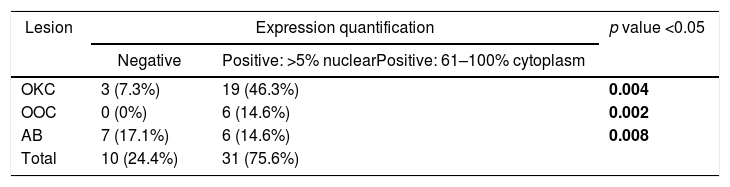
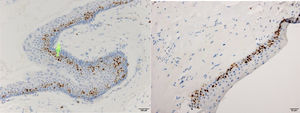
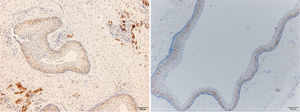
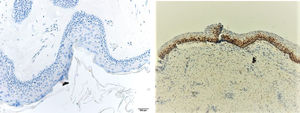
ResultsPositive results were quantified in nuclear staining (Ki-67 and Cyclin D1) (Figs. 1 and 2) when more than 5% of nuclei were positive and in cytoplasmic staining (COX-2) (Fig. 3) when greater than 61% were positive. Markers that demonstrated a positive correlation between COX-2 expression (p=0.005) are summarized in Table 2; Ki-67/MIB-1 (p=0.004) in Table 3 and Cyclin D1 (p=0.020) in Table 4. OKC versus AB presented higher values in their expression.
Fig. 1 shows the highest expression of Ki-67 in OKC mainly in the most basal layers, with greater cell proliferation, in contrast to AB, where mainly the suprabasal layers were positive and less cell proliferation and mitoses were found. In Fig. 2 the same is observed with the Cyclin D1 marker and in Fig. 3 with the COX-2 marker.
DiscussionDespite the different quantifications of IHC expression in different studies such as Gani et al., Alsaegh et al. or Mendes et al.,21–23 the number of cells positive for Ki-67 is always higher in OKCs than in ABs or OOCs; this was also found in the present study.
De Vicente et al. found that Ki-67 expression measured in percentage for dentigerous cysts was 17%, 15.5% for radicular cysts and 7.8% in ABs. However, the percentage rose to 40% in OKCs, leading them to conclude that the cell proliferation index was significantly higher in OKCs than in other types of lesions.
This finding would also point to an intrinsic growth potential of the OKC and a defective function of the tumour suppressor genes relating to a possible mutation of the PTCH 1 gene as well as an increase in cell proliferation, leading to more aggressive behaviour of these lesions.24
Both Alsaegh et al and Mendes et al, observing ABs and OKCs respectively and comparing COX-2 expression, obtained very high percentages of positive cells. However, the mean of positive cells (for COX-2) in our research is lower, approximately ±2. A possible explanation for these differences could be that they used Clones BA0738 with a 1:100 dilution, while we used the CX-294 clone at a 1:50 dilution.22,23
Finally, Gani, Vera-Sirera et al and De Vicente et al found a high expression of Cyclin D1 in OKCs vs ABs, coinciding with our results (p<0.05).17,21.24
In conclusion, statistically significant differences have been found for all three markers, COX-2, MIB-1/Ki-67 and Cyclin D1, which would suggest that they could prove to be useful tools in the differential diagnosis between OKCs, OOCs and ABs and also in predicting the behaviour of the cystic lesions. Furthermore, they could be of help in planning more aggressive surgery for OKC lesions.
FundingNo funding was received.
Ethics approval and consent to participateThis study is approval for the ethics committee Hospital 12 de Octubre and following the Helsinki Declaration.
Conflict of interestThe authors declare that they have no competing interest
We would like to thank the pathology department of the University Hospital “12 de Octubre” for their help with this study.