The incidence of periprosthetic joint infection (PJI) in hip surgeries has significantly decreased thanks to intravenous (IV) antibiotic prophylaxis. However, in patients colonised with methicillin-resistant Staphylococcus aureus (MRSA) or those at risk of colonisation, it is necessary to include vancomycin in the prophylaxis. Intraosseous administration of vancomycin could enhance its effectiveness in total hip arthroplasty (THA).
Materials and methodsA retrospective study was conducted between March and December 2023 involving 53 patients scheduled for primary THA with colonisation risk factors. The median age of the patients was 67 years (range 61–75), and all received treatment with intraosseous vancomycin (500mg). Detailed records and documentation of complications during hospitalisation and the first three months post-surgery were maintained. As a secondary outcome measure, the incidence of PJI was explored.
ResultsWe administered 500mg of intraosseous vancomycin, injected into the greater trochanter, along with standard IV prophylaxis. The incidence of complications was 1.64%. The PJI rate at 90 days was 0%.
ConclusionsIntraosseous administration of low-dose vancomycin in THA for patients at risk of MRSA colonisation, combined with standard IV prophylaxis, was shown to be safe and did not present significant adverse effects. Furthermore, this strategy eliminates the logistical challenges associated with timely vancomycin administration.
Level of evidence IV: Case series.
La incidencia de infección articular periprotésica (IAP) en cirugías de cadera ha disminuido significativamente gracias a la profilaxis antibiótica intravenosa (IV). Sin embargo, en pacientes colonizados con Staphylococcus aureus resistente a meticilina (SARM), o en aquellos con riesgo de colonización, es necesario incluir vancomicina en la profilaxis. La administración intraósea de vancomicina podría mejorar su eficacia en la artroplastia total de cadera (ATC).
Materiales y métodosSe realizó un estudio retrospectivo entre marzo y diciembre de 2023 con la participación de 53 pacientes programados para ATC primaria con factores de riesgo de colonización. La mediana de edad de los pacientes fue de 67 años (rango de 61 a 75), y todos recibieron tratamiento con vancomicina intraósea (500mg). Se llevaron a cabo registros detallados y documentación de las complicaciones que se presentaron tanto durante la hospitalización como en los 3 primeros meses posteriores a la cirugía. Como medida de resultado secundaria, se exploró la incidencia de IAP.
ResultadosAdministramos 500mg de vancomicina intraósea, inyectada en trocánter mayor, junto a profilaxis IV habitual. La incidencia de complicaciones fue del 1,64%. La tasa de IAP a 90 días fue del 0%.
ConclusionesLa administración intraósea de dosis bajas de vancomicina en ATC en pacientes con factores de riesgo de colonización por SARM, combinada con profilaxis IV estándar, se mostró segura y no presentó efectos adversos significativos. Además, esta estrategia elimina los desafíos logísticos asociados con la administración oportuna de vancomicina.
Total hip arthroplasty is arguably one of the most successful surgical procedures in orthopaedics, although it can be affected by periprosthetic infection. This feared complication presents therapeutic challenges and a substantial socioeconomic burden. The geographic variability in causative agents highlights the complexity of the problem. Approximately two-thirds of periprosthetic joint infections are caused by Staphylococcus.1,2 Although cephalosporins are commonly used as prophylaxis, their efficacy has been questioned due to often insufficient tissue concentrations.3
The increase in antibiotic resistance among coagulase-negative Staphylococcus aureus is prompting medical professionals to re-evaluate their approach.4,5 In this context, vancomycin is emerging as the recommended prophylactic antibiotic to address these circumstances.
However, the use of intravenous vancomycin has been associated with various adverse effects, and its proper administration has become a truly logistical challenge.6,7 For this reason, in recent years, the administration of low doses of intraosseous vancomycin in knee arthroplasty using a haemostatic cuff has gained popularity. This technique has been shown to achieve high tissue concentrations, presenting reduced complication rates and overcoming the technical difficulties associated with intravenous administration.8
Its application in hip arthroplasty has recently been addressed in a publication, which demonstrated significant increases in vancomycin tissue concentrations compared to intravenous administration, with minimal adverse effects reported.9
The purpose of this study was to evaluate the complications associated with the intraosseous administration of a single dose of vancomycin in the greater trochanter during primary total hip arthroplasty, used as an additional prophylactic measure to standard cefazolin in patients with risk factors for methicillin-resistant Staphylococcus aureus (MRSA) colonisation during the hospitalisation period and the first 3 postoperative months.
Material and methodsWe conducted a retrospective study between April and December 2023 with the participation of 53 patients. All patients who underwent primary elective hip arthroplasty with a diagnosis of coxarthrosis or avascular bone necrosis at our institutions and who had one or more risk factors for MRSA colonisation were included. These factors included a body mass index (BMI)≥35; insulin-dependent diabetes; IV dependence; haemodialysis; chronic kidney disease (CKD); chronic skin disease/furunculosis; prolonged hospitalisation and/or transfer from another institution (including closed communities); hospitalisation for more than 24h in the 3 months prior to evaluation; prior joint surgeries; antibiotic use for more than 1 month in the 6 months prior (cephalosporins, quinolones, multiple prior antibiotics); a history of prior S. aureus infection or colonisation; the presence of intravascular catheters, and immunosuppression. Exclusion criteria included patients undergoing hip arthroplasty for fractures, prosthetic revisions, and known hypersensitivity to vancomycin.
Uncemented metaphyseal fixation implants were used in 46 patients; For acetabular cups measuring 52mm or larger, we used highly cross-linked polyethylene and mostly 36mm heads, and for acetabular cups measuring 50mm or smaller, 32mm heads were used. Hybrid fixation using heads of the same diameter and tapered polished stems was used in 7 patients. Spinal anaesthesia was performed, and all patients received routine intravenous antibiotic prophylaxis with 1g of cefazolin (2g if the patient weighed more than 70kg or 3g if the weight was more than 120kg), administered in 3 doses every 8h. Diluted intraosseous vancomycin was administered following the protocol published by Harper et al.9
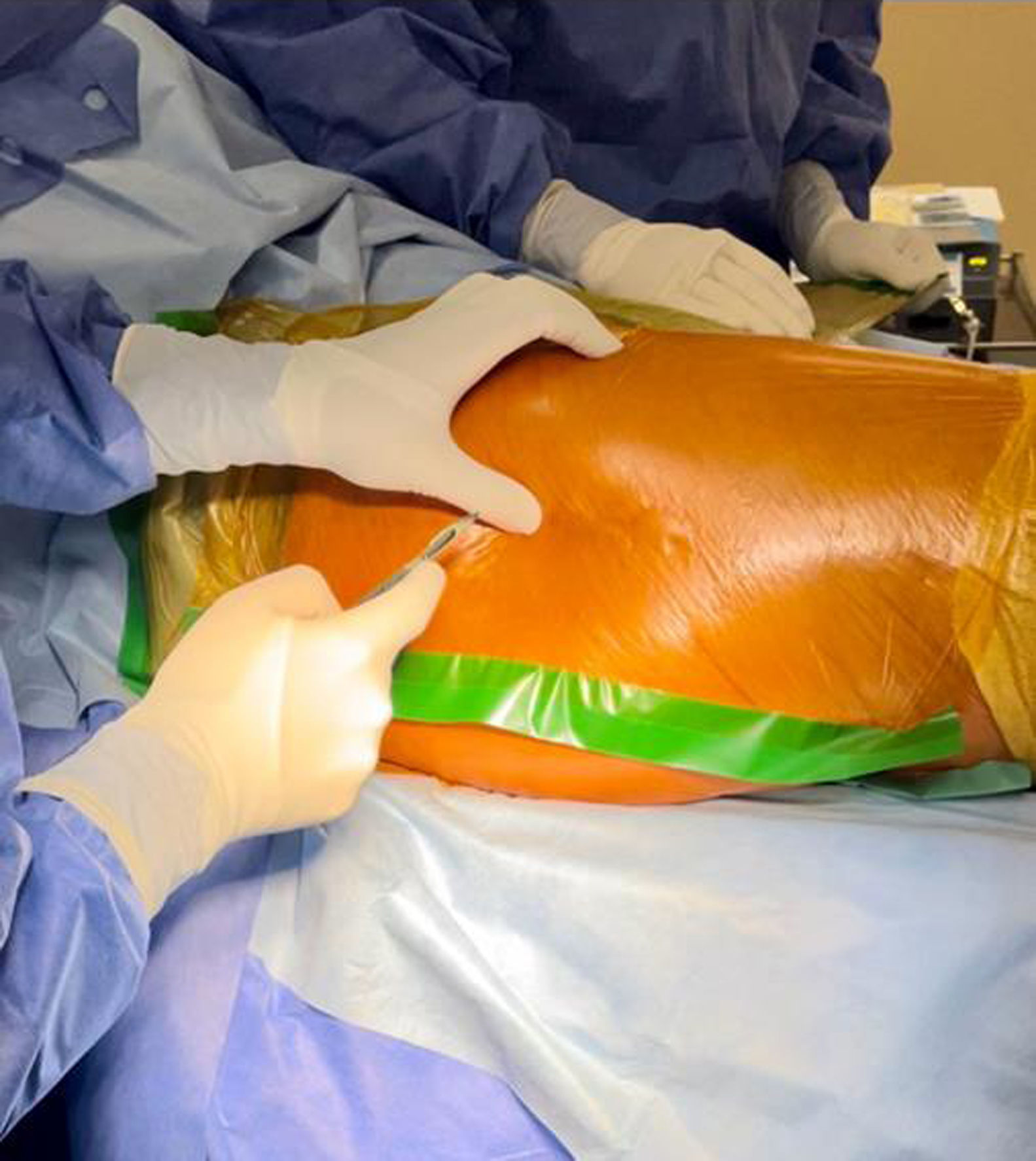
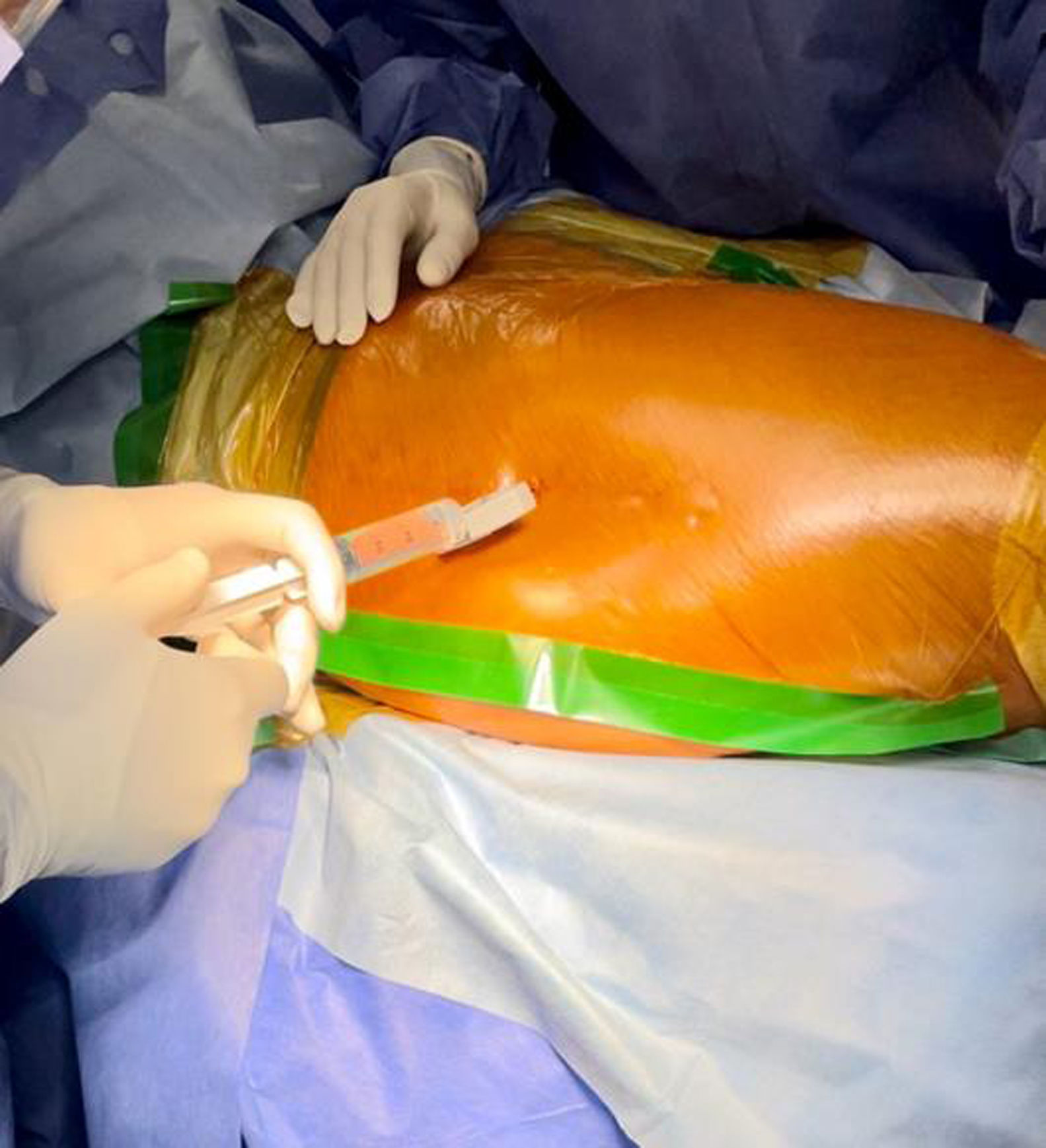
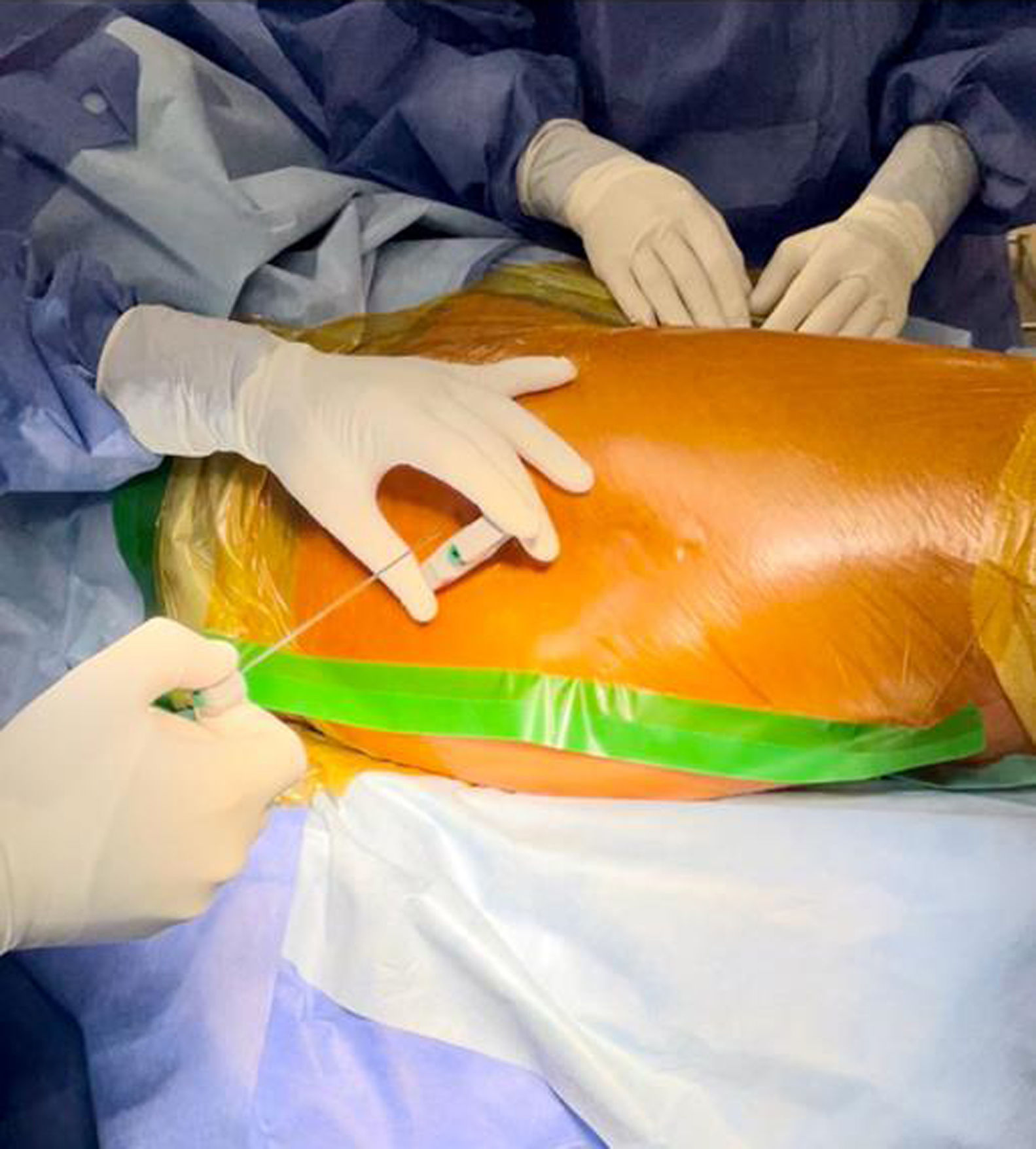
500mg of vancomycin was dissolved in 10ml of physiological saline; the mixture was then diluted in 90ml of saline solution. An incision was made in the adhesive field and the skin (Fig. 1), the cancellous bone on the lateral aspect of the greater trochanter was accessed with a Jamshidi needle (Fig. 2), and 100ml of the preparation was injected (Fig. 3). Intraosseous antibiotics were administered immediately before the skin incision for the procedure, and the dosage was not adjusted for obese patients. In obese patients with a large fat pad, we incised the skin down to the fascia and inserted the bone puncture needle.
Clinical safety was assessed by investigating the incidence of vancomycin-associated adverse reactions, including post-infusion hypotension; dyspnoea; pruritus; red man syndrome, related to massive histamine release due to rapid administration of the antibiotic; acute kidney injury, and neutropenia. Detailed records and documentation of complications occurring both during hospitalisation and during the first month after surgery were maintained. As a secondary outcome measure, the incidence of periprosthetic infection was investigated in the first 3 months after surgery.
We assessed blood pressure immediately after vancomycin infusion, throughout surgery (at 15, 30, and 60min after administration), and during hospitalisation. Hypotension was defined as a decrease in systolic blood pressure of more than 20mmHg and in diastolic blood pressure of more than 10mmHg. The records were taken with a manual blood pressure monitor.
We performed postoperative evaluations in all patients for upper body redness, itching, burning sensation, chest pain, wheezing, dyspnoea, and muscle spasms, all signs associated with red man syndrome.
We determined the diagnosis of acute kidney injury following the Kidney Disease Improving Global Outcomes guidelines, which are based on changes in serum creatinine levels and urine output.10 Acute kidney injury was considered present when serum creatinine increased 1.5-fold or more compared to baseline, or when the increase was equal to or greater than .3mg/dL compared to the patient's baseline. In addition, urinary flow was used as a criterion, considering a value less than 05ml/kg/h for a period of 6–12h.
In our study, we defined neutropenia as an absolute neutrophil count below 1000/μl.11
We monitored urinary flow in all patients and performed laboratory tests before and after surgery to assess the incidence of acute kidney injury and neutropenia. Monitoring included serum urea, creatinine, and white blood cell counts. Postoperative laboratory tests were performed at 48h and at 1 month for comprehensive follow-up.
Finally, we recorded the incidence of periprosthetic joint infection up to 3 months postoperatively, considering the diagnostic criteria for periprosthetic joint infection defined by the Musculoskeletal Infection Society (MSIS).12
ResultsThe mean patient age was 67 years (range, 61–75); 18 patients were men and 35 were women. All patients had risk factors for MRSA colonisation; 18 had two or more comorbidities that predisposed them to colonisation (Table 1).
Risk factors for MRSA colonisation in patients undergoing intraosseous vancomycin infusion.
| BMI≥35 | 12 |
| Diabetes | 13 |
| IV addiction | 2 |
| CKD/haemodialysis | 6 |
| Chronic skin diseases | 4 |
| Hospitalisation for more than 24h in the previous 3 months | 17 |
| Prolonged hospitalisation/transfer from another institution | 9 |
| Previous joint surgeries | 6 |
| Antibiotics in the previous 6 months | 1 |
| History of S. aureus infection or colonisation | 1 |
Eight patients in our study experienced hypotension; five of them during the BP measurement following the intraosseous vancomycin infusion, two during the measurement taken 15min after the intraosseous infusion, and one patient experienced hypotension half an hour after administration. In all cases, hypotension was mild, and all patients partially or completely corrected previous values without the need for vasoactive drugs (Table 2).
Blood pressure recording after intraosseous vancomycin infusion.
| Patients | Pre-infusion BP (mm/hg) | Post-infusion BP | BP 15min after | 30min after | 1h after | Maximum variation (mm/hg) |
|---|---|---|---|---|---|---|
| 1 | 119/83 | 96/63 | 102/70 | 104/74 | 113/79 | 23/20 |
| 2 | 109/74 | 85/60 | 89/64 | 87/62 | 102/72 | 24/14 |
| 3 | 132/83 | 109/71 | 121/80 | 118/77 | 122/76 | 24/12 |
| 4 | 98/70 | 77/56 | 84/61 | 84/62 | 92/68 | 22/14 |
| 5 | 114/81 | 93/70 | 104/77 | 102/72 | 118/94 | 21/13 |
| 6 | 143/92 | 134/90 | 122/79 | 129/84 | 134/89 | 21/13 |
| 7 | 108/71 | 103/68 | 88/60 | 90/62 | 115/79 | 20/11 |
| 8 | 128/82 | 119/80 | 120/71 | 107/67 | 119/72 | 21/15 |
No cases of red man syndrome were recorded; however, two patients reported mild symptoms of pruritus, which resolved spontaneously. The first patient experienced these symptoms 28min after the infusion, while the other experienced them 22h after the infusion. In addition, one patient reported mild dyspnoea 40min after the infusion, which improved with oxygen therapy.
One patient presented with acute kidney injury according to the criteria established by the Kidney Disease Improving Global Outcomes clinical guidelines. This patient was an insulin-dependent diabetic, and after correction of the postoperative hyperglycemia and electrolyte imbalances observed, his kidney function levels recovered to levels similar to those prior to surgery.
We had no cases of neutropenia in our case series.
One patient presented with persistent wound drainage, defined as a drainage area greater than 2cm×2cm on the incisional dressing that persisted for more than 72h postoperatively. Nonsurgical measures were implemented, including modification of venous thromboembolism prophylaxis (low-molecular-weight heparins were discontinued and aspirin was prescribed), nutritional supplementation, and restriction of range of motion. No surgical intervention was necessary in this patient.
The periprosthetic infection rate in the 90 days after surgery was 0% in our series.
The overall incidence of complications was 15% (Table 3), although all were mild and of little impact on the patients.
Complications associated with intraosseous vancomycin dosing in our series.
| Complications associated with the use of intraosseous vancomycin | Number of patients | Time until symptom onset |
|---|---|---|
| Low blood pressure | 8 | 0–30min |
| Pruritus | 2 | 28min and 22h |
| Acute kidney injury | 1 | 24h |
| Persistent wound discharge | 1 | 0–72h post-administration |
In the context of total hip arthroplasty, prevention of periprosthetic joint infection is crucial, and antibiotic prophylaxis plays a key role. However, the increase in MRSA and surgical site infections has raised uncertainties about the optimal surgical prophylaxis regimen. Our research suggests that low-dose vancomycin administration to the greater trochanter, along with intravenous cephalosporins, in patients at risk for colonisation, represents an effective and safe strategy for preventing infection. Furthermore, this approach has fewer adverse effects than intravenous vancomycin administration and avoids the logistical challenges associated with the latter.
Antibiotic prophylaxis should cover bacterial strains commonly associated with periprosthetic joint infection. Cephalosporins are a leading choice for preventing coagulase-negative S. aureus infections, which account for up to 70% of cases of periprosthetic joint infection.13 Despite their efficacy, an increase in beta-lactam resistance is observed with these agents. Studies, such as that by Stevoska et al., indicate that coagulase-negative Staphylococcus, with a high incidence of oxacillin resistance (50.0–100.0%),14 are the most common microorganisms in periprosthetic hip and knee infections. Furthermore, an increase in MRSA infections has been observed, reaching approximately 50% of infected patients, according to data from the US and the UK.15–17
Fortunately, both oxacillin-resistant coagulase-negative Staphylococcus and MRSA remain susceptible to vancomycin. However, intravenous administration of this antibiotic has been associated with multiple adverse effects. Complications associated with its use include nephrotoxicity. Courtney et al. reported an increased incidence of acute kidney injury in patients receiving intravenous cefazolin and vancomycin together as dual prophylaxis for total joint replacement.18 However, a study by Harper et al. highlighted that the use of intraosseous vancomycin in total knee replacement did not increase the incidence of acute kidney injury compared with intravenous vancomycin.19
Notwithstanding, the most feared complication of rapid intravenous vancomycin administration is the histaminergic release response, which can manifest from generalised flushing to severe hypotension, respiratory distress, and even shock and cardiorespiratory arrest.20 This anaphylactic reaction is attributed to the release of histamine following mast cell degranulation.
In recent years, a safe and effective technique for administering vancomycin as antibiotic prophylaxis in knee arthroplasty has gained popularity: intraosseous injection into the proximal tibial metaphysis.21 This technique has several notable advantages. First, the dose used (500mg) is half that used with intravenous administration (1g or 15mg/kg). Second, vancomycin concentrations in bone and subcutaneous fat have been observed to be much higher, reaching 10 to 15 times higher than those achieved with intravenous administration.22 Finally, this technique eliminates the logistical challenges associated with systemic vancomycin administration, such as timing of the infusion, appropriate dosing based on weight, and the slowness of dosing this antibiotic for antibiotic prophylaxis in joint replacements.8
Recently, a study by Harper et al. demonstrated that intraosseous infusion of this antibiotic into the greater trochanter in total hip arthroplasty just prior to surgical incision achieved statistically higher antibiotic concentrations in bone samples and all tissue samples compared to intravenous administration. This was achieved even without the ability to restrict blood flow to the area, as a haemostatic cuff was not possible. Furthermore, systemic vancomycin levels were significantly lower when administered intraosseously, and no adverse effects were observed with its use.9
The limitations of our study include the retrospective nature of the study, the small sample size, the specific characteristics of the patients, and the outcome measures. Ultimately, the results do not allow us to conclude whether the antibiotic administration method used results in a long-term reduction in periprosthetic infections. However, a significant strength lies in the fact that all surgeries were performed following a uniform protocol and technique.
ConclusionIntraosseous administration without blood flow restriction minimises the systemic complications commonly associated with vancomycin usage and eliminates the logistical challenges associated with its administration, marking a promising step towards improving the outcomes of total hip arthroplasty. Future research will focus on determining whether this technique translates to clinical reductions in infection, as has been previously demonstrated in total knee arthroplasty.
Level of evidenceLevel of evidence iv.
Ethical considerationsWe obtained approval from our institution's Research Ethics Committee (CPHA Research Ethics Committee, dated 9/02/23, reference no. 0000098657). The principles of the World Medical Association Code of Ethics (Declaration of Helsinki) were adhered to. All sensitive patient data were protected. We obtained informed consent from the patients included in this study.
FundingWe received no funding for this study from any public or private institution.
Conflict of interestsNone.