Hip fracture (HF) in the elderly has a high prevalence and risk of morbidity and mortality in the short and long term. It can reduce life expectancy by almost 2 years, and require permanent socio-sanitary assistance in one in 5 patients. Its management as a process where the patient takes priority over the activities of the organisation can bring new perspectives, optimisation tools and redesign of the workflow to make it more efficient.
Objectiveto develop an in-hospital clinical guide for the management of patients with HR adapted to the environment, clear and concise, to be able to intervene in the best possible conditions and favour their adequate recovery.
Material and methods407 patients divided into 3 groups: pre-implementation (knowing the scope of the problem and areas for improvement); implementation (after the development of the management guide) and post-implementation (valued its implementation).
ResultsThe clinical results obtained with this guide allowed improving surgical programming and reducing delay times (increasing the proportion of patients operated in the first 48h and reducing the average hospital stay in 3 days), raising awareness of the problem at all Services involved, improve the management of drugs that altered hemostasia, optimise transfusion therapy and reduce hospital stay and perioperative complications.
ConclusionThe implementation of this guide, with integrated global criteria, has improved the results of this process, and achieved a more efficient management, reducing the consumption of resources and as a consequence, health expenditure.
La fractura de cadera (FC) en el anciano tiene elevada prevalencia y riesgo de morbimortalidad a corto y largo plazo. Puede disminuir la esperanza de vida casi 2años y precisar asistencia socio-sanitaria permanente en uno de cada 5pacientes. Su gestión como un proceso donde prime el paciente sobre las actividades de la organización puede aportar nuevas perspectivas, herramientas de optimización y rediseño del flujo de trabajo para hacerlo más eficiente.
ObjetivoDesarrollar una guía clínica intrahospitalaria para el manejo de pacientes con FC adaptada al entorno, clara y concisa, para poder intervenir al paciente en las mejores condiciones posibles y favorecer su adecuada recuperación.
Material y métodosCuatrocientos siete pacientes divididos en 3grupos: preimplantación (conocer el alcance del problema y las áreas de mejora); implantación (tras el desarrollo de la guía de manejo) y postimplantación (valoró la implementación de esta).
ResultadosLos resultados clínicos obtenidos con la presente guía permitieron mejorar la programación quirúrgica y reducir los tiempos de demora (aumentando la proporción de pacientes intervenidos en las primeras 48h y reducir la estancia media hospitalaria en 3 días), concienciar del problema a todos los servicios implicados, mejorar el manejo de los fármacos que alteraban la hemostasia, optimizar la terapia transfusional y reducir la estancia hospitalaria y las complicaciones perioperatorias.
ConclusiónLa implantación de esta guía, con criterios globales integrados, ha logrado mejorar los resultados de este proceso y conseguido una gestión más eficiente, reduciendo el consumo de recursos y, como consecuencia, el gasto sanitario.
Hip fracture (HF), or fracture of the proximal third of the femur from the femoral head to 5cm below the lesser trochanter, is a current major public health problem in the older population in developed countries. It is the most frequent cause of hospital admission to trauma services,1 and the World Health Organisation expects its incidence to triple over the next 50 years. In 2015, according to the Minimum Basic Data Set of the Spanish National Health System (SNS), there were 53,867 hospitalisations due to HF in Spain, primarily in women (72.6%) and at over 65 years of age (92.6%).
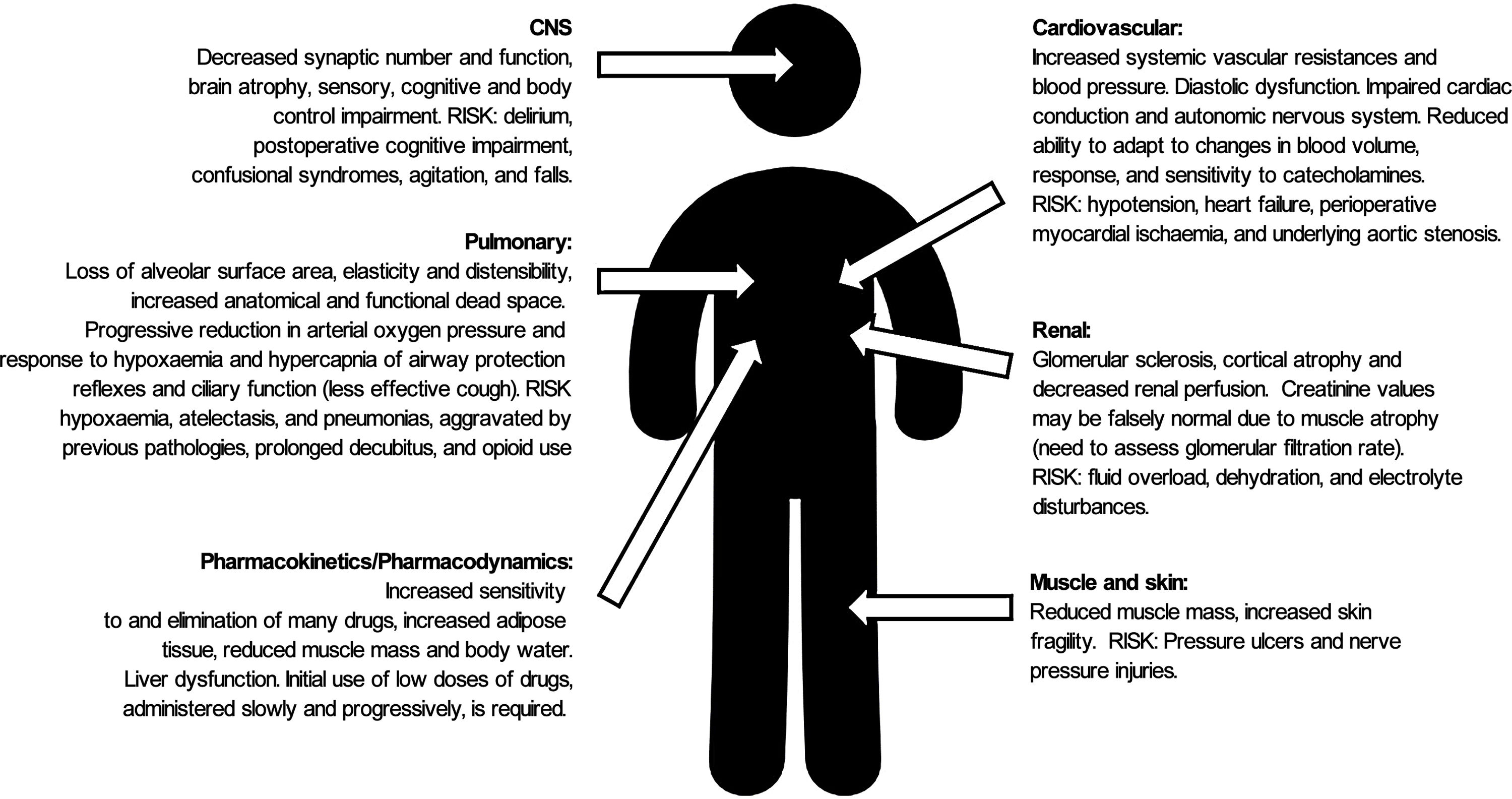
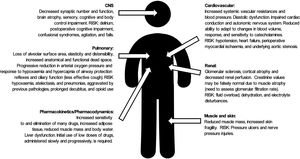
HF (together with the physiological changes of ageing (Fig. 1), presents a high risk of morbidity and mortality in the short and long term (reducing life expectancy by almost 2 years), functional deterioration, dependence (one in every 5 patients will require permanent health and social care2) and the need for institutionalisation following the fracture.3
HF surgery has increased in the last 20 years, from 86% in 1997 to 91.48% in 2007, and conservative treatment of these fractures is currently uncommon. Surgery enables early mobilisation, avoiding bed rest and the risk of developing respiratory or urinary infections, deep vein thrombosis or pressure ulcers. Surgery should be performed early (preferably within 48h) after the patient's medical condition has been optimised and their medication adjusted. Surgical delay is associated with a worse prognosis and higher mortality at one year, sometimes due to a lack of consensus on haemostasis-altering drugs or difficulty in scheduling operating theatres.
HF also has a high economic impact on healthcare (in Spain, the average cost is between euro7031 and euro12,331, depending primarily on the number of days spent in hospital, and is double the average cost of other causes). To this must be added the costs generated in the patient's environment and the non-quantitative costs derived from changes in lifestyle and loss of productivity for the patient, their families and society.
For all these reasons, an action protocol is needed for all professionals involved in this process to ensure comprehensive care from admission to discharge from hospital, and even after the process is over, to help these patients maintain their quality of life for a few more years. Orthogeriatric services have established themselves as the most efficient in the comprehensive treatment of the older patient.4,5
This article describes the experience in implementing an intrahospital clinical guideline for the perioperative management of patients with HF at the Hospital Puerta de Hierro Majadahonda in Madrid. Annex 1 shows the summary of the guidelines with the most important aspects to be considered for the comprehensive care of HF patients and the measures to be adopted. It was drawn up after a prior analysis of the situation that assessed the existing shortfalls in the process in our setting.
The aim of the clinical guideline was to set out clearly and concisely the necessary perioperative care to ensure patients are operated as soon as possible in the best possible conditions with a more efficient recovery.
Material and methodsStudy conducted in 3 phases after approval by the Ethics Committee (Act n. 309, dated 27 April 2015) at the Hospital Universitario Puerta de Hierro Majadahonda (HUPHM), a tertiary level hospital of the Public Health Service of the Community of Madrid, which complies with the ethical standards of the Research Committee and the Helsinki Declaration of 1975 with the revision of October 2013 (https://jamanetwork.com/journals/jama/fullarticle/1760318).
Inclusion criteria: patients diagnosed with HF aged 65 years or older, admitted to the emergency department of HUPHM and scheduled for surgery.
Exclusion criteria: patients under 65 years of age, pathological or periprosthetic HF, polytrauma patients, conservative treatment, and failure to obtain informed consent for data collection.
Timeframe: the study was divided into 3 periods, with analysis of the data between the periods prior to taking measures:
First period (pre-implementation group [PRE], n=88): retrospective study of patients undergoing HF surgery in 2013, to establish the extent of the problem and possible areas for improvement. From the data obtained, a clinical guideline was drawn up by the anaesthetics and trauma departments, with the support of internal medicine and emergency medicine.
Second period (implementation group [IMPL], n=112): prospective study of patients undergoing HF surgery from February to June 2016, applying the guideline developed.
Third period (post-implementation group [POST], n=207): prospective study analysing the degree of compliance with the clinical guideline implemented from November 2016 to June 2017.
Demographic data were collected (age, sex), social data (place of residence at the time of HF, home or care home), data on dependency (ability to perform basic activities of daily living without assistance), presence of coexisting diseases (high blood pressure [HBP], diabetes mellitus [DM], atrial fibrillation, heart failure, cerebrovascular disease [CVA]), respiratory failure and chronic renal failure), cognitive impairment (information obtained during anamnesis and clinical history), chronic consumption of antiaggregant and anticoagulant drugs, and anaesthetic (American Society of Anesthesiologists (ASA) classification) and cardiovascular (Goldman criteria) risks.
Data were collected on the type of HF (extracapsular or intracapsular), surgical parameters (osteosynthesis with intramedullary nail, cannulated screws or partial or total arthroplasty) and anaesthetic (general anaesthesia [GA], regional subarachnoid anaesthesia [RA] and femoral and femoral cutaneous nerve blocks).
Laboratory data were collected, including serial haemoglobins (Hb) (on admission, at 24h, at 48h, post-surgery and before discharge), transferrin saturation (values <20% were considered indicative of depleted iron stores), International Normalised Ratio (INR) in case of chronic treatment with acenocoumarol on admission, at 24h and at 48h (an INR below 1.5 is required for neuroaxial anaesthesia) and creatinine (as a marker of renal function, on admission, at 48h and before discharge from hospital).
In terms of transfusion therapy, the number of packed red blood cell units, time of administration and transfusion threshold were recorded. Optimisation with IV iron for anaemia correction was also recorded; ferric carboxymaltose was administered to anaemic patients (Hb on admission below 13g/dl−1 in men and 12g/dl−1 in women) and to those who although they had Hb levels in range had transferrin saturation <20% and vitamin K (to accelerate the correction of coagulation parameters).
Dates of admission, surgery and discharge were recorded to calculate the surgical delay (determining whether it was for medical reasons or due to operating theatre management), hospital stay, discharge destination (home, care home or intermediate care centre).
The intrahospital morbidity parameters collected were the degree of anaemia (assessment of decrease in Hb levels in relation to time and according to fracture type), impairment of renal function (assessed by creatinine and urea), morbidity after hospital discharge (readmission within the first 3 months after surgery) and intrahospital mortality rate, at 6 weeks (first post-surgical check-up) and at 3 and 6 months after surgery.
Statistical analysis: a descriptive analysis was performed for categorical variables using absolute and relative frequencies, and for numerical variables, using mean and standard deviation or median and 25th and 75th percentiles, minimum and maximum values, according to compliance with the assumption of normality. The Mann–Whitney U test or Student's t-test were used for the univariate analysis to contrast numerical variables and the chi-squared test or Fisher's exact statistic to contrast hypotheses of categorical variables, as appropriate. For comparisons between the 3 groups, the Kruskall–Wallis non-parametric test or one-way ANOVA was performed, as appropriate. The Bonferroni correction was used to eliminate accumulated type I error because of multiple comparisons and multivariate logistic regression analysis was performed to assess the association between being transfused, being anaemic on admission and fracture type. To assess the risk factors associated with overall mortality, a multivariable logistic regression analysis was performed, plotting odds ratios (OR) and their respective 95% confidence intervals (CI). The significance level was set at .05. The statistical package used was Stata/IC v.14.1. (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX, USA: StataCorp LP).
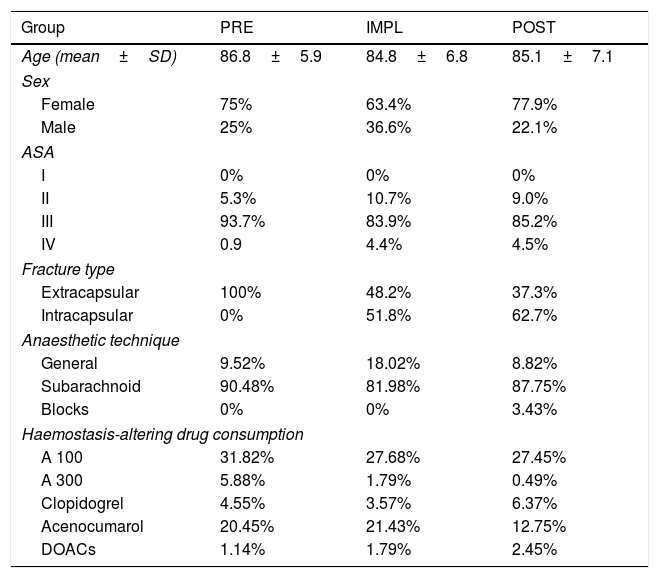
ResultsThere were no differences between the groups in terms of demographic characteristics (Table 1).
Demographic characteristics.
| Group | PRE | IMPL | POST |
|---|---|---|---|
| Age (mean±SD) | 86.8±5.9 | 84.8±6.8 | 85.1±7.1 |
| Sex | |||
| Female | 75% | 63.4% | 77.9% |
| Male | 25% | 36.6% | 22.1% |
| ASA | |||
| I | 0% | 0% | 0% |
| II | 5.3% | 10.7% | 9.0% |
| III | 93.7% | 83.9% | 85.2% |
| IV | 0.9 | 4.4% | 4.5% |
| Fracture type | |||
| Extracapsular | 100% | 48.2% | 37.3% |
| Intracapsular | 0% | 51.8% | 62.7% |
| Anaesthetic technique | |||
| General | 9.52% | 18.02% | 8.82% |
| Subarachnoid | 90.48% | 81.98% | 87.75% |
| Blocks | 0% | 0% | 3.43% |
| Haemostasis-altering drug consumption | |||
| A 100 | 31.82% | 27.68% | 27.45% |
| A 300 | 5.88% | 1.79% | 0.49% |
| Clopidogrel | 4.55% | 3.57% | 6.37% |
| Acenocumarol | 20.45% | 21.43% | 12.75% |
| DOACs | 1.14% | 1.79% | 2.45% |
A: aspirin; ASA: American Society of Anaesthesiologists anaesthetic risk classification.
No statistically significant differences between the groups in age, sex, or ASA grade.
In the PRE group all the fractures were extracapsular, due to the code used to locate the medical records (this part of the study was carried out retrospectively).
Unit of age: years.
No statistically significant differences between groups in distribution or percentage of antiaggregant (p=.165) or anticoagulant (p=.318) drugs consumed.
DOACs: direct acting oral anticoagulants.
The distribution of medical diseases is typical for older patients (median age 88 years (PRE) and 86 (IMPL and POST), with 25% over 90 and only 25% under 80 years of age). The most frequent were: HBP, DM and heart rhythm disturbances, followed by heart failure, stroke and chronic respiratory disease.
Of the patients, 35% had 2 or more cardiovascular risk factors (class II-III of the Goldman criteria for cardiovascular risk in non-cardiac surgery), implying a risk of cardiac death of 1–2% and of serious cardiac complications of 3–10%.
Of the patients, 51.14% were institutionalised prior to admission for HF, 57.5% required some form of assistance in performing the basic activities of daily living and 40.8% had some degree of cognitive impairment.
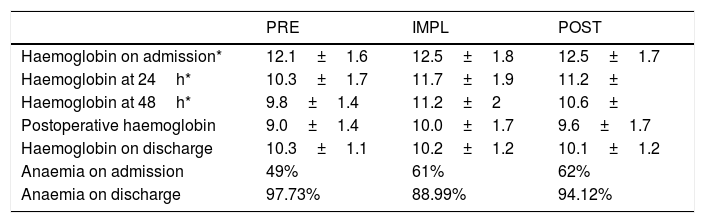
Anaemia on admission (Table 2) was a conditioning factor for transfusion (Table 3) in the IMPL and POST groups, but not in the PRE group (p=.218), probably due to the high percentage of patients transfused.
Perioperative anaemia.
| PRE | IMPL | POST | |
|---|---|---|---|
| Haemoglobin on admission* | 12.1±1.6 | 12.5±1.8 | 12.5±1.7 |
| Haemoglobin at 24h* | 10.3±1.7 | 11.7±1.9 | 11.2± |
| Haemoglobin at 48h* | 9.8±1.4 | 11.2±2 | 10.6± |
| Postoperative haemoglobin | 9.0±1.4 | 10.0±1.7 | 9.6±1.7 |
| Haemoglobin on discharge | 10.3±1.1 | 10.2±1.2 | 10.1±1.2 |
| Anaemia on admission | 49% | 61% | 62% |
| Anaemia on discharge | 97.73% | 88.99% | 94.12% |
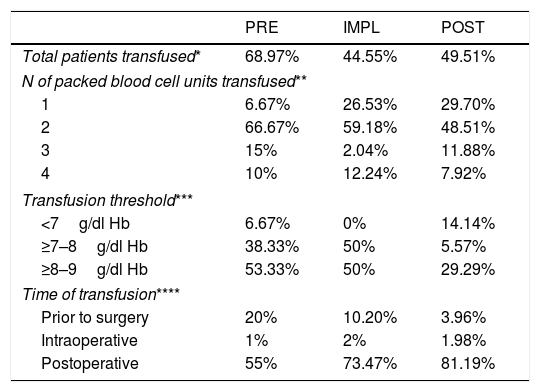
Transfusion therapy.
| PRE | IMPL | POST | |
|---|---|---|---|
| Total patients transfused* | 68.97% | 44.55% | 49.51% |
| N of packed blood cell units transfused** | |||
| 1 | 6.67% | 26.53% | 29.70% |
| 2 | 66.67% | 59.18% | 48.51% |
| 3 | 15% | 2.04% | 11.88% |
| 4 | 10% | 12.24% | 7.92% |
| Transfusion threshold*** | |||
| <7g/dl Hb | 6.67% | 0% | 14.14% |
| ≥7–8g/dl Hb | 38.33% | 50% | 5.57% |
| ≥8–9g/dl Hb | 53.33% | 50% | 29.29% |
| Time of transfusion**** | |||
| Prior to surgery | 20% | 10.20% | 3.96% |
| Intraoperative | 1% | 2% | 1.98% |
| Postoperative | 55% | 73.47% | 81.19% |
In the IMPL group there were differences for both extracapsular (p=.008) and intracapsular fractures (p=.001), with a 48% lower risk for transfusion compared to the PRE group (OR .52; 95% CI .28–.98; p=.045). In the POST group there were differences for intracapsular fractures (p=.042), but not for extracapsular fractures (p=.053), although in both cases there was less transfusion than in the former 2 groups, with a 42% lower risk of transfusion compared to the PRE group (OR .58; 95% CI, .33–1.01; p=.055). In all groups, extracapsular HF increased the risk of transfusion 2.08-fold over intracapsular fracture.
Iron deficiency (transferrin saturation <20%) was observed in the patients with anaemia on admission (81.65%) and in those with Hb in the normal range (80.89%), with no differences between groups (p=.876). Iron administration did not influence the transfusion rate (p>.9), but did affect the number of patients with anaemia on admission (81.65%) and those with Hb in the normal range (80.89%), 9), and it did influence the number of bags transfused, with a 64% decreased risk for transfusion of a second packed red blood cell bag compared to the patients who were not given iron (OR=.36, 95% CI .15–.84; p=.018).
The implementation of the clinical guideline led to changes in the form of transfusion (Table 3), both in the transfusion threshold and in the number of packed red blood cells transfused.
Multivariate analysis of predictors of mortality showed that only age (p<.001) was a risk indicator, with an OR of 1.11 (95% CI, 1.05–1.19). Sex (p=.369), Hb on admission (p=.369), transfusion (p=.136), patient provenance (p=.105), surgery in under 48h (p=.977), anaesthetic technique (p=.867), use of antiplatelet agents (p=.807) or anticoagulants (p=.68) were not indicators of risk.
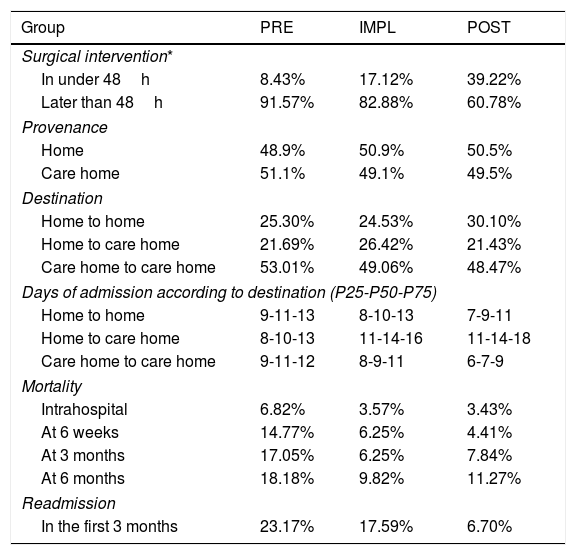
Regarding surgical delay (Table 4), there was increased mortality in patients undergoing surgery later than the first 48h of admission; however, this is not statistically significant (p=.4). In the PRE group, the surgical delay was 6 days, and a quarter of the patients underwent surgery with a delay of >7 days. In the IMPL group the delay was reduced to 4 days and in the POST group to 3 days. During the study, the delay in surgery was halved. The proportion of patients undergoing surgery within the first 48h in the PRE group was below 10%; however, in the POST group it was near 40%.
Management data relating to hip fracture surgery.
| Group | PRE | IMPL | POST |
|---|---|---|---|
| Surgical intervention* | |||
| In under 48h | 8.43% | 17.12% | 39.22% |
| Later than 48h | 91.57% | 82.88% | 60.78% |
| Provenance | |||
| Home | 48.9% | 50.9% | 50.5% |
| Care home | 51.1% | 49.1% | 49.5% |
| Destination | |||
| Home to home | 25.30% | 24.53% | 30.10% |
| Home to care home | 21.69% | 26.42% | 21.43% |
| Care home to care home | 53.01% | 49.06% | 48.47% |
| Days of admission according to destination (P25-P50-P75) | |||
| Home to home | 9-11-13 | 8-10-13 | 7-9-11 |
| Home to care home | 8-10-13 | 11-14-16 | 11-14-18 |
| Care home to care home | 9-11-12 | 8-9-11 | 6-7-9 |
| Mortality | |||
| Intrahospital | 6.82% | 3.57% | 3.43% |
| At 6 weeks | 14.77% | 6.25% | 4.41% |
| At 3 months | 17.05% | 6.25% | 7.84% |
| At 6 months | 18.18% | 9.82% | 11.27% |
| Readmission | |||
| In the first 3 months | 23.17% | 17.59% | 6.70% |
Twenty percent of the patients previously living at home undergoing HF surgery had to be admitted to an intermediate care hospital for functional recovery. The patients who returned home needed support from social workers to adapt their home to their new situation. It was possible to reduce this aspect by 18.2%.
In the patients who were institutionalised beforehand, hospital stay was reduced by 36.4%, from an average of 11 days (PRE) to 7 days (POST).
It should be added that the criteria for admission to medium-stay hospitals in the Community of Madrid changed during the study, resulting in a one-day increase in hospital stay in the IMPL and POST groups, due to the centralised management of these hospitals and increased administrative bureaucracy. This influenced hospital stays, which is the major economic cost of HF in its acute phase.
Given that the average daily cost of a trauma bed is euro177–euro199/day,6 the cost was reduced by euro708–euro796/day, which means a saving in 6 months of euro144,556–euro164,772. Transfusion therapy is also important in the economic cost of this process, both due to the number of packed red blood cells (it is estimated that each packed red blood cell bag costs euro243), and due to prolonged hospital stay, not counting the indirect costs of complications associated with blood product transfusion. The reduction in transfusion rate and number of packed red blood cell bags transfused results in an average saving of 40% (42% POST [euro23,575.86]) and 38% IMPL [euro21,865.14] in transfusion therapy-related costs compared to the PRE group [euro37,725.75]).
DiscussionThe predominance of women in our study is comparable to national and world registers,8 mainly due to their longer life expectancy9 and postmenopausal bone loss.8 The high rate of institutionalised patients is due to the large number of care homes in the study population area. This helps to explain the high degree of dependency prior to admission, more than half the patients needed help with the basic activities of daily living (57.5%) and the advanced age of the patients, values similar to those of Castilla-La Mancha (2016),2 Ávila (2015)6 or Guadalajara (2017)10; on the other hand, there is the advantage that hospital stays can be shortened by facilitating discharge back to their care homes.
More than 80% of the patients were classified as ASA III anaesthetic risk (“major risk”), not differentiating risk between the different patients as most of them had the same level, and therefore other scales should be used, such as The Nottingham Hip Fracture Score (NHFS), which predicts the risk of mortality in the first 30 days after HF,11 an aspect that has been considered in successive revisions of the clinical guideline.
The best practice guidelines on haemostasis-altering drugs are constantly being revised and the clinical guideline should be updated, informing all the actors involved in this process, as this is an essential aspect in the appropriate management of HF patients.
Aspirin does not pose a problem in surgical delay because it is maintained during admission indefinitely.12
Clopidogrel must be discontinued to perform neuroaxial anaesthetic techniques safely and, following studies13 that demonstrate greater efficacy than aspirin, its use is expected to increase in the future. The recommendations of the Spanish Society of Anaesthesiology indicate that the hypothetical risk of bleeding caused by clopidogrel maintenance cannot justify delaying the intervention until its effect wears off, as thromboembolic complications and mortality increase..14 The Association of Anaesthesiologists of Great Britain and Ireland recommend not stopping clopidogrel on admission, not delaying surgery and not prophylactically transfusing platelets.15
Anticoagulants contraindicate neuroaxial anaesthesia and should be discontinued. In the case of acenocoumarol, vitamin K and INR monitoring are recommended, since coagulation can be corrected within 24–48h.
The recommendations on direct-acting oral anticoagulants (DOACs) are based on their pharmacokinetics.16 The plasma level drops by about 90% within 3 half-lives after the peak plasma level of the drug is reached following administration (36h for apixaban, 27h for rivaroxaban and 48h for dabigatran). The use of coagulation tests for monitoring is not appropriate; however, they should be performed once the safe withdrawal times for these drugs have been met, as normal test results ensure haemostatic competence. It is recommended17 to discontinue DOACs 2 days before surgery in patients with normal kidney function (http://qxaapp.secardiologia.es/landing/).
Patient blood management (PBM) programmes are based on three pillars: optimising preoperative erythropoiesis, minimising intraoperative blood loss and improving tolerance to anaemia. PBM has excellent results in elective surgeries, however, in HF the priority is early intervention, and therefore blood management is very limited in these patients.
Anaemia on admission correlates with increased morbidity, mortality, hospital stay and risk for blood product transfusion.7 Our data (incidence of 40–50%, and higher prevalence in patients over 85 years of age) are similar to other studies.18
Anaemia in the older patient is of mixed aetiology: nutritional (iron, folic acid, and vitamin B12 deficiencies), of inflammatory origin, or due to chronic disorders. There is also iron deficiency without anaemia. The European guideline on management of perioperative bleeding19 recommends treating iron deficiency by intravenous administration in non-delayable surgeries. Several studies20 have found a correlation between IV iron administration and reduced transfusion requirements, it being most effective in patients with Hb>12g/dl−1. In studies conducted in anaemic patients, the transfusion rate is maintained; however, the number of packed red blood cells transfused decreases, as in our study.
The “Seville” Consensus Document21 on alternatives to transfusion indicates that, in patients with HF, the preoperative administration of IV iron and application of restrictive criteria in transfusion therapy improve transfusion rates and postoperative morbidity, especially in non-anaemic patients or those with subcapital fracture. Vitamin B12 and folic acid are also recommended for nutritional deficit. There are studies comparing the isolated administration of iron with combined administration with erythropoiesis-stimulating agents (rHuEPO at a dose of 40,000IU or half dose in the case of kidney failure).22 The problem is that rHuEPO is contraindicated in patients with uncontrolled HBP, a history of venous or arterial thromboembolic disease, or in those who cannot receive prophylactic antithrombotic treatment. Therefore, their administration has not become widespread, due to the high number of associated comorbidities in these patients.
The third pillar of PBM is the reduction of intraoperative blood loss with the use of antifibrinolytic drugs, such as tranexamic acid, which has been shown to reduce blood loss in orthopaedic surgery23 (off-label use). It is contraindicated in patients with a history of arterial thrombosis, severe kidney failure and a history of seizures, and the dose should be adjusted in cases of mild or moderate kidney failure due to the increased risk of thrombotic complications. Given the contraindications to tranexamic acid and erythropoietin, only IV iron administration was considered in this study to optimise anaemia. Some studies also recommend prescribing iron on discharge to improve recovery.24
Transfusion of blood products should always be assessed on an individual basis, since it increases morbidity and mortality, hospital stay and risk for infection, possibly due to its immunomodulatory effect.25Anaemia on admission is the main factor that increases the risk for transfusion,26 which was also an aspect in our study. Extracapsular HF (doubling the risk for transfusion) and surgical delay were also risk factors (reduced from 20% of patients transfused preoperatively in the PRE group to 3% in the IMPL group). The transfusion threshold has been gradually restricted to the current levels27 of Hb<8g/dl−1. Our protocol has reduced the number of patients transfused with Hb>8g/dl−1 by 40%.
In terms of mortality, RA was the most common in the 3 groups, similar to other studies.2,6 It is important to highlight that there are no differences28 in morbidity and mortality between GA and RA, maintaining adequate intraoperative haemodynamic stability being essential. Delay in surgery has a greater influence on morbidity and mortality than the anaesthetic technique itself.18
The intrahospital mortality rate in Spain29 is between 5% and 8% (4.8% in women and 8.9% in men). In the first month it is between 6% and 12% and at one year it reaches 30%. The Agency for Healthcare Quality and Research (AHQR) considers intrahospital mortality associated with HF an indicator of quality of care. The risk factors for mortality vary according to the study (advanced age, male sex, frail patient and institutionalisation,29 age and dependency5). In our study, only age was a risk factor (a mean of 5 years more in deceased patients).
The mean hospital stay for HF in Spain30 is 12–14 days and is associated with delay in surgery, transfusion rates and perioperative infectious and cardiorespiratory complications, especially heart failure.31 Delayed HF surgery increases morbidity and mortality.32,33 The factors involved in surgical delay can be divided into medical (Charlson comorbidity index >2,34 patient blood management, use of haemostasis-altering drugs (our main factor in delay) and organisational factors35 (day of the week of admission, surgery being delayed if the patient was admitted towards the end of the week36 (operating theatre planning is responsible for 20% of surgical delay37). The different studies and guidelines vary on the recommended period between patient blood management and time of surgery (from 1 to 4 days).38 The Spanish Society of Trauma and Orthopaedic Surgery, within the recommendations of the Ministry of Health, recommend not delaying surgery for more than 48h in the absence of a formal medical contraindication, in line with current national39 and international40 guidelines. However, the latest editions of the New Zealand41 and Scottish42 guidelines consider reducing this time to 24–36h, although there appear to be no particularly noteworthy benefits, as demonstrated by the Canadian Health System.43 Our results are far from these recommendations, but coincide with the median obtained in the Spanish national health system data.
Regarding clinical and interventional parameters, the surgical delay for the PRE group is similar to Sánchez-Hernández and Sáez-López in 2010,6 while it is lower for the POST group. Surgical stay, transfusion rate and the anaesthetic technique used do not differ much from other published articles, but mortality in the POST group is lower.2,6,31,35,37,44
In the PRE group, surgical programming was performed weekly, with a delay of almost 6 days until the intervention, depending on the day of the week when admitted, which increased by an average of one more day in antiplatelet-anticoagulated patients, and 53% with Hb>8g/dl−1 were transfused. In light of the data obtained in the second period, a new distribution of the trauma operating theatres was achieved with the approval of management, and one was enabled for fracture interventions, giving priority to hip fractures.
The intrahospital clinical guideline for the perioperative management of patients admitted with HF was disseminated to all the services involved, with the collaboration of Management. Patient collaboration was sought through an information sheet on the process that they were given on their arrival at the emergency department.
The limitations of the present study can be summarised into 3: assuming a previous diagnosis of cognitive deterioration of the patient, and not carrying out a specific assessment on admission. No data were collected on the worsening of cognitive impairment after surgery, resulting in a lack of depth in the analysis of morbidity. With respect to the causes of mortality, the computer system only recorded deaths, not their aetiology, and therefore it was not possible to analyse the main causes of mortality. Finally, the mortality risk factors were not statistically significant for some of the variables because the sample size was not sufficient. A study with a larger number of patients is advisable to reach conclusions.
We should point out that, after completing this study, the hospital opened the orthogeriatrics department, starting the comprehensive treatment of patients with HF, assessing cognitive function, functional status, degree of dependence and nutritional status by means of scales, to provide care for these patients. These data will allow results to be measured.
ConclusionThe implementation of a guideline for the management of patients with HF has achieved more efficient management, reducing resource consumption and healthcare costs. It has reduced the rates of perioperative complications, transfusion, and readmission, as well as the number of days of hospitalisation and intraoperative mortality and mortality in the first 6 months. It has optimised surgical scheduling, increased the number of trauma operating theatres, and reduced surgical delay. It has also improved results and the patients’ and their relatives’ understanding of this process.
However, we must continue to work to ensure that more patients benefit from the reduction in surgical delay, especially those admitted at the end of the week, where results have been less satisfactory.
Level of evidenceLevel of evidence II.
FundingThis work has not been funded or received grants or subsidies of any kind.
Conflict of interestsThe authors have no conflict of interest to declare.
| Considerations/risks | Measures to adopt | |
|---|---|---|
| Perioperative anaemia | Mixed aetiology | Daily controls of IV ferric carboxymaltose (500–1.000mg) in the case of Hb 9–3g/dl−1 |
| Transfusion | Restrictive criteria (Hb of 8g/dl−1) lack significant differences in morbidity and mortality | Based on clinical symptoms, comorbidities, and Hb levels. One at a time administration and reassessment |
| Antiaggregation and anticoagulation | Increase surgical bleeding, contraindicate neuroaxial anaesthesia | Defined substitution guidelines, stratifying haemorrhagic and thrombotic risk |
| Thrombosis | Deep vein thrombosis and pulmonary thromboembolism | Mechanical or pharmacological measures (LMWH somewhat increases bleeding risk) |
| Blood glucose monitoring | Important factor in reducing morbidity | Target: blood glucose between 60–180mg×dl−1, with insulin guidelines |
| Water and electrolyte control | Dehydration or fluid overload and electrolyte disturbances | Na+ levels between 120–150meq×dl−1 and K+ levels between 2.8–6meq×dl−1 |
| Kidney function | Falsely normal creatinine values due to muscle atrophy | Assess glomerular filtration rate |
| Nutrition | Albumin <3.5g×dl−1 indicates increased perioperative mortality | High protein nutritional supplements |
| Haemodynamic status | Quantifiable by Goldman index. Murmurs and arrhythmias without haemodynamic instability do not require specialist assessment | Atrial fibrillation: a ventricular rate <100bpm is recommended, correcting the causal factors and if this is not sufficient, administering beta-blockers or the verapamil of choice |
| Respiratory infection | Risk due to prolonged bed rest and coexisting diseases. | Antibiotic prophylaxis and respiratory physiotherapy. Subarachnoid anaesthesia, early mobilisation, and physiotherapy analgesia |
| Oxygen therapy | There are episodes of hypoxia from admission to postoperative day 5 | SpO2>92% and ideally >95%. Supplemental oxygen at 2–3bpm from admission until at least 48h postoperatively |
| Perioperative analgesia | Reduces respiratory, cardiovascular, gastrointestinal morbidity, risk of delirium and hospital stay and facilitates early mobilisation | Underestimated, especially in disorientated or dementia patients. Should be multimodal, avoiding opioids and excess NSAIDs. The use of peripheral nerve blocks is advisable |
| Urinary catheter | Both urinary retention and catheterisation favour urinary tract infection | Avoid routine catheterisation (use intermittent voiding catheterisation in the event of retention). If a catheter is placed during surgery, encourage its removal 24h after starting mobilisation |
| Intraoperative | Complications, hospital stay, and mortality are reduced as the experience of the surgical and anaesthetic team increases | Antibiotic prophylaxis should be used correctly 30–60min before surgery. Subarachnoid anaesthesia is recommended whenever conditions permit |
| Delirium | 60% incidence, especially if there are preconditions. Detect patients at risk | Recommend preventive measures and early treatment (haloperidol [.25–.5mg×6h−1], olanzapine [2.5mg 24h−1 SC or PO] or risperidone [.25–.5mg PO]), avoiding mechanical restraint |
| Early mobilisation | Reduces pressure ulcers, respiratory complications and DVT risk | Early mobilisation the day following surgery, if possible |
| Secondary prophylaxis | There is a high risk of contralateral fracture2 | Treatment on hospital discharge: calcium with vitamin D, unless contraindicated |
Please cite this article as: Hormaechea Bolado L, Ortiz Gómez JR, Fornet Ruiz I, Guijarro Valdueña A, del Valle Quintans S, Álvarez Bartolomé A, et al. Desarrollo e implementación de una guía de manejo perioperatorio de pacientes con fractura de cadera: gestión sanitaria e impacto clínico. Rev Esp Cir Ortop Traumatol. 2021;65:294–304.