Traumatic spinal cord injury (SCI) leads to increased intraspinal pressure that can be prevented by durotomy and duroplasty. The aim of the study was to evaluate fibrosis and neural damage in a porcine model of SCI after duroplasty and application of hyaluronic acid (HA) in the tissue cavity.
Materials and methodsExperimental study. We created a porcine SCI model by durotomy and spinal cord hemisection of a cervical segment (1cm). Six pigs (Sus scrofa domestica) were used to evaluate three surgical scenarios: (1) control injury with dural reparative microsurgery, (2) duroplasty using bovine pericardium (BPD), and (3) previous method plus HA applied at the lesion. Animals were sacrificed one-month post-injury to assess fibrotic responses and neural tissue damage using conventional histological and immunohistochemical methods.
ResultsIn the control case, dural suture prevented invasion of the lesion by extradural connective tissue, and the dura mater showed a 1-mm thickening in the perilesional area. The bovine pericardium patch blocked the entrance of extradural connective tissue, decreased dura-mater tension, and satisfactorily integrated within the receptor tissue. However, it also enhanced subdural and perilesional fibrosis, which was not inhibited by filling the lesion cavity with low- or high-molecular-weight HA.
ConclusionsDuroplasty prevents collapse of the dura-mater over the spinal cord tissue, as well as invasion of the lesion by extramedullary fibrotic tissue, without creating additional neural damage. Nevertheless, it enhances the fibrotic response in the spinal cord lesion and the perilesional area. Additional antifibrotic strategies are needed to facilitate spinal cord repair.
La lesión medular traumática (LM) conlleva aumento de la presión intramedular pudiéndose reducir mediante durotomía y duroplastia. El objetivo del estudio es evaluar la fibrosis y la extensión de la lesión en cerdos con LM sometidos a duroplastia y relleno del defecto tisular con ácido hialurónico (AH).
Material y métodosEstudio experimental. Elaboramos un modelo de LM porcino mediante durotomía y hemisección medular con exéresis de un segmento cervical (1cm). Se emplearon 6 cerdos (Sus scrofa domestica), evaluándose tres escenarios quirúrgicos: (1) Lesión control con microcirugía reparativa dural; (2) Duroplastia mediante pericardio bovino (DPB); y (3) Situación anterior asociando aplicación de AH en la zona de lesión. Los animales fueron sacrificados 1 mes post-lesión para evaluar la fibrosis y el estado del tejido neural por técnicas de histología convencional e inmunohistoquímica.
ResultadosEn la situación control, la sutura dural previno la invasión de la lesión por tejido conectivo extradural, presentándose engrosamiento meníngeo de 1mm en la zona perilesional. El parche de pericardio bovino también bloqueó la entrada de fibroblastos extradurales, relajó la tensión de la duramadre y se integró satisfactoriamente con el tejido receptor. Sin embargo, también incrementó la fibrosis subdural y perilesional, y dicha fibrosis no fue inhibida por la aplicación de AH de bajo o alto peso molecular en el defecto medular.
ConclusiónLa DPB previene el colapso de la duramadre sobre el tejido medular lesionado, así como la invasión de la lesión por tejido fibrótico extramedular, sin causar daño neural adicional. Sin embargo, incrementa la fibrosis dentro y alrededor del defecto medular. Se requieren tratamientos antifibróticos adicionales para facilitar la reparación de la médula espinal.
Peridural (PF) and intradural fibrosis is a scarring phenomenon with a high incidence after spinal trauma or elective spinal surgery, and there is no known effective clinical procedure to prevent its onset. The cellular origin of fibrosis is diverse, and primarily involves fibroblasts from extramedullary connective tissue and meningeal cells, as well as pericytes and perivascular fibroblasts from the neural parenchyma itself.1–4
Fibrosis is a major contributor to failure of axonal regeneration in the central nervous system (CNS), constituting a mechanical and molecular barrier to neural regeneration while limiting the expression of molecular cues that would otherwise aid tissue repair.3,5,6 Following injury, fibrotic tissue contributes to the restoration of tissue boundaries, creating a CNS/connective tissue interface that is generally impenetrable to neural cells.1–6 This is essentially because on the neural side of the interface neural cells possess adhesion molecules for neurons and glial cells, plus trophic factors and many other biomolecules that retain homotypic cellular elements; whereas in the connective tissue not only are pro-regenerative molecules scarce, but there is also an abundance of negative regulators of neural growth, including semaphorins, proteoglycans, and a dense web of extracellular matrix.3–5
Therapeutic measures for established and correctly diagnosed PF are scarce. Some studies have evaluated the efficacy of various agents in the partial prevention of PF after surgery, such as duroplasty with pericardial patches,7,8 and antifibrotic gels, but this is currently an unresolved clinical problem.
Hyaluronic acid (HA) gels are currently used strategy.9–12 HA is highly biocompatible and does not cause a foreign body reaction. There are data that show that in the early stages of tissue repair, high concentrations of HA in the extracellular space may partially prevent collagen matrix deposition.9–12 These effects can be explained by the physical barrier that is generated, as well as by inflammatory regulation. However, the molecular mechanism by which HA prevents epidural adhesion has not been described in the literature. Various types of pericardial patch have also been used.7,8 Both strategies have been relatively successful in significantly decreasing scar tissue, but do not prevent the development of an interface with separation between neural and connective tissue, and have not yet been effective in promoting neural repair.
The aim of this experimental work is to develop a methodology to control scarring phenomena after spinal cord injury by reconstructive microsurgery of the meninges and using a dural patch. We also investigated the use of low molecular weight and high molecular weight HA as antifibrotic therapies in the area of the injury.
Material and methodsAnimalsRandomised experimental study. The population of the present project are 6 prepubertal female pigs (Sus scrofa domestica), 2 months old, with an average weight of 12–15kg at the time of injury, distributed into three experimental groups. The pig was used because of its suitability for the size of the spinal cord, and its neuroanatomy and histopathology, which are similar to those of humans.3,13,14
The experimental procedures were conducted in compliance with animal welfare standards, European Commission recommendations and Spanish regulations for the protection of experimental animals (86/609/EEC, 32/2007 and 223/1988). The protocols were approved by our centre's Ethical Committee for Animal Experimentation and by the Junta de Comunidades de Castilla-La Mancha (resolution 2859425).
The animals were kept in our facilities for at least 3 weeks from their arrival for acclimatisation and training before any procedure was performed. During this period of time, they were accustomed to being handled by the animal facility staff and by the researchers, and to wearing harnesses that were later required for their rehabilitation.
General methodology and description of the experimental groupsThe first two experimental animals were used to fine tune the different surgical techniques and procedures, and the following four animals (numbered 1–4) were used to evaluate the experimental treatments. Animal 1 underwent C6 spinal cord hemisection and dural reparative microsurgery, the latter being used as the control injury. In animal 2, after durotomy and medullary hemisection, repair was performed with bovine pericardium with continuous 7-0 monofilament suture under an operating microscope. In animals 3 and 4, after spinal hemisection was performed, .5cc of high molecular weight HA (6×106Da) (SynviscOne©) or low molecular weight HA (Anti AD©), respectively, was instilled into the spinal defect and the dura mater was then repaired with bovine pericardium with 7-0 continuous suture.
Surgical proceduresAfter the adaptation period, the animals were transferred to the experimental operating theatre and received inhalation anaesthesia to perform the spinal cord hemisection, the anaesthetic process was performed by our centre's veterinarian. Anaesthesia was induced with an intramuscular (i.m.) injection of ketamine (10mg/kg), midazolam (.1mg/kg), and medetomidine (.02mg/kg), followed by intravenous (i.v.) administration of propofol (3mg/kg). The animals were then intubated with an endotracheal tube and anaesthesia was maintained with sevoflurane (1.7–2%) together with remifentanil (26mg/kg/h i.v.) and rocuronium (1.2mg/kg/h i.v.). Mechanical ventilation (Fabius Tiro, Dräger) was set at 12–14breaths/min with a tidal volume of 10–15ml/kg. In addition, heart rate, blood pressure, exhaled carbon dioxide, blood oxygen saturation, and inspired and exhaled sevoflurane levels were monitored throughout the procedure.
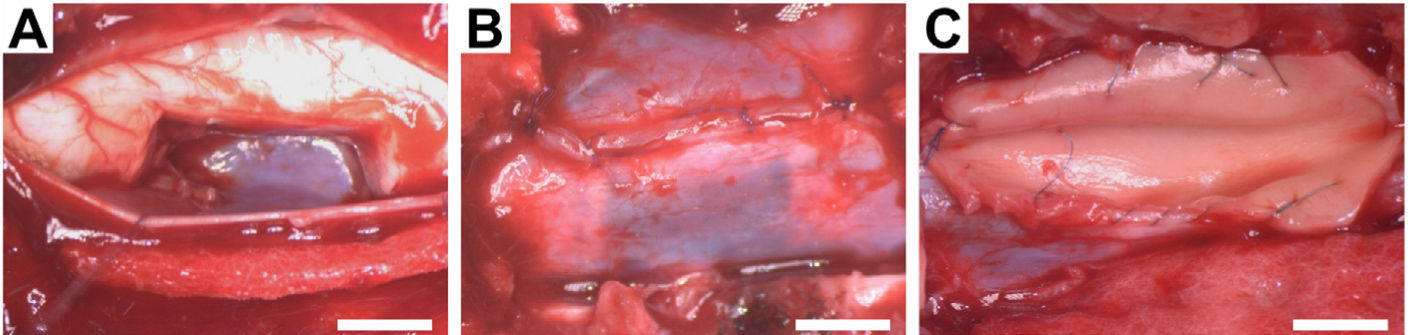
The animals were placed in the prone position. The surgical field was prepared by shaving the dorsal cervical area and applying 2% chlorhexidine as a disinfectant. The posterior cervical approach was then used from C4 to C7. The C5 cervical vertebra was located and a hemilaminectomy was performed with a 2° bone punch and, under an operating microscope, a midline durotomy was performed with exposure of the arachnoid, and the dorsal and ventral roots of the right side of the C6 spinal segment. Once the C6 segment was correctly identified, the dorsal and ventral roots were cut. The midline was located and the hemisection was performed by excising a 1cm long portion of the right side of the spinal cord (Fig. 1). Once the bleeding caused by the excision of the tissue was controlled, dural reconstructive microsurgery with 7-0 prolene was performed, and the application of bovine dural plasty alone or with HA depending on randomisation. No cervical spinal stabilisation system was required after the therapy, given the low instability and the strong cervical musculature of the pig. Layered closure was performed, using an intradermal skin suture to prevent the animal from self-injury. After surgery, the animals received meperidine (4mg/kg) subcutaneously (s.c.) as analgesia every 12h for two days, marbofloxacin (2mg/kg i.m.) as antibiotic, and meloxicam (.2mg/kg s.c.) as an anti-inflammatory agent for 7 days.
Intraoperative photographs of the surgical procedures. (A) Cavity resulting from excision of 1cm length of the right side of the C6 spinal cord segment. (B) Dural reconstructive microsurgery with continuous suturing. (C) Application of hyaluronic acid to the spinal cord defect and spinal dural patch with bovine pericardium and continuous suture. Scale bar, 5mm.
Afterwards in their pens the animals were provided the necessary analgesic care and assisted with feeding and hydration. The injured pigs required wheelchairs to begin limb mobility and prevent muscle atrophy.
Spinal cord removal, processing, and histologyOne month after injury, the animals received inhalational anaesthesia and underwent dorsal laminectomy of the C1 to T2 vertebrae. They were then administered a lethal dose of pentobarbital (120mg/kg i.v.), and the cervical spinal cord was rapidly removed and immediately immersed in 4% paraformaldehyde solution in phosphate buffered saline (PBS) for fixation for 3 days at 4°C. Once the tissue fixation was complete, the medullary segments were identified and individualised, and cryoprotected by immersion in 30% sucrose in PBS for 4 days. Finally, each medullary segment was embedded in freezing medium and stored at −20°C until sectioning in the cryostat. Parasagittal sections of 50μm thickness were obtained and stained with cresyl violet, as well as 10μm sections for combined neurofilament (NF) and platelet-derived growth factor receptor-β (PDGFRβ) immunohistochemistry. Cresyl violet stains Nissl bodies and nuclei, providing an overall picture of the state of the spinal cord, neuronal death, and the presence of cells and tissue cavitation. Neurofilament stains neurons and their axons, and PDGFRβ, fibroblasts and pericytes, allowing visualisation of the injury borders and fibrosis. For immunohistochemistry, tissue sections were incubated 30min in PBS with 1% triton and 2% goat serum, rinsed three times with PBS, and then incubated overnight at 4°C with primary antibodies (NF, Sigma–Aldrich N0142, 1:500; PDGFRβ, Abcam AB32570, 1:100) dissolved in PBS with 1% triton and 1% serum. The sections were then washed and incubated for 2h with Alexa 488- and 594-labelled secondary antibodies (Molecular Probes, 1:500), and cell nuclei were also labelled with Hoechst 33342 (Molecular Probes, 1.5mg/ml in PBS for 15min).
ResultsGeneral condition of the animalsNo infectious process or visible skin necrosis was observed in the post-operative follow-up period of the experimental animals. No animals were lost during the study period.
The degree of motor dysfunction after cervical spinal cord hemisection in pigs has been previously described.14 In the present study, no functional loss in addition to that caused by the spinal cord injury itself was observed in any of the animals, indicating that the new surgical procedures for reconstruction of the dura mater have no detrimental effect on the course of the disease.
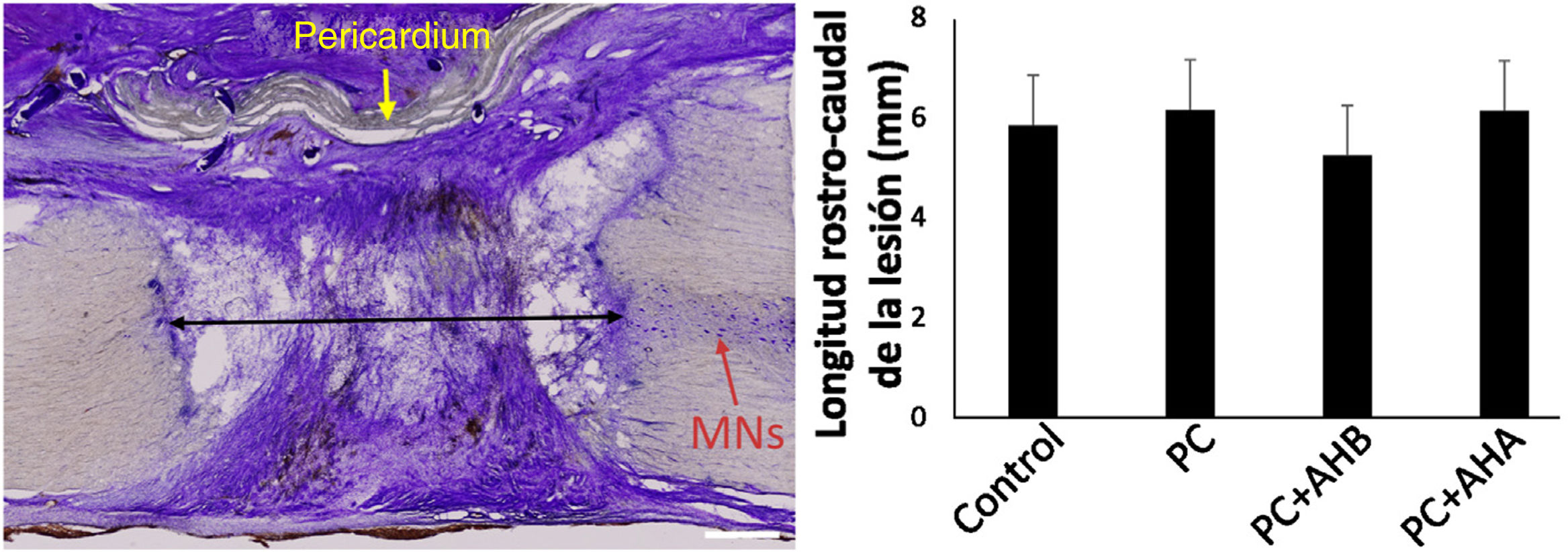
Extent of injuryOn study of the samples by cresyl violet staining 1 month after injury, in the control hemisection a tissue defect of approximately 6mm was found in the rostro-caudal plane of the spinal cord, this length was similar in the animals that received the bovine pericardial suture with or without added HA (Fig. 2), irrespective of molecular weight. At this point, the bovine pericardium had not completely resorbed, creating an effective separation between the extramedullary connective tissue and the interior of the lesion, and allowing analysis of cellular responses to surgical procedures. Moreover, no necrosis, cysts, or other signs of neural pathology attributable to the duroplasty or HA administration were observed.
Rostro-caudal extent of spinal cord damage and general appearance of the injured area. Left, tissue section centred on the spinal injury, processed for cresyl violet. The extent of the lesion was measured as the distance to healthy tissue between the rostral and caudal stumps (black arrow), with no differences between the different study groups (right). MNs, motor neurons. Note the dense connective tissue that has invaded the lesion area. Scale bar, 1mm.
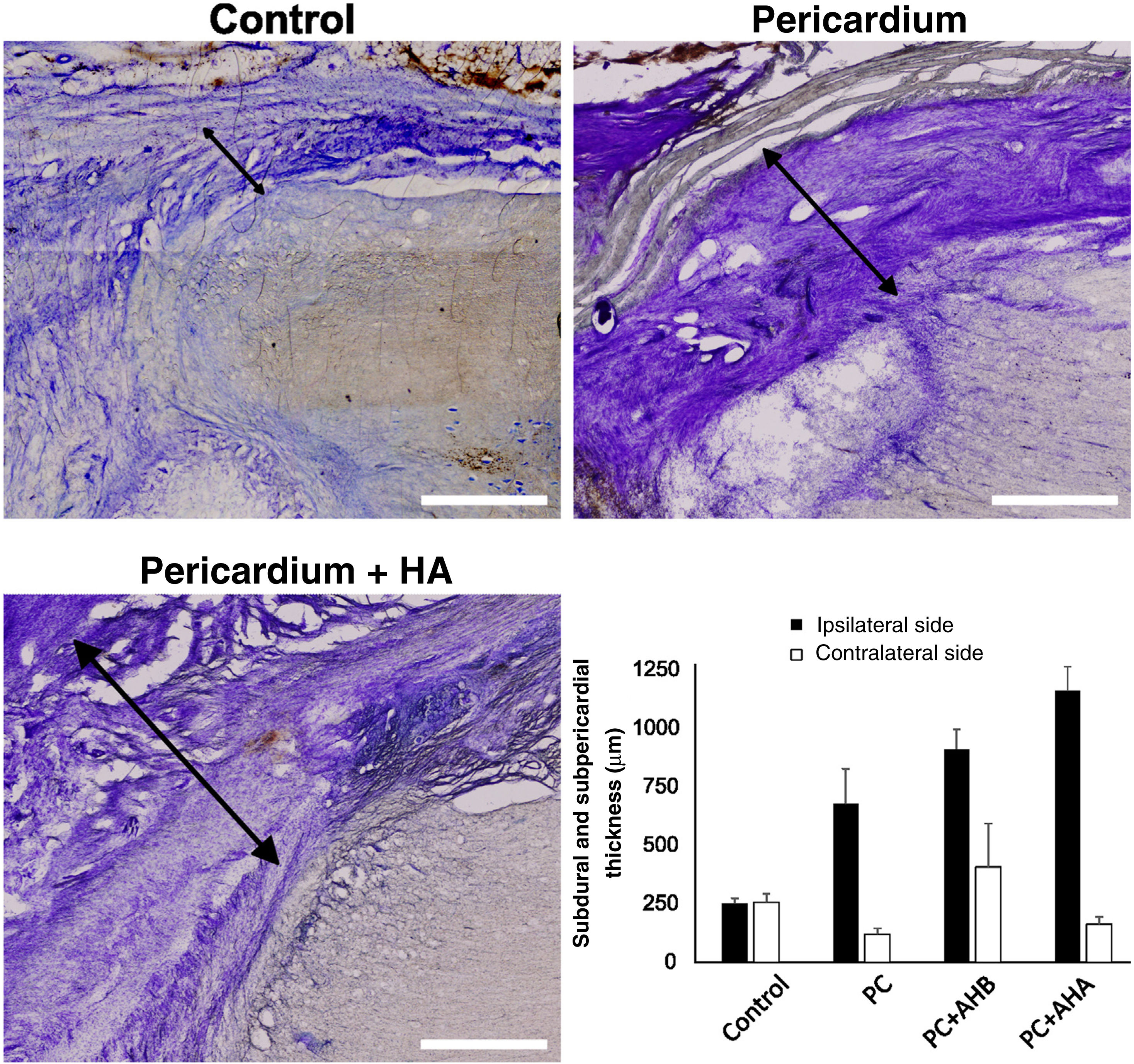
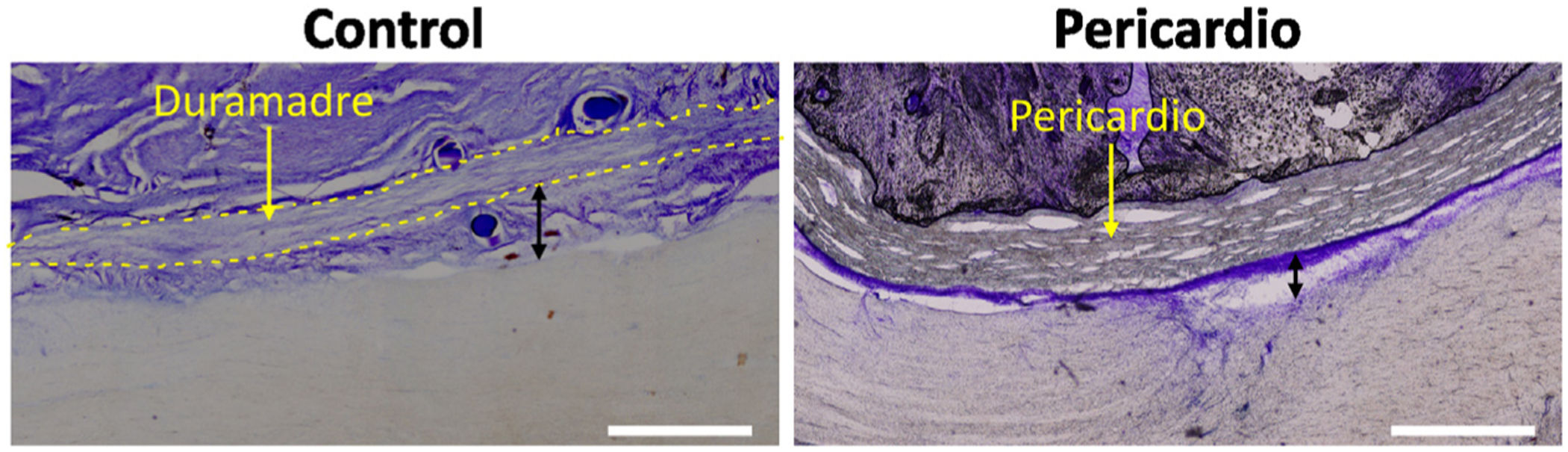
Conventional histology showed that the injury induced a proliferation of meningeal cells, with extensive meningeal fibrosis extending into the tissue defect and fusing dorsoventrally to the spinal cord. The thickness of the subdural meningeal fibrotic meningeal tissue was approximately 250μm on the side of the lesion in the control case, tripling in the case of the bovine pericardial graft (Fig. 3). This subpericardial thickening was almost 1mm after using low molecular weight HA and more than 1mm with high molecular weight HA.
Subdural and subpericardial scar connective tissue, ipsilateral and contralateral to the lesion. Histological sections ipsilateral to the lesion are shown exemplifying subdural and subpericardial fibrosis in the different groups and their respective quantification. Fibrotic thickening was measured between the spinal cord and dura/pericardium (black arrows), being, for the side ipsilateral to the lesion, smaller in the control and increasing with additional procedures. Scale bar, 1mm.
Despite increased fibrosis in the animals treated with bovine pericardium alone or in combination with HA, this tissue reaction was mostly observed on the injured side of the spinal cord, while the uninjured side induced a minimal response, similar or even less than in the control situation (Fig. 4).
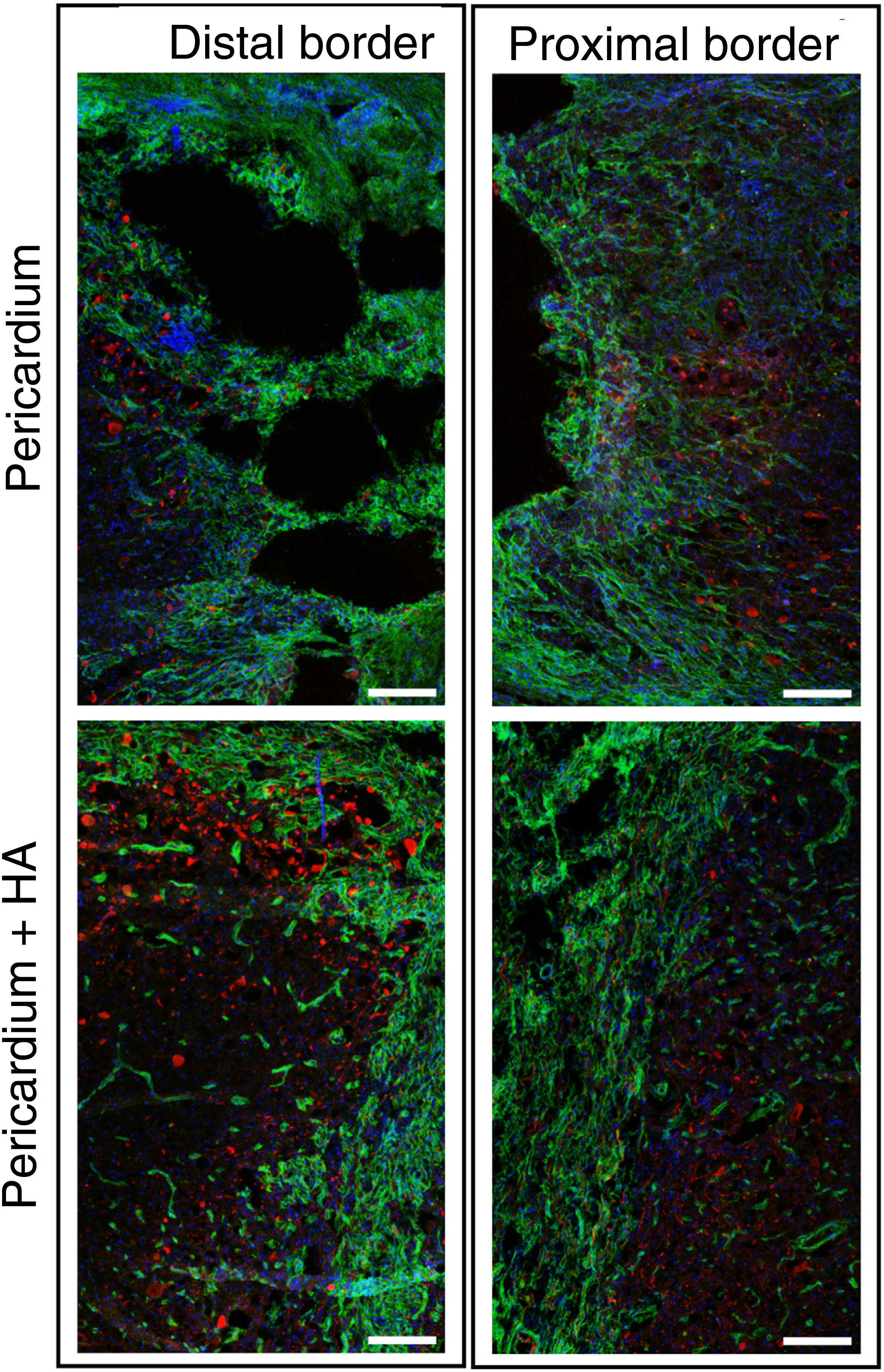
Healing and axonal growthTo obtain better visualisation of the cells present in the scar and the neural growth response, double immunohistochemistry was performed by identifying axons by staining for neurofilament (NF), and fibrotic cells (fibroblasts and pericytes) by staining for platelet-derived growth factor beta receptor (PDGFRβ). As in rodents15 and humans,16 in porcine spinal cord injury, fibrotic cells expressed PDGFRβ and formed an impenetrable scar for most axons, irrespective of the treatment (Fig. 5). In HA-treated animals, an even greater fibrotic reaction with transverse orientation was observed at the border of the lesion (Fig. 5).
Fluorescence images using a 10× objective on a confocal microscope, centred on the distal and proximal borders of the lesion, taken from tissue sections processed for PDGFRβ (green) and NF (red). Hoechst-stained blue nuclei are shown. A dense fibrotic scar positive for PDGFRβ was observed that prevented axonal growth (red). The scar was denser and more impenetrable to axons when hyaluronic acid was applied to the lesion. Scale bar, .2mm.
Peridural and intramedullary fibrosis is a frequent phenomenon after spinal cord trauma and reducing it poses a therapeutic challenge. Various therapeutic strategies have been proposed, but have failed to prevent it.
In the absence of a treatment to induce neural growth and block intraspinal fibrosis, the subdural space and cavities resulting from spinal cord injury are filled with fibrotic tissue.3 Direct dural suturing attenuates this process in rats,5,17 preventing infiltration of the injured area by connective tissue from ligaments, periosteum, and other spinal structures. However, it does not completely eliminate it due to the proliferation of fibrotic cells from intradural tissues, including the meningeal cells themselves,1,3,18 pericytes,2,6 and perivascular fibroblasts.15 Finally, fibrotic tissue adheres the borders of the spinal cord to the meninges and limits the successful application of neuroreparative therapies.3,19
Furthermore, dural suturing decreases the space available for the insertion of potential neuroreparative implants, and carries the risk of increased intraspinal pressure, associated with ischaemia and neurological deterioration.20
This potentially negative effect could be prevented by duroplasty. Previous studies have evaluated the fibrotic response in pigs following laminectomy, opening of the dura mater, and application of different types of dural patches.7,8,21 To our knowledge, there is no history of evaluating BPD in a porcine model of spinal cord injury. Based on the results obtained in our study, this therapeutic alternative is of interest for the treatment of spinal cord injury and should be further explored.
In this preliminary study, we evaluated subdural and perilesional fibrosis and lesion extent in pigs with spinal cord injury that underwent bovine pericardial duroplasty (BDP) and filling of the tissue defect with HA. Although BDP blocked fibrotic tissue entry from extradural structures without evidence of immune rejection, the procedure increased fibrosis within the lesion and this fibrosis was not inhibited by applying HA to the tissue defect. The environment of the traumatised spinal cord area is pro-inflammatory in the acute phase, and under these conditions the opening of the dura mater and the bovine pericardial graft probably enhanced the proliferation and migration of fibroblasts and other connective tissue cells into the lesion. Furthermore, in the absence of neural growth-inducing treatment, the increased space available after duroplasty filled with fibrotic tissue. These results indicate that BDP can be used to reduce mechanical stress after spinal cord trauma and incorporate implants in the area of injury, but must be accompanied by effective anti-fibrotic treatments to facilitate neural repair.
This preliminary study focused on evaluating the effect of BDP on subdural, perilesional, and intralesional fibrosis, as these scar elements contribute to the failure of axonal regeneration after spinal cord injury and their control is essential for successful neuroreparative treatment.3,5 However, it is to be expected that BDP and its barrier effect also influence histological reactions of the dura mater itself and extradural fibrosis. These aspects of great clinical relevance22 were not addressed in the present study and require a specific experimental methodology, including the use of specific labelling for the different types of meningeal and connective tissue cells.23
The limitations of the study also include the small number of experimental subjects used, as well as the follow-up of only one month post injury. The use of single cases in each group does not allow for adequate control of individual variability in the response to surgical procedures. Interpersonal variations in the formation of hypertrophic surgical scars have been reported,24 and we do not know whether a similar phenomenon could occur in our porcine model. However, the use of animals from the same supplier, as well as the reproducibility of the extent of fibrosis observed in the same type of animals subjected to contused spinal cord injury,3 reduce this possibility. It is also noteworthy that the main histological responses were similar in the three animals that underwent BDP, including the absence of increased tissue damage, chronic inflammation around the graft, spinal cord compression, and increased fibrosis.
Moreover, the animals were sacrificed at one-month post-injury, at which time the bovine pericardium had not yet been completely resorbed. Histological evaluation in the longer term would be desirable, when there are no more implant remnants, and to use a larger number of animals and sacrifice them at different times to analyse the evolution of the fibrosis. Despite these limitations, it is relevant to mention that, in the prepubertal pigs used, one month's evolution is equivalent to approximately two human years.13 Therefore, the histological responses observed probably correspond to the chronic stage and provide clinically relevant information.
Finally, it is worth mentioning the possibility that other gels22 or pharmacological schemes that have demonstrated an antifibrotic effect after spinal cord injury in rodents19 may allow use of BDP without increasing the invasion of the damaged area by connective tissue. Thus, the clinical utility of BDP could be increased, limiting the negative consequences of fibrosis, and facilitating neuroreparative responses in spinal cord injury.
ConclusionsFibrosis in the spinal cord defect was not inhibited by the application of low or high molecular weight HA. BDP prevents the collapse of the dura mater over the injured spinal cord tissue, as well as invasion of the lesion by extramedullary fibrotic tissue, without causing additional neural damage. However, it increases fibrosis within the spinal cord defect, necessitating the use of additional anti-fibrotic treatments in conjunction with neuroreparative therapies for spinal cord injury.
Level of evidenceLevel of evidence i.
FundingThis research project received specific support from research grants from the Spanish Society of Orthopaedic Surgery and Traumatology (SECOT) and the Spanish Society for Spine Specialists (GEER) for its elaboration.
Conflict of interestsThe authors have no conflict of interests to declare.