There is level iv evidence that the preoperative administration of antibiotics helps in the prevention of prosthetic infection.
There is controversy on whether the ischaemia applied during surgery may affect the minimum inhibitory concentration of the antibiotic in the peri-prosthetic tissues.
The aim of this study is to review this phenomenon through the determination of antibiotic concentration in the synovial tissue.
Material and methodA prospective observational clinical study was conducted on 32 patients undergoing total knee replacement. Cefonicid 2g was administered as prophylaxis, with a tourniquet used for all patients. The antibiotic concentration was quantified by high performance liquid chromatography in samples of synovial tissue collected at the beginning and at the end of the intervention.
ResultsThe mean concentration of antibiotic was 23.16μg/g (95% CI 19.19–27.13) in the samples at the beginning of the intervention and 15.45μg/g (95% CI 13.20–17.69) in the final samples, being higher than the minimum inhibitory concentration of cefonicid, set at 8μg/g. These results were statistically significant for both concentrations (P<.00001).
DiscussionThe antibiotic concentration throughout the standard total knee prosthesis surgery performed with tourniquet gradually decreases throughout the intervention.
The concentration determined at the end of the intervention was higher than the minimum inhibitory concentration required for the antibiotic studied.
In conclusion, the use of a tourniquet does not increase the risk of infection.
Existe evidencia científica grado iv de la importancia que el antibiótico administrado preoperatoriamente tiene en la prevención de la infección protésica.
Hay controversia en si la isquemia aplicada en la cirugía de estos pacientes puede afectar a la concentración mínima inhibitoria del antibiótico en los tejidos periprotésicos.
Para estudiar este fenómeno hemos diseñado un estudio basado en la determinación de la concentración de antibiótico en el tejido sinovial.
Material y métodoEstudio clínico prospectivo observacional de 32 pacientes intervenidos de prótesis total de rodilla. Se administró 2g de cefonicid como profilaxis y se utilizó el manguito de isquemia en todos los pacientes, cuantificándose la concentración antibiótica mediante la cromatografía líquida de alta resolución en muestras de tejido sinovial del inicio y del final de la intervención.
ResultadosLa concentración media de antibiótico fue de 23.16μg/g (IC del 95%, 19.19-27.13) en las muestras del inicio de la intervención y de 15.45μg/g (IC del 95%, 13.20-17.69) en las muestras del final, mostrándose superiores a la concentración mínima inhibitoria del cefonicid, establecida en 8μg/g, siendo estos resultados estadísticamente significativos para ambas concentraciones (p<0.00001).
DiscusiónLa concentración de antibiótico a lo largo de una intervención estándar de prótesis total de rodilla realizada con isquemia preventiva varía a lo largo de la intervención sufriendo un descenso paulatino.
Aun así, la concentración determinada al final de la intervención no fue inferior a la concentración mínima inhibitoria del antibiótico estudiado.
Como conclusión, la utilización del manguito de isquemia no aumenta el riesgo de infección.
The surgical technique for total knee replacement (TKR) has experienced significant advances and improvements, but is not without complications. Infection, together with aseptic loosening, is the main complication, as its consequences entail significant repercussions for the patient, as well as in terms of hospitalization, and social and economic aspects.1–3 The most frequent origin of contamination is during the surgical procedure. The existence of infection depends on the conditions of the patient and the environment, as well as the presence of an implant allowing infection by minor bacterial inoculums and microorganisms with low virulence. Antibiotic prophylaxis aims to maintain high concentrations of antibiotic throughout the entire intervention in order to prevent the multiplication of germs contaminating the surgical field.4,5 Specifically, in traumatology and orthopaedic surgery, it has been established that the administration of a dose of antibiotic against the majority of the contaminating flora 30min prior to the intervention significantly decreases the risk of infection of the surgical wound.6–9
In clinical practice, many of the interventions affecting the limbs use a pneumatic ischaemia cuff or tourniquet to prevent bleeding during the operation. This system drains the blood from the surgical field, thus preventing blood loss and facilitating the work of the surgeon, although it has also raised doubts regarding its negative effect in terms of the delivery of prophylactic antibiotic agents.
The objective of this study is to evaluate whether the concentration of prophylactic antibiotic is maintained over the minimum inhibitory concentration (MIC) throughout the entire TKR intervention when an ischaemia cuff is used.
Material and methodStudy designThis was an observational clinical study of 32 patients intervened at Hospital Arnau de Vilanova in Lleida (Spain) for primary TKR with arthrosic causes. All the patients signed an informed consent form and the study was approved by the Ethics Committee of the Hospital. The cases were collected consecutively as long as they did not fulfil any of the following exclusion criteria: patients who were allergic to penicillin, patients who had undergone prior surgeries on the same knee being operated and patients treated with another antibiotic in the 7 days prior to the intervention.
A single dose of 2g cefonicid EFG® was administered intravenously in the reception area of the operating room. The ischaemia cuff was applied in the base of the limb to be intervened, placing a soft cushioning between the skin and the cuff to prevent skin lesions and fixing the cuff with straps of Velcro and a bandage. The venous drain was always carried out by inclination, with the limb kept elevated while the surgical field was being prepared. The tourniquet was then inflated using the air outlet of the operating room, just before starting the intervention. The pressure applied by the cuff ranged between 350 and 400mmHg in all cases. We registered the time of exposure to the antibiotic, the starting time of ischaemia and the starting time of the intervention.
Once the intervention was started, we collected a first sample of synovial tissue at the time of opening the joint capsule of the knee (M1). The second sample of synovial tissue (M2) was collected after the prosthesis was implanted, just before closing the joint capsule, and the ischaemia was removed once the intervention had been completed. The times of collection of each sample were registered, as well as the ending time of the intervention and removal of ischaemia.
The samples were collected and manipulated under aseptic conditions and were weighed before being frozen at −80°C until the time they were manipulated.
Minimum inhibitory concentrationRegarding the MIC associated to cefonicid, some studies10,11 determined that the MIC for Staphylococcus aureus (S. aureus) was 6.3μg/ml, whilst another work12 concluded that 8μg/ml of cefonicid were required to inhibit the growth of S. aureus by 90%.
In this study we established a reference value of 8μg/ml as MIC of cefonicid.
Determination of antibiotic concentration in synovial tissueIn order to determine the concentration of antibiotic in the samples of synovial tissue we used the method of high-resolution liquid chromatography (HPLC), following the various references found in the literature.13–16 As mobile phase we used an aqueous solution of ammonium acetate (0.385%) and methanol (10%). The chromatographic separation was carried out in a 4.6mm×250mm Ultrasphere IP column with 5μ particles (Beckmann Coulter®). We used pure antibiotic to determine the optimal conditions for detection of cefonicid, with the following parameters: wavelength of 265nm, chromatography time of 35min, flow of 1.3ml/min, retention time of the peak between minutes 22 and 28 and injection volume of the samples of 10μl.
Secondly, we used samples of synovial tissue obtained from the knee before antibiotic administration, and added different known doses of cefonicid at the time of processing, labelled as standard synovial tissue, with the dual objective of validating the HPLC technique for the determination of cefonicid in synovial tissue (carrying out the corresponding tests for linearity, precision of the system, robustness and stability of the analytical solution) and also estimating a reference equation to relate the areas of the peaks obtained in the chromatogram with the known doses of antibiotic.
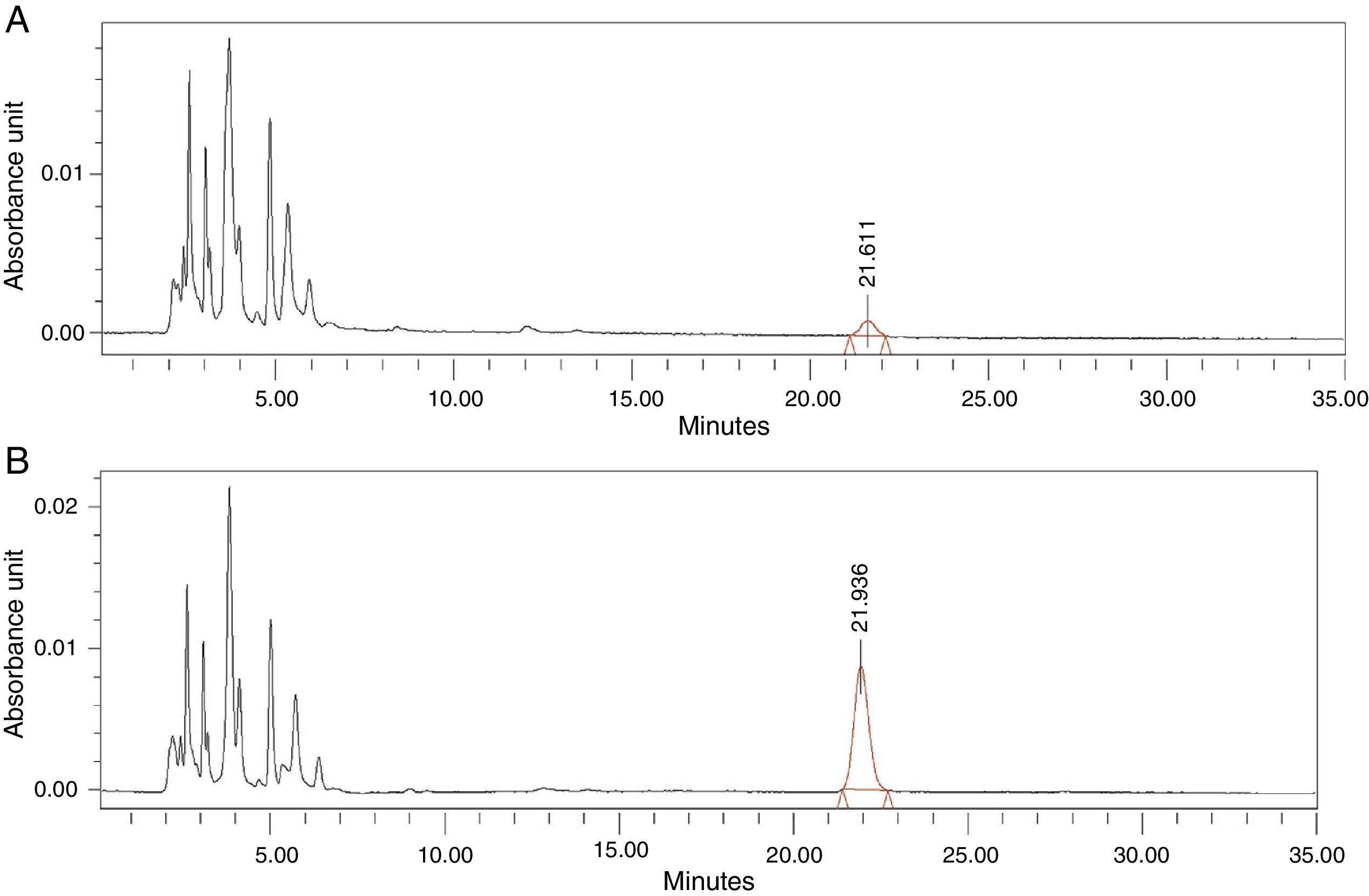
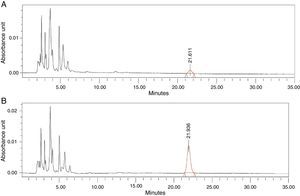
Thirdly, we proceeded to interpolate the concentrations of antibiotic in the synovial tissue of the M1 and M2 samples of the patients based on the areas obtained in the chromatogram, using the equation calculated. Each sample was injected into the HPLC in duplicate: the first injection to obtain the area corresponding to the antibiotic studied and the second injection after administering an extra dose of cefonicid, to verify that the peak detected with the first injection corresponded to the antibiotic studied, by verifying an increase in its area (Fig. 1A and B).
Statistical methodsThe general description of the sample was done by calculating the usual descriptive statistics, using the mean±standard deviation and the range for the quantitative variables, as well as the absolute and relative frequencies in percentages for the categorical variables. We used the Mann–Whitney U test to study the differences between the body mass indexes (BMI) by gender and the Student's t test and Wilcoxon nonparametric test to evaluate whether the average levels of antibiotic concentration in synovial tissue were higher than the MIC (8μg/g) in M1 and M2. We used the statistics software package SPSS version 18, and established the level of statistical significance at 5% (α=0.05).
ResultsRegarding the studied cohort, out of the 32 patients included, 9 were males (28%) and 23 were females (72%), with a mean±standard deviation age of 71.5±6.65 years (range: 54–84 years). The mean weight of the patients was 80.43±11.38kg (range: 65–125.5kg), with a mean BMI of 32.55±4.49kg/m2 (range: 23.59–45.36kg/m2). Two patients presented normal weight (6%), 6 patients were overweight (19%), 18 patients suffered mild obesity (56%), 4 moderate obesity (13%) and 2 patients morbid obesity (6%). The mean BMI of the males and females were 28.55 and 34.11kg/m2, respectively, with this difference being statistically significant (P=.001). A total of 22 cases affected the right side (69%) and 10 the left (31%). According to the model of prosthesis implanted, 26 were hybrid press-fit total knee prostheses (81%), 3 were posterior stabilized total prostheses (9.5%) and 3 were Endo-model rotational prostheses (9.5%). The mean days of admission were 8.1±2.89 days (range: 4–19 days). A total of 7 patients were monitored by the home hospitalization service (22%). The mean duration of monitoring was 34.84±21.75 months (range: 4–72 months). None of the patients were diagnosed with infection of the prosthesis, acute or chronic, during their follow-up periods in the clinic or in the subsequent revision of the clinical histories. The mean time of exposure to antibiotic was 47.88±20.84min (range: 13–113min), the mean duration of the intervention was 93.69±17.65min (range: 54–151min) and the mean duration of ischaemia was 96.34±15.84min (range: 65–124min). The mean period from antibiotic administration until sample collection was 56.41±20.41min (range: 22–119min) for the M1 sample, and 121.5±23.61min (range: 83–192min) for the M2 sample.
Regarding the validation of the HPLC method for the determination of cefonicid, the results obtained indicated a high linearity (determination coefficient R2=0.99), a high precision of the system (variation coefficient of the replications regarding the sample 2.76%) and an acceptable reproducibility, with dispersion levels ranging between 4%, 23% and 34% for antibiotic concentrations of 6, 20 and 40μg/g, respectively. The intraday and interday robustness of the analytical method presented a variation coefficient lower than 4% and 2%, respectively, and the stability of the analytical solution had a variation coefficient of 7.46%, 5% and 3.24% for concentrations of 10, 16 and 20μg/g, respectively.
The reference pattern obtained with the standard samples of synovial tissue allowed us to extrapolate the areas obtained to the samples from the patients in antibiotic concentration.
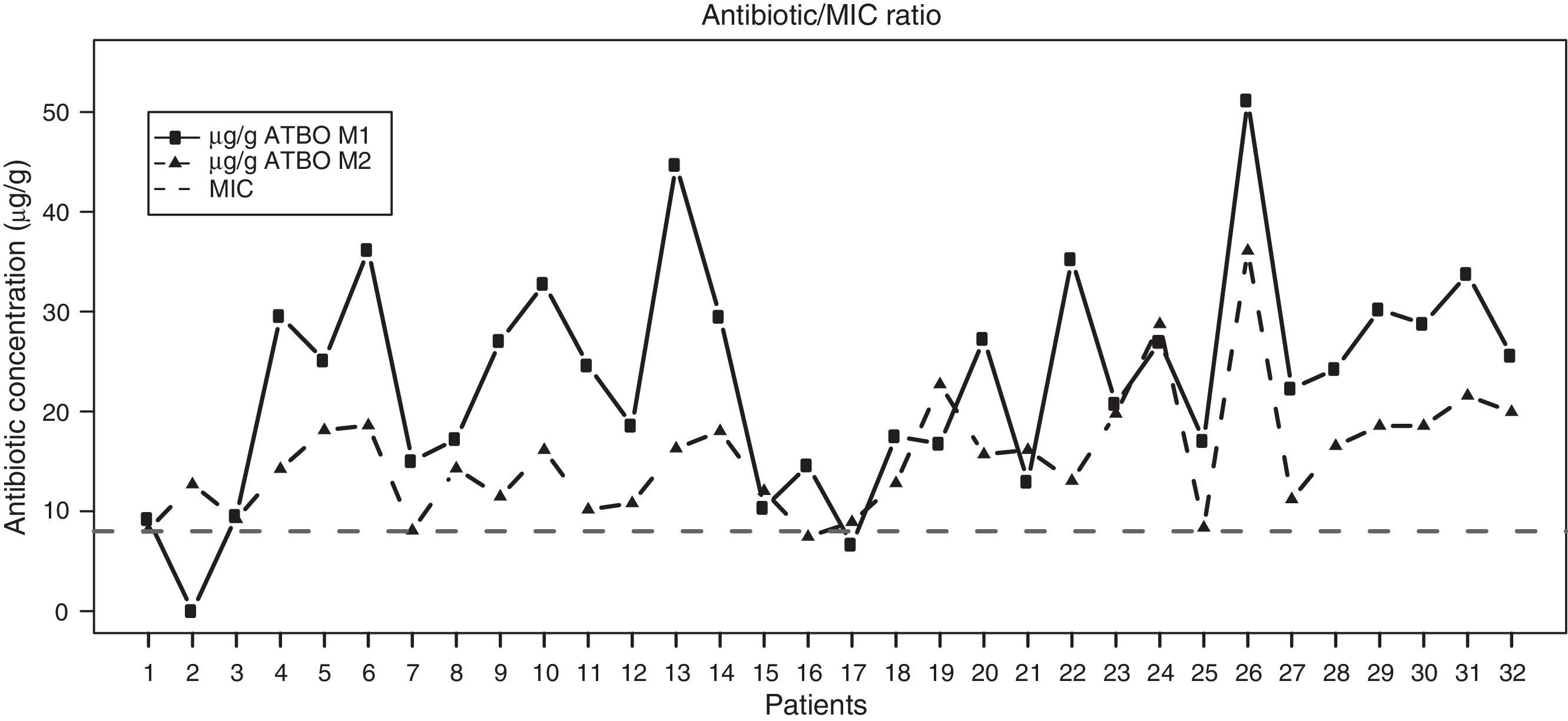
Regarding the results of our study, Fig. 2 shows the antibiotic concentrations detected in samples M1 and M2 in the synovial tissue of intervened patients, with a clear trend towards higher concentrations in the M1 samples versus the M2 being observed, along with a predominance of concentrations above the MIC in both cases. Table 1 shows the descriptive statistics of the antibiotic concentration at the analyzed times. The mean concentrations were 23.16μg/g (95% CI 19.19–27.13) and 15.45μg/g (95% CI 13.20–17.69) in M1 and M2, respectively, both higher than the MIC of cefonicid, established at 8μg/g. These results clearly reached statistical significance (P<.00001 for both concentrations). Moreover, the levels of antibiotic registered in M1 were significantly higher than those registered in M2 (P=.00002).
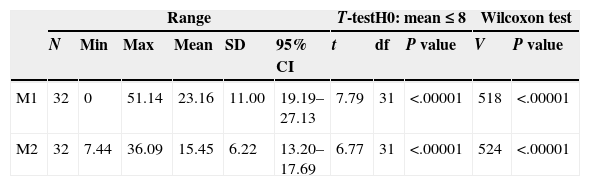
Descriptive statistics for the antibiotic values of M1 and M2.
| Range | T-testH0: mean≤8 | Wilcoxon test | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Min | Max | Mean | SD | 95% CI | t | df | P value | V | P value | |
| M1 | 32 | 0 | 51.14 | 23.16 | 11.00 | 19.19–27.13 | 7.79 | 31 | <.00001 | 518 | <.00001 |
| M2 | 32 | 7.44 | 36.09 | 15.45 | 6.22 | 13.20–17.69 | 6.77 | 31 | <.00001 | 524 | <.00001 |
Range. N: sample size; Min: minimum; Max: maximum; 95% CI: 95% confidence interval; SD: standard deviation.
Results of the Student's t parametric test. df: degrees of freedom; t: t statistic.
Results of the Wilcoxon nonparametric test. V: V statistic.
Unilateral statistical tests to evaluate the differences of the mean values with regard to a MIC of 8μg/g.
In relation to the levels of antibiotic in the M1 and M2 samples and the different clinical and experimental variables studied, we did not find statistically significant associations for the variables time of application of ischaemia, time of exposure to antibiotic, time of M1 sample collection, time of M2 sample collection, duration of the intervention, age, weight, days of admission and duration of follow-up in outpatient clinic. Neither did we find significant differences when we analyzed the different levels of antibiotic according to the categorized BMI (normal weight, overweight, mild obesity, moderate obesity and morbid obesity).
DiscussionThe most relevant direct clinical implication of this eminently observational study is the validation of antibiotic prophylaxis administered before surgery and the demonstration that the levels of antibiotic were maintained above the MIC throughout the intervention even when the ischaemia cuff was applied.
Regarding other articles published on the detection of cefonicid in tissue, the study most similar to ours in terms of dose, administration route and antibiotic detection method was that conducted by Nightingale et al.,13 even though it studied a different tissue (femoral head bone in patients undergoing total hip replacement). We verified that the mean concentrations of cefonicid detected (18.7μg/g) were similar to those in our study. We also reviewed different medical studies which investigated the effect of applying a tourniquet on the prophylactic action of antibiotics, and noted a certain controversy in terms of the results published. We found no cases of antibiotic prophylaxis using cefonicid, but it was interesting to examine the bases on which they supported their conclusions. Three studies17–19 determined the effect of ischaemia on antibiotic prophylaxis based on the presence of postoperative infection, with 2 studies17,19 registering a higher incidence of infection in the group with ischaemia, although none of these studies determined the antibiotic concentration in tissue. Other works compared patients undergoing knee and hip replacements,20–22 and related the antibiotic concentration in tissue to the time from antibiotic administration, obtaining conclusions regarding the concentration with times around 90min from the initial administration. We believe that this time does not correspond exactly to the last stage of the intervention, which in our opinion is the most objective way of determining whether the ischaemia interfered with the possibility of reducing the antibiotic concentration which would be particularly evident at the end of the intervention.
Regarding the antibiotic concentration required to prevent infection during the intervention, we found different criteria in the literature, with certain studies establishing an antibiotic concentration equal to or greater than the MIC,7,23,24 twice that amount,22 and even up to 4 times the MIC.25 In this sense, we also argue that the antibiotic concentration required to prevent infection would be equal to or greater than the MIC, since, if we apply the premise of requiring twice the MIC in synovial tissue, in our study we would obtain 8 cases of M1 samples with insufficient concentrations of antibiotic, and 16 cases in M2. This risk is too high not to find any cases of infection.
We highlight the case of patient 2, in whom no antibiotic was detected in the M1 sample and 13mg/g were registered in the M2 sample. Furthermore, this patient had a time of exposure to the antibiotic of 13min. The fact that no antibiotic was detected in the M1 sample could be explained either by a very low time of exposure or else by a chromatography error. In the majority of patients studied we detected a decrease in antibiotic levels at the end of the intervention (M2) compared to the start. We considered that this was due to the interruption of the blood supply caused by the application of ischaemia and the subsequent metabolization of the antibiotic. In any case, the concentration detected at the end of the intervention was higher than the MIC, so we consider that the level of protection against infection was clinically adequate.
Among the limitations of this study we can mention that, although the size of the studied sample was clearly sufficient to evaluate the main hypothesis, the statistical power for the analysis stratified by different clinical and experimental variables was low.
While it is true that the results obtained cannot be extrapolated to a general clinical framework, it is also true that our clinical practice is the habitual for TKR. Thus, the results obtained provide a general view for this kind of interventions and could serve as reference for other type of interventions using an ischaemia cuff. In any case, the results of this study prove that ischaemia does not compromise the levels of antibiotic prophylaxis.
Level of evidenceLevel of evidence iv.
Ethical responsibilitiesProtection of people and animalsThe authors declare that this investigation adhered to the ethical guidelines of the Committee on Responsible Human Experimentation, as well as the World Medical Association and the Declaration of Helsinki.
Confidentiality of dataThe authors declare that they have followed the protocols of their workplace on the publication of patient data and that all patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors assume the responsibility of guaranteeing the right to privacy of patients, and protecting their identity both in the text of the article and in the images shown.
FundingThe authors declare that they have not received any financial support from any public or private entity to conduct this work.
Conflict of interestThe authors have no conflict of interest to declare.
Please cite this article as: Prats L, Valls J, Ros J, Jover A, Pérez-Villar F, Fernández-Martínez JJ. Influencia del manguito de isquemia en la profilaxis antibiótica en prótesis total de rodilla. Rev Esp Cir Ortop Traumatol. 2015;59:275–280.