To describe mortality and complications of patients seen in the emergency room, diagnosed with necrotising soft tissue infection (NSTI) and the correlation of such complications with the Laboratory Risk Indicator for Necrotising fasciitis scale (LRINEC).
MethodsRetrospective observational study including patients with a diagnosis of NSTI in the emergency room of a tertiary hospital over 7 years. The results are shown as median, interquartile range and absolute range for quantitative variables. In the case of qualitative variables, the results are shown as absolute and relative frequency. The comparison between the categories of the LRINEC scale was performed through a post hoc comparison from a non-parametric ANOVA analysis. Comparisons between LRINEC groups in the qualitative variables were performed using Fisher's Exact test.
Results24 patients with a mean age of 51.9 years were identified. The LRINEC scale was used on 21 patients: in 10, the value indicated low risk (<6), in 4 it indicated intermediate risk (6 or 7) and in 7 it indicated high risk (≥8). The amputation rate in patients with low, intermediate and high risk was 10%, 25% and 66% respectively with a mortality of 4.2%. There was an increase in hospital stay between the low and high level of the scale (p=0.007).
ConclusionsIn general, a change in the prognosis between the medium and high levels of the LRINEC scale could not be recorded, but was recorded in hospital stay between the low and the high level, practically tripling the median of days of hospital stay.
Describir la mortalidad y las complicaciones de pacientes atendidos en urgencias, con diagnóstico de infección necrosante de partes blandas (INPB) y su correlación con la escala Laboratory Risk Indicator for Necrotising Fasciitis (LRINEC).
MétodoEstudio observacional retrospectivo con inclusión de pacientes con diagnóstico de INPB en urgencias de un hospital terciario durante 7 años. Los resultados se muestran como mediana, rango intercuartílico y rango absoluto para las variables cuantitativas. En el caso de las variables cualitativas, como frecuencias absoluta y relativa. La comparación se ha llevado a cabo mediante comparación post hoc a partir de un análisis ANOVA con aproximación no paramétrica. Las comparaciones entre grupos de LRINEC en las variables cualitativas se han realizado con la prueba exacta de Fisher.
ResultadosSe identificaron 24 pacientes con edad media de 51,9 años. La escala LRINEC se determinó en 21 pacientes: en 10 indicó bajo riesgo (<6), en 4 riesgo intermedio (6 o 7) y en 7 alto riesgo (≥8). La tasa de amputación en riesgos bajo, intermedio y alto fue del 10, 25 y 66%, respectivamente, con una mortalidad del 4,2%. Se observa un aumento en la estancia hospitalaria entre los niveles bajo y alto de la escala (p=0,007).
ConclusionesEn general no se ha podido constatar un cambio en el pronóstico entre los niveles medio y alto de la escala LRINEC. Pero sí entre la estancia hospitalaria entre el nivel bajo y el alto, triplicando prácticamente la mediana de días de estancia hospitalaria.
Necrotising soft tissue infection (NSTI) is an entity that is characterised by necrosis of the skin, subcutaneous cellular tissue, fascia, muscle or several of the said structures simultaneously. It progresses very swiftly, compromising limb viability and even the patient's life.1–3 Although this is a rare infection it still has a high rate of morbimortality which can be reduced by early diagnosis and treatment.4,5 Nevertheless, it is very hard to distinguish between NSTI and cellulitis in its early phases. The Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) scale has been validated in an attempt to ensure the early diagnosis of NSTI. This is based on levels of haemoglobin, glucose, C-reactive protein (CRP), creatinine, natremia and the number of leukocytes.6,7 This work aims to describe the mortality and complications of patients seen in the emergency room with a diagnosis of NSTI, together with their correlation with the LRINEC scale.
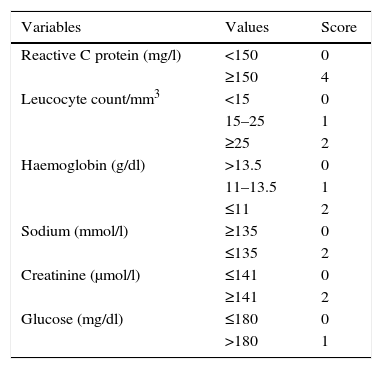
Material and methodsThe clinical records of all the patients with a confirmed diagnosis in our hospital of NSTI in a limb from January 2001 to February 2008 were reviewed retrospectively. This study included all of the patients who, in debridement following the clinical diagnosis of suspected NSTI by the emergency department, displayed surgical findings that are characteristic of necrotising infection: devitalised fascia easily dissected by the fingers between planes, or the finger test, the presence of purulent exudate or “dish-water pus”, the absence of bleeding and regional vascular thrombosis, and who were diagnosed histopathologically as necrotising fasciitis. The following data were recorded: age, sex, comorbidity, infection location, possible entry site of the infection, main symptoms, analytical results at admission, septic shock and the diagnosis of suspicion at admission. Additionally, the antibiotic treatment given at admission was recorded, together with the time passed between the arrival of the patient at the emergency department to surgical debridement, resected tissue histology, the number of operations, isolated microorganism, whether or not the limb was amputated, the duration of hospitalisation and mortality. The LRINEC score was calculated in all of the cases for which the necessary data were available (Table 1).
Laboratory risk indicator for necrotising fasciitis scale (LRINEC).
| Variables | Values | Score |
|---|---|---|
| Reactive C protein (mg/l) | <150 | 0 |
| ≥150 | 4 | |
| Leucocyte count/mm3 | <15 | 0 |
| 15–25 | 1 | |
| ≥25 | 2 | |
| Haemoglobin (g/dl) | >13.5 | 0 |
| 11–13.5 | 1 | |
| ≤11 | 2 | |
| Sodium (mmol/l) | ≥135 | 0 |
| ≤135 | 2 | |
| Creatinine (μmol/l) | ≤141 | 0 |
| ≥141 | 2 | |
| Glucose (mg/dl) | ≤180 | 0 |
| >180 | 1 |
LRINEC: Low risk: under 6. Moderate risk: 6–7. High risk: over 7.
The results are shown as the median, interquartile range and absolute range for the quantitative variables. The results of qualitative variables are shown as absolute and relative frequencies. The categories of the LRINEC scale were compared by post hoc based on an ANOVA analysis by the non-parametric transformation approximation of independent variables into ranges. Comparisons between LRINEC groups in qualitative variables were carried out using Fisher's exact test. All analyses used the SPSS v. 20 programme, and a type I bilateral error of 5% was applied in all of the statistical tests.
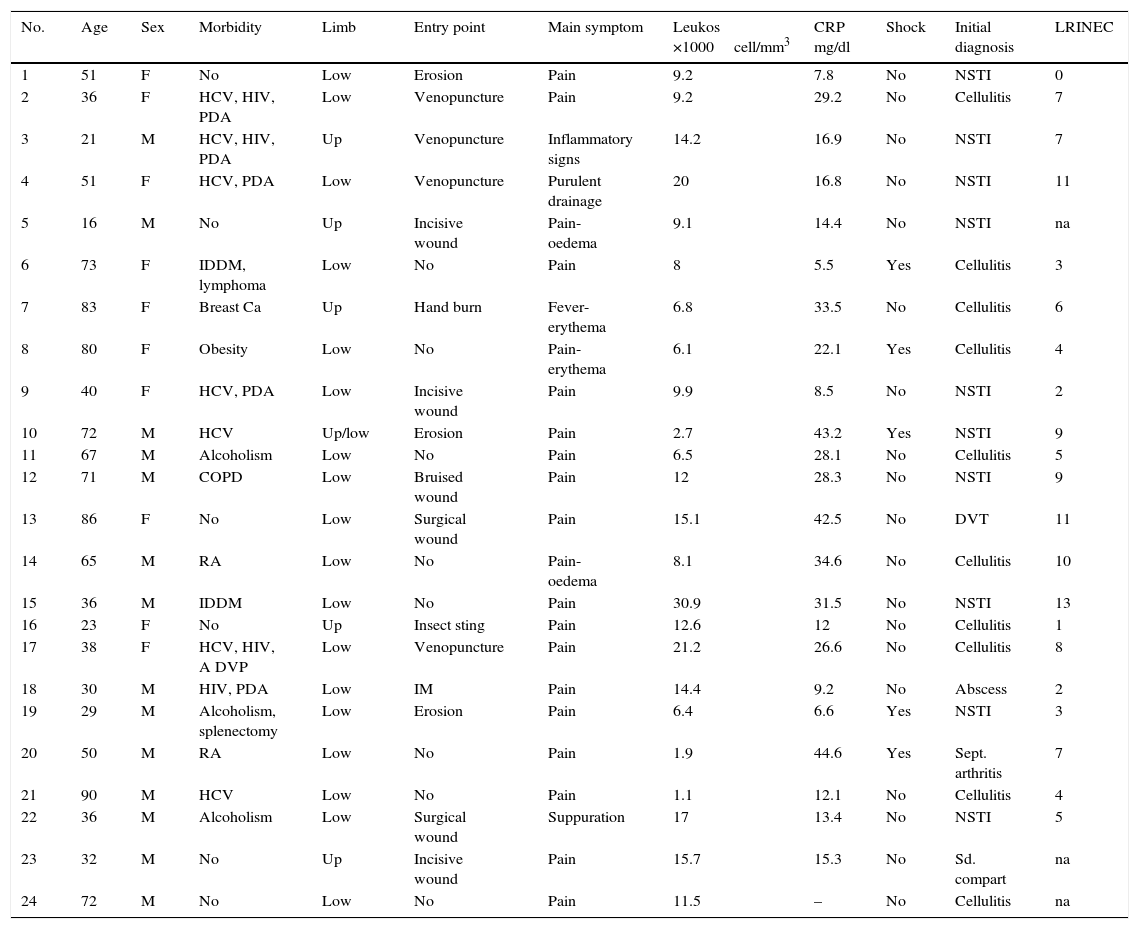
ResultsA total of 24 patients were identified. Their average age was 51.9 years old, and 13 of them were men. Seven patients (29.1%) were infected with the hepatitis C virus, 6 (25%) were parenteral drug addicts, 4 (16.6%) were HIV carriers, 3 (12.2%) were active alcoholics, 2 (8.3%) were insulin-dependent diabetics and 2 (8.3%) received chronic treatment for rheumatoid arthritis. Thirteen patients (54.1%) had 2 or more pathological antecedents. Six patients (25%) had no relevant pathological antecedent. In 6 cases (25%) the location was in an upper limb, while it was in a lower limb in 19 cases (79.1%). The chief clinical and evolutionary characteristics of the patients studied are shown in Tables 2 and 3.
Clinical characteristics and LRINEC scale score of the patients studied.
| No. | Age | Sex | Morbidity | Limb | Entry point | Main symptom | Leukos ×1000cell/mm3 | CRP mg/dl | Shock | Initial diagnosis | LRINEC |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 51 | F | No | Low | Erosion | Pain | 9.2 | 7.8 | No | NSTI | 0 |
| 2 | 36 | F | HCV, HIV, PDA | Low | Venopuncture | Pain | 9.2 | 29.2 | No | Cellulitis | 7 |
| 3 | 21 | M | HCV, HIV, PDA | Up | Venopuncture | Inflammatory signs | 14.2 | 16.9 | No | NSTI | 7 |
| 4 | 51 | F | HCV, PDA | Low | Venopuncture | Purulent drainage | 20 | 16.8 | No | NSTI | 11 |
| 5 | 16 | M | No | Up | Incisive wound | Pain-oedema | 9.1 | 14.4 | No | NSTI | na |
| 6 | 73 | F | IDDM, lymphoma | Low | No | Pain | 8 | 5.5 | Yes | Cellulitis | 3 |
| 7 | 83 | F | Breast Ca | Up | Hand burn | Fever-erythema | 6.8 | 33.5 | No | Cellulitis | 6 |
| 8 | 80 | F | Obesity | Low | No | Pain-erythema | 6.1 | 22.1 | Yes | Cellulitis | 4 |
| 9 | 40 | F | HCV, PDA | Low | Incisive wound | Pain | 9.9 | 8.5 | No | NSTI | 2 |
| 10 | 72 | M | HCV | Up/low | Erosion | Pain | 2.7 | 43.2 | Yes | NSTI | 9 |
| 11 | 67 | M | Alcoholism | Low | No | Pain | 6.5 | 28.1 | No | Cellulitis | 5 |
| 12 | 71 | M | COPD | Low | Bruised wound | Pain | 12 | 28.3 | No | NSTI | 9 |
| 13 | 86 | F | No | Low | Surgical wound | Pain | 15.1 | 42.5 | No | DVT | 11 |
| 14 | 65 | M | RA | Low | No | Pain-oedema | 8.1 | 34.6 | No | Cellulitis | 10 |
| 15 | 36 | M | IDDM | Low | No | Pain | 30.9 | 31.5 | No | NSTI | 13 |
| 16 | 23 | F | No | Up | Insect sting | Pain | 12.6 | 12 | No | Cellulitis | 1 |
| 17 | 38 | F | HCV, HIV, A DVP | Low | Venopuncture | Pain | 21.2 | 26.6 | No | Cellulitis | 8 |
| 18 | 30 | M | HIV, PDA | Low | IM | Pain | 14.4 | 9.2 | No | Abscess | 2 |
| 19 | 29 | M | Alcoholism, splenectomy | Low | Erosion | Pain | 6.4 | 6.6 | Yes | NSTI | 3 |
| 20 | 50 | M | RA | Low | No | Pain | 1.9 | 44.6 | Yes | Sept. arthritis | 7 |
| 21 | 90 | M | HCV | Low | No | Pain | 1.1 | 12.1 | No | Cellulitis | 4 |
| 22 | 36 | M | Alcoholism | Low | Surgical wound | Suppuration | 17 | 13.4 | No | NSTI | 5 |
| 23 | 32 | M | No | Up | Incisive wound | Pain | 15.7 | 15.3 | No | Sd. compart | na |
| 24 | 72 | M | No | Low | No | Pain | 11.5 | – | No | Cellulitis | na |
PDA, parenteral drug addict; RA, rheumatoid arthritis; Ca, cancer; IDDM, insulin dependent diabetes mellitus; COPD, chronic obstructive pulmonary disorder; Low, lower; NSTI, necrotising soft tissue infection; LRINEC, Laboratory Risk Indicator for Necrotising Fasciitis; F, female; na, not available; CRP, C-reactive protein; Up, upper; DVT, deep vein thrombosis; M, male; HCV, hepatitis C virus; HIV, human immunodeficiency virus.
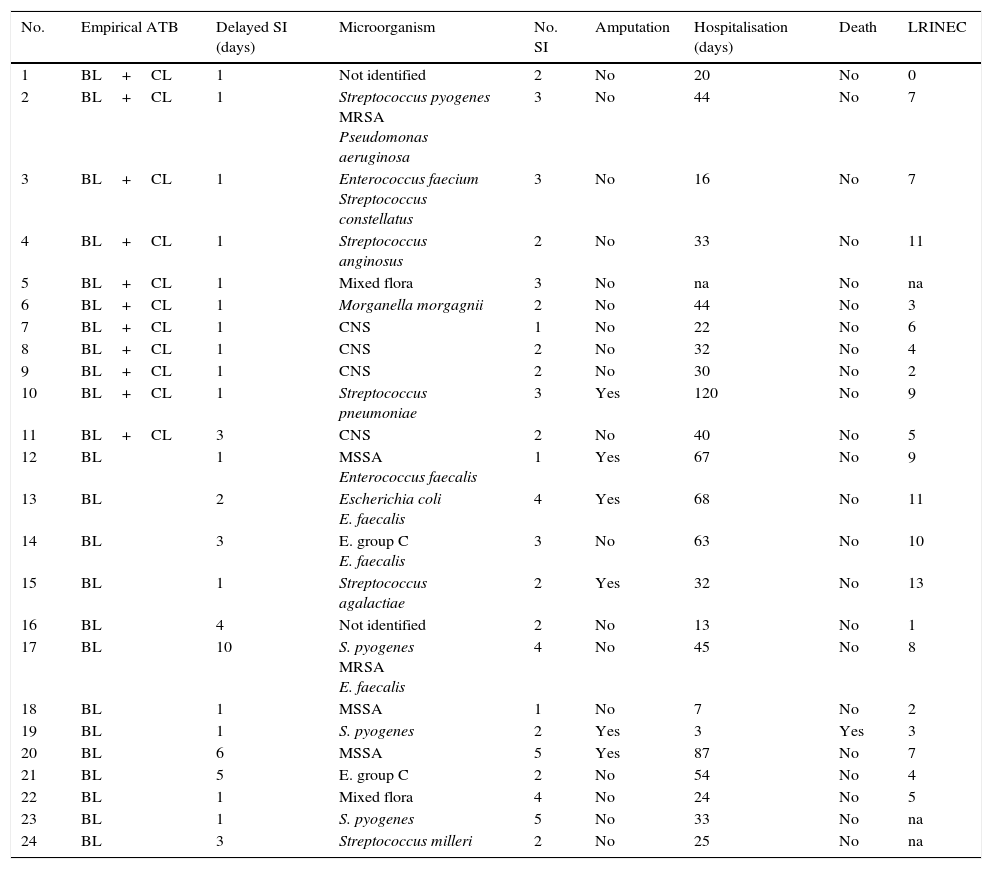
Diagnosis, treatment and evolution of the patients studied.
| No. | Empirical ATB | Delayed SI (days) | Microorganism | No. SI | Amputation | Hospitalisation (days) | Death | LRINEC |
|---|---|---|---|---|---|---|---|---|
| 1 | BL+CL | 1 | Not identified | 2 | No | 20 | No | 0 |
| 2 | BL+CL | 1 | Streptococcus pyogenes MRSA Pseudomonas aeruginosa | 3 | No | 44 | No | 7 |
| 3 | BL+CL | 1 | Enterococcus faecium Streptococcus constellatus | 3 | No | 16 | No | 7 |
| 4 | BL+CL | 1 | Streptococcus anginosus | 2 | No | 33 | No | 11 |
| 5 | BL+CL | 1 | Mixed flora | 3 | No | na | No | na |
| 6 | BL+CL | 1 | Morganella morgagnii | 2 | No | 44 | No | 3 |
| 7 | BL+CL | 1 | CNS | 1 | No | 22 | No | 6 |
| 8 | BL+CL | 1 | CNS | 2 | No | 32 | No | 4 |
| 9 | BL+CL | 1 | CNS | 2 | No | 30 | No | 2 |
| 10 | BL+CL | 1 | Streptococcus pneumoniae | 3 | Yes | 120 | No | 9 |
| 11 | BL+CL | 3 | CNS | 2 | No | 40 | No | 5 |
| 12 | BL | 1 | MSSA Enterococcus faecalis | 1 | Yes | 67 | No | 9 |
| 13 | BL | 2 | Escherichia coli E. faecalis | 4 | Yes | 68 | No | 11 |
| 14 | BL | 3 | E. group C E. faecalis | 3 | No | 63 | No | 10 |
| 15 | BL | 1 | Streptococcus agalactiae | 2 | Yes | 32 | No | 13 |
| 16 | BL | 4 | Not identified | 2 | No | 13 | No | 1 |
| 17 | BL | 10 | S. pyogenes MRSA E. faecalis | 4 | No | 45 | No | 8 |
| 18 | BL | 1 | MSSA | 1 | No | 7 | No | 2 |
| 19 | BL | 1 | S. pyogenes | 2 | Yes | 3 | Yes | 3 |
| 20 | BL | 6 | MSSA | 5 | Yes | 87 | No | 7 |
| 21 | BL | 5 | E. group C | 2 | No | 54 | No | 4 |
| 22 | BL | 1 | Mixed flora | 4 | No | 24 | No | 5 |
| 23 | BL | 1 | S. pyogenes | 5 | No | 33 | No | na |
| 24 | BL | 3 | Streptococcus milleri | 2 | No | 25 | No | na |
ATB, antibiotic; BL, betalactamic; CL, clindamycin; CNS, coagulase negative staphylococcus; SI, surgical intervention; LRINEC, Laboratory Risk Indicator for Necrotising Fasciitis; na, not available; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, meticillin-sensitive S. aureus.
A simple X-ray was taken at the moment of admission, and it showed soft tissue gas in only 4 cases. All of the patients were operated, and the average time to this after their admission to the emergency department was one day (range 1–10). The average number of operations per patient was 2.6 (range 1–5). The infection was monomicrobian in 16 cases, and the most frequent microorganisms were beta-haemolytic streptococcus (2 Streptococcus pyogenes, one Streptococcus agalactiae and one group C streptococcus). The infection was polymicrobian in 8 cases: in 4 of these there were different species of gram positive cocci and in 3 there were gram-negative bacilli (together with gram positive bacilli in 2 cases). An amputation took place in 6 patients (25%) as a part of the treatment, and in one case an upper limb and a lower limb were amputated. Eleven patients (45.8%) were treated initially using empirical antibiotic treatment with betalactamic (BL) medication with a clindamycin (CL) and 13 (54.1%) were given a BL as monotherapy. There was one amputation in the first group (of a lower limb) and there were no deaths. 5 amputations took place in the second group (20.8%) and one patient died (4.2%). The average duration of hospitalisation was 39.9 (range 7–120) days, and the overall mortality of the series was 4.2% (1 case).
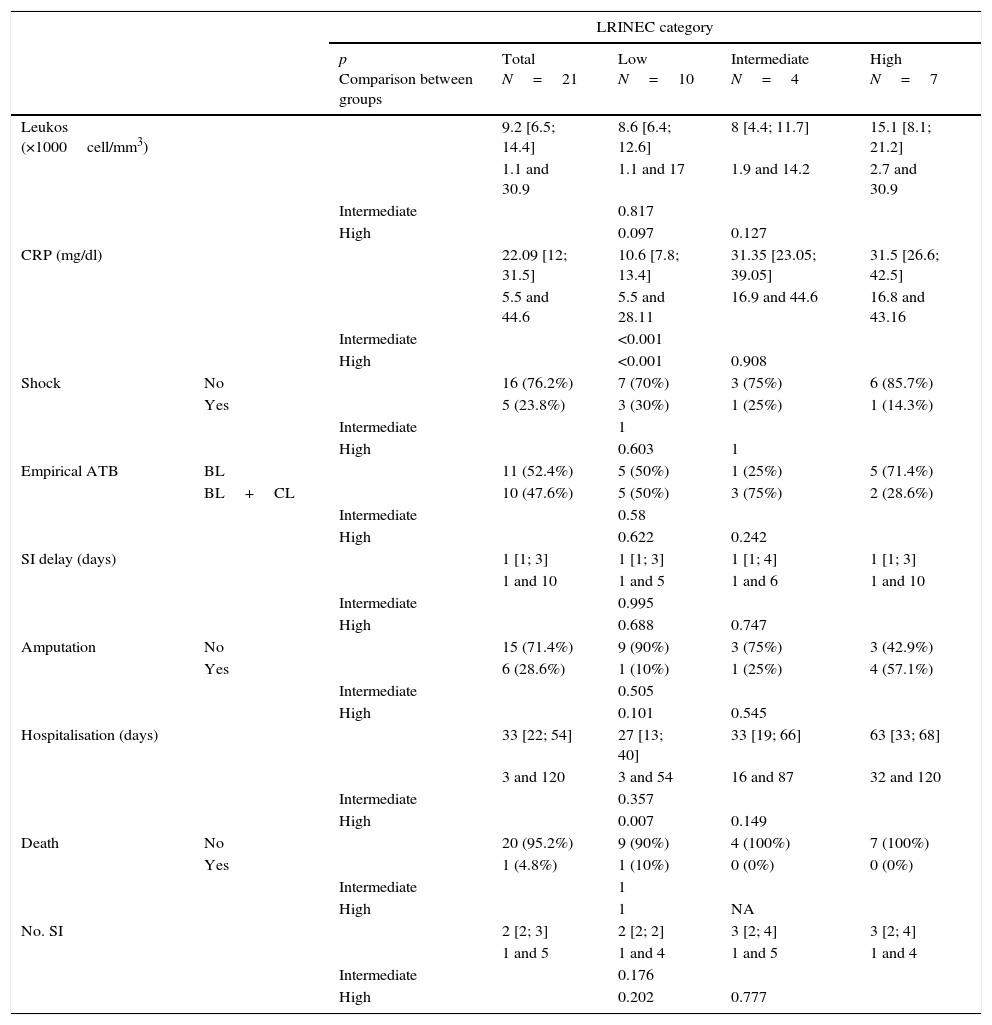
The LRINEC scale was calculated for 21 patients when they were admitted to the emergency department (Table 1). For 10 patients the score was <6 (low risk), for 4 patients it was 6 or 7 (intermediate risk) and for 7 patients it was ≥8 (high risk). The evolution of the patients according to the risk group where they were classified using the LRINEC scale was: in the low risk group, 10% required one amputation, with a surgical average of 2.1 (range 1–4) during their average of 26.7 days of admission (range 3–54); in the intermediate risk group amputation increased to 25% with an average of 3 surgical operations (range 1–5), during an average hospitalisation of 42.2 days (range 16–87), without any deaths; lastly, in the high risk group the need for amputation during the 65.8 days of hospitalisation (range 32–120) amounted to 66.6%, with an average of 2.8 surgical operations (range 1–4), without any deaths. A very slight tendency was detected for the leucocyte count to increase from low grade risk to high grade risk in the scale (p=0.097). Respecting the CRP results, a clear increase was observed for the intermediate and high risk groups compared to the low risk group on the scale (p<0.001 in both comparisons). The length of hospitalisation was also found to increase in the high level on the scale in comparison with the low level (p=0.007) (Table 4).
Results.
| LRINEC category | ||||||
|---|---|---|---|---|---|---|
| p Comparison between groups | Total N=21 | Low N=10 | Intermediate N=4 | High N=7 | ||
| Leukos (×1000cell/mm3) | 9.2 [6.5; 14.4] | 8.6 [6.4; 12.6] | 8 [4.4; 11.7] | 15.1 [8.1; 21.2] | ||
| 1.1 and 30.9 | 1.1 and 17 | 1.9 and 14.2 | 2.7 and 30.9 | |||
| Intermediate | 0.817 | |||||
| High | 0.097 | 0.127 | ||||
| CRP (mg/dl) | 22.09 [12; 31.5] | 10.6 [7.8; 13.4] | 31.35 [23.05; 39.05] | 31.5 [26.6; 42.5] | ||
| 5.5 and 44.6 | 5.5 and 28.11 | 16.9 and 44.6 | 16.8 and 43.16 | |||
| Intermediate | <0.001 | |||||
| High | <0.001 | 0.908 | ||||
| Shock | No | 16 (76.2%) | 7 (70%) | 3 (75%) | 6 (85.7%) | |
| Yes | 5 (23.8%) | 3 (30%) | 1 (25%) | 1 (14.3%) | ||
| Intermediate | 1 | |||||
| High | 0.603 | 1 | ||||
| Empirical ATB | BL | 11 (52.4%) | 5 (50%) | 1 (25%) | 5 (71.4%) | |
| BL+CL | 10 (47.6%) | 5 (50%) | 3 (75%) | 2 (28.6%) | ||
| Intermediate | 0.58 | |||||
| High | 0.622 | 0.242 | ||||
| SI delay (days) | 1 [1; 3] | 1 [1; 3] | 1 [1; 4] | 1 [1; 3] | ||
| 1 and 10 | 1 and 5 | 1 and 6 | 1 and 10 | |||
| Intermediate | 0.995 | |||||
| High | 0.688 | 0.747 | ||||
| Amputation | No | 15 (71.4%) | 9 (90%) | 3 (75%) | 3 (42.9%) | |
| Yes | 6 (28.6%) | 1 (10%) | 1 (25%) | 4 (57.1%) | ||
| Intermediate | 0.505 | |||||
| High | 0.101 | 0.545 | ||||
| Hospitalisation (days) | 33 [22; 54] | 27 [13; 40] | 33 [19; 66] | 63 [33; 68] | ||
| 3 and 120 | 3 and 54 | 16 and 87 | 32 and 120 | |||
| Intermediate | 0.357 | |||||
| High | 0.007 | 0.149 | ||||
| Death | No | 20 (95.2%) | 9 (90%) | 4 (100%) | 7 (100%) | |
| Yes | 1 (4.8%) | 1 (10%) | 0 (0%) | 0 (0%) | ||
| Intermediate | 1 | |||||
| High | 1 | NA | ||||
| No. SI | 2 [2; 3] | 2 [2; 2] | 3 [2; 4] | 3 [2; 4] | ||
| 1 and 5 | 1 and 4 | 1 and 5 | 1 and 4 | |||
| Intermediate | 0.176 | |||||
| High | 0.202 | 0.777 | ||||
Data shown as median, interquartile range [25 and 75 percentiles] and absolute range in quantitative variables. In the qualitative variables, as n (%).
ATB, antibiotic; BL, betalactamic; CL, clindamycin; SI, surgical intervention; LRINEC, Laboratory Risk Indicator for Necrotising Fasciitis; NA, not applicable; CRP, C-reactive protein.
In our series of 24 patients diagnosed NSTI of the limbs, mortality was 4.2%, 6 patients (25%) were amputated and the average length of hospitalisation was 39.9 days (range 7–120). Majeski and Alexander6–8 compared mortality in 2 groups with NSTI, one of 20 patients (group A) treated from 1965–1980, and another 10 (group B) treated from 1980 to 2000. Their clinical and epidemiological characteristics were similar. Nevertheless, mortality in group A was 50%, while in group B it was 0%. This improvement in survival was attributed to early diagnosis and immediate and extensive debridement of the necrotic tissue. In the year 1998, Bilton et al.6,7,9 reviewed their experience in 68 patients. In 21 patients correct surgical treatment took place after 24h hospitalisation, while the second group of 47 patients were subjected to early extensive debridement. Although the average numbers of surgical procedures in both groups were similar, at 3.4 and 3.3, the average length of hospitalisation in the first group was 46 days (range 11–104), in the second group it was 28 days (range 4–45). Mortality in the first group stood at 38%, while in the second group it was 4.2%. The study by Wong et al.5,9 of a series of 89 consecutive patients from 1997 to 2002 found that delaying surgical treatment by more than 24h was the sole independent factor associated with higher mortality. In the study by Wong et al. the rate of mortality was 21% and the amputation rate was 22%. The lower mortality observed in our series (4.2%) cannot be attributed to any of the factors studied on the basis of the available data. The only patient in our series who died scored as low risk on the LRINEC scale. He was initially diagnosed NSTI and debrided in the first 24h, and we are unable to offer any explanation apart from the supposition that splenectomy was the fundamental aggravating factor that led to his death. We found no statistically significant differences in the number of amputations according to risk on the LRINEC scale.
The initial diagnosis was NSTI in only 10 of the 24 (41.7%) cases reviewed, and cellulitis was the most frequent alternative diagnosis.10–21 It is highly important to search for objective parameters that will make it possible to identify NSTI at an early stage.8,20,22–25 The LRINEC scale uses laboratory parameters to identify the risk that a patient who seems to have an infection of the skin and soft tissues really has a necrotising infection.2,6,7,25 In our series, 11 of the 21 patients whose LRINEC scale score was calculated scored ≥6 (intermediate-high risk of NSTI), while 10 patients were not properly classified. A possible explanation for this is that it is easy to access the emergency department in our environment, so that patients arrive in early stages of the infection with few biological alterations. We therefore believe that it is necessary for clinicians to maintain a high degree of initial suspicion when faced with possible cases of NSTI, most especially when (i) there is major pain, (ii) the infection progresses rapidly in spite of having commenced correct antibiotic treatment, or (iii) the general state of the patient is affected or they are in shock, all independently of the apparent severity of the lesion or score on the LRINEC scale. In all cases when NSTI is suspected it is necessary to monitor evolution of the lesion and vital signs every 3–4h.
The score of patients on the scale was correlated with their evolution, so that 66% of cases in the high risk group needed amputation, as opposed to 10% in the low risk group; hospitalisation in the first group was for 65.8 days vs 26 days, and the number of surgical operations necessary to control the infection was 30% higher in the intermediate-high risk group vs the low risk group. In general it was not possible to detect a change in prognosis between the intermediate and high levels of the LRINEC scale. Nevertheless, this was possible for the length of hospitalisation in the low and high risk groups, as in the latter the average number of days of hospitalisation practically tripled. Respecting the parameters used to create the scale, the one the most closely associated with outcome was CRP. This tripled from the low risk group to the intermediate and high risk groups, without any significant changes between the intermediate and high risk groups (Table 4). Given the time periods during which data were gathered in our series it may be considered to be representative. However, its low number of cases means that clinically relevant differences, such as those between the low and intermediate-high risk groups for leucocyte count or the high rate of amputations (more than half) in the high risk group are not statistically significant.
Several NSTI experimental models caused by S. pyogenes have shown that association with a BL or CL or linezolid vs BL monotherapy reduces animal mortality by more than 50%.24–31 Our series supports the combined use of antibiotics, and although they were not administered based on randomisation criteria, we found that in the group of patients treated with BC+CL (54.1%) one amputation took place and there were no deaths. In the group treated only with BL (45.8% of the patients) there were 5 amputations and one patient died.
In the majority of series the microorganism that was isolated the most often was beta-haemolytic streptococcus. However, in our series 3 cases were caused by gram-negative bacilli. The patient in whom Pseudomonas aeruginosa was isolated had a history of HIV and HCV infection, and was a parenteral drug addict; the second case, in which Morganella morgagnii was isolated, was a lymphoma patient, and in both situations there is increased predisposition to infection by gram-negative germs due to immune system alterations. Nevertheless, the third case of gram-negative bacilli caused by Escherichia coli in association with Enterococcus faecalis had no relevant medical history.
It should be underlined that we present 3 cases caused by gram-negative bacilli, given that they are rare in infections of the limbs. However, one author has pointed out a tendency to change in the microorganisms that cause NSTI,32 and this fact should be taken into account in the future.
To conclude, in our study the LRINEC scale accurately identified only 52% of NSTI cases. We therefore have to insist that a low score on the scale does not rule out the possible presence of this entity; nevertheless, it does correlate with the infection prognosis. We recognise the limitations of our study: firstly, the low number of patients, even though with a rare entity it is hard to achieve a sufficiently large sample size; secondly, as a result of the small sample we were unable to perform an inferential study that would make it possible to draw all of the statistically significant conclusions that we would need, and thirdly, this is a retrospective observational cohort study. Nonetheless, we believe that we currently lack a tool that would make it possible to extraordinarily identify a case of NSTI. We therefore have to monitor the evolution of skin and soft tissue infections, and given the suspicion of NSTI or progression to the same, indicate early and aggressive debridement as well as starting suitable combined intravenous antibiotic treatment, as these are the most important measures to be implemented to modify the prognosis of this entity.
Level of evidence1 – Centre for Evidence-Based Medicine (CEBM) Level of evidence: 2C.
2 – Agency for Healthcare Research and Quality (www.ahrq.gov/clinic/uspstfix.htm). Level of evidence: II 2.
3 – Scottish Intercollegiate Guidelines Network (SIGN). Level of evidence: 2++.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments took place in human beings or animals for this research.
Confidentiality of dataThe authors declare that no patient data appear in this paper.
Right to privacy and informed consentThe authors declare that no patient data appear in this paper.
Conflict of interestWe the authors have not received any economic support whatsoever for this work. Nor have we entered into any agreement due to which we will receive any benefits or fees from any commercial body. No commercial body has paid nor will pay the foundations, educational institutions or other not-for-profit organisations of which we are members.
Please cite this article as: Ballesteros-Betancourt JR, García-Tarriño R, Ríos-Guillermo J, Rodriguez-Roiz JM, Camacho P, Zumbado-Dijeres A, et al. Infecciones necrosantes de partes blandas atendidas en un servicio de urgencias de tercer nivel: evolución y correlación con la escala laboratory risk indicator for necrotising fasciitis (LRINEC). Rev Esp Cir Ortop Traumatol. 2017;61:265–272.