Recently, a new approach of autologous chondrocyte implantation technique (using as biomaterial a collagen type I/III membrane) based on increasing cell density called HD-ACI (high density autologous chondrocyte implantation) has been described. The objective of this paper was to study the clinical outcome and incidence of subchondral bone oedema in patients with cartilage lesions in the knee treated with HD-ACI at 1–2 years of follow-up.
MethodsThis is a retrospective study performed with forty patients with chondral injuries grade III-IV. All patients were treated with HD-ACI with a cellular dose of 5×106 chondrocytes/cm2 of lesion. The subjective perception of improvement of symptoms and functionality was measured with the IKDC score (International Knee Documentation Committee). The presence of bone oedema was assessed at 6, 12 and 24 months of follow-up by magnetic resonance imaging.
ResultsIKDC values showed a significant improvement at 12 and 24 months (p<.001). The mean difference of IKDC between the baseline visit and 12 months was 26.3 points, and 31.6 points at 24 months. Twenty-seven point five percent of the patients presented subchondral bone oedema at 2 years of follow-up.
ConclusionsHD-ACI is an effective and safe treatment that improves pain, clinical perception and functionality of the joint. No correlation was found between the presence of bone oedema and the patients’ clinical outcome.
Recientemente se ha descrito una nueva modalidad de la técnica de implante de condrocitos autólogos sobre membrana de colágeno I/III llamada HD-ACI (High Density Autologous Chondrocyte Implantation) que está basada en el aumento de la densidad celular. El objetivo de este trabajo fue estudiar la evolución clínica y la incidencia de la aparición de edema óseo en pacientes con lesiones de cartílago en la rodilla tratados con HD-ACI al año y a los 2 años de la intervención.
MétodosSe trata de un estudio retrospectivo en 40 pacientes con lesiones condrales grado III-IV. Todos los pacientes fueron tratados con HD-ACI con una dosis celular de 5×106 condrocitos/cm2 de lesión. La percepción subjetiva de la mejora de los síntomas/funcionalidad se valoró mediante la escala del Comité Internacional de Documentación de la Rodilla (IKDC, International Knee Documentation Committee). La presencia de edema óseo se evaluó a los 6, 12 y 24 meses de seguimiento por resonancia magnética. Comité Internacional de Documentación de la Rodilla (IKDC)
ResultadosLos valores de IKDC mostraron una mejoría significativa a los 12 y 24 meses (p<0,001). La diferencia media de IKDC entre la visita basal y los 12 meses fue de 26,3 puntos y de 31,6 puntos a los 24 meses. El 27,5% de los pacientes presentaron edema óseo subcondral a los 2 años de seguimiento.
ConclusionesHD-ACI es un tratamiento efectivo y seguro que mejora el dolor, la percepción clínica y la funcionalidad de la articulación. No se ha encontrado correlación entre la presencia de edema óseo y la evolución clínica de los pacientes.
The year 2018 marked the 24th anniversary of the application of regenerative medicine techniques, specifically cell therapy, for the treatment of joint cartilage injuries. Since Hunter1 stated in 1743 that cartilage, once damaged, is almost impossible to repair, this problem has been viewed quite pessimistically from the perspective of orthopaedics. In recent years techniques have been emerging for the repair of focal joint cartilage lesions2 such as replacement (mosaicplasty)3 or stimulation procedures (microfractures and perforations),4 the latter based on stimulation of the mesenchymal cells of the underlying bone marrow. The problem can be solved when the chondral defect is small (less than 1cm in diameter) using the technique of mosaicplasty and only one cylinder is required for the repair. When the defect is larger, more than one cylinder is required and fibrocartilaginous tissue may form between the cylinders, which are incapable of providing the repaired cartilage its biomechanical characteristics. Furthermore, stimulation methods do not solve the problem in the long term as they lead to the formation of fibrocartilaginous repair tissue, rich in type I collagen that cannot restore the functions of highly specialised tissue such as joint cartilage (hyaline cartilage with high type II collagen content).2
It was not until the end of the last century, 1994 to be specific, that Brittberg et al.5 described the use of chondrocytes as a therapeutic agent and clearly opened the doors of regenerative medicine to the effective treatment of these lesions. Regenerative medicine seeks to re-establish the organ's own function, replacing damaged cells with healthy cells, so that the underlying cause disappears, and the original function of the organ is restored indefinitely. Autologous chondrocyte implantation (ACI) is currently the only cell therapy capable of generating hyaline cartilage.6 Much information has been published on the long-term clinical benefits of this treatment.7–9 The ACI technique is based on isolating healthy chondrocytes from a biopsy of cartilage taken from the patient from a non-weightbearing area, which are then cultured and implanted in the region of the damaged cartilage, where they help towards its regeneration. In the original ACI technique a suspension of 20 million chondrocyte cultured in vitro was used, which was implanted in the cartilage lesion (irrespective of its size) under a periosteal patch. Despite its good outcomes in some cases the technique can present problems since the cells being in liquid medium makes their handling complicated and the surgical procedure difficult. Moreover, the use of periosteum sometimes involves rescue surgery, because using living tissue can result in hypertrophic growth and even delamination of newly formed tissue.10 Given the difficulty of the ACI technique and associated morbidity, work started on a second generation of the procedure using a collagen membrane that acts as a biological support in which the chondrocytes are integrated, and thus the MACI was born (membrane-based autologous chondrocyte implantation).11 One of the advantages of incorporating the membrane is that the technique can be performed arthroscopically, and in a high percentage of cases total regeneration of the joint surface can be achieved, by the formation of hyaline cartilage rich in type II collagen.12 With the MACI technique, the cell density is one million cultured chondrocytes per cm2 of lesion. Therefore, depending on the size of the lesion, the number of implanted cells is different and less than when using the ACI technique. A new ACI method has recently been described, HD-ACI (high density autologous chondrocyte implantation)13,14 that also uses a collagen membrane as a transporter but at 5 times greater cell density. With HD-ACI, the lesion is measured during the second surgery, and the membrane is trimmed to its size and shape. The cultured chondrocytes are sown in the membrane at a density of 5 million cells per cm2 of lesion, and the membrane with the chondrocytes is fixed to the defect. In this regard, Guillén-García et al.13 compared the histological and molecular characteristics of the repaired tissue after treatment of cartilage lesions with MACI or HD-ACI in a sheep model. The histological results showed that the newly formed tissue after HD-ACI was more similar in structure, cellularity and organisation to the control tissue (normal cartilage) than the synthesised tissue after treatment with MACI. In addition, the levels of expression of type II collagen and aggrecan after HD-ACI were closer to the levels of expression of the control cartilage than those found after MACI.13 Results observed in patients treated with HD-ACI on knee joint cartilage lesions showed the technique to be effective and safe, with good outcomes in terms of pain and mobility.14 Moreover, with regard to subjective perception of knee functionality measured using the IKDC (International Knee Documentation Committee) score, a statistically significant improvement at 1-year and 2-year follow-up was observed, and therefore the increased cell density seems to indicate improvement for patients.14
Bone oedema is one of the most common complications after ACI.15 Bone oedema is an inflammatory process that occurs after overload or trauma has caused a bone injury. As the bone's response, an inflammatory infiltrate is generated in the medulla coming from the blood vessels present in spongy bone. This infiltrate presses against the nerve endings irritating them and causing pain. It is often caused by response to an injury, bone fracture or contusion. Although bone oedema can present in different joints, it occurs more commonly in the knees and, in this case, is the principal cause of localised knee and joint pain and can only be diagnosed by magnetic resonance imaging (MRI).
The percentage of patients with bone oedema after chondrocyte implantation (as per the literature following ACI in liquid medium or MACI) is very disparate according to the different authors15,16 and ranges between 50% and 79%. It has been published that bone oedema is present during the first stages of cartilage maturation for up to 2 years of follow-up, it then tends to disappear, and in some cases reappears after a few years.15,17 In this paper we studied the incidence on the onset of bone oedema in 40 patients with knee joint cartilage lesions using the HD-ACI technique and its correlation with the knee's functional outcome at 1 year and 2 years following the treatment.
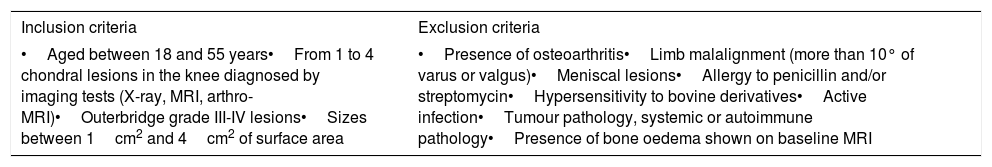
Patients and methodsThis retrospective study included a series of 40 patients with knee cartilage lesions and treated with HD-ACI in our hospital. All the patients signed their informed consent, the study was undertaken in compliance with the principles of the Declaration of Helsinki for medical research on human beings and was approved by the hospital's Teaching and Research Committee. The study's inclusion/exclusion criteria are shown in Table 1. All the patients underwent a cartilage biopsy from an non-weight bearing area. The samples were put into the DMEM culture medium (Lonza Group Ltd., Basil, Switzerland) and were processed in under 24h in a GMP (Good Manufacturing Practices) accredited laboratory, approved and certified by the Agencia Española del Medicamento y Productos Sanitarios (Spanish Medicines and Health Products Agency). To isolate the chondrocytes, the cartilage biopsies were digested at 37°C and stirred for 16h in a 1mg/ml solution of collagenase A (Roche Diagnostics GmbH, Mannheim, Germany). Once isolated, the cells were cultured in monolayer in complete DMEM (10% autologous serum, L-glutamine and penicillin-streptomycin) and incubated at 37°C, with 5% CO2 and 95% relative humidity. Once the culture had reached 80% confluency, the cells were separated from the bottom of the culture flask with trypsin–EDTA (Hyclone Utah, U.S.A.). The cells were counted in a Neubauer chamber using the trypan blue exclusion method. An aliquot of cells was frozen (retention sample) at this point and stored in liquid N2 as a sample of culture safety. The remaining cells were subcultured a maximum of 3 passes until 40–50 million cells were obtained.
Study inclusion/exclusion criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
| •Aged between 18 and 55 years•From 1 to 4 chondral lesions in the knee diagnosed by imaging tests (X-ray, MRI, arthro-MRI)•Outerbridge grade III-IV lesions•Sizes between 1cm2 and 4cm2 of surface area | •Presence of osteoarthritis•Limb malalignment (more than 10° of varus or valgus)•Meniscal lesions•Allergy to penicillin and/or streptomycin•Hypersensitivity to bovine derivatives•Active infection•Tumour pathology, systemic or autoimmune pathology•Presence of bone oedema shown on baseline MRI |
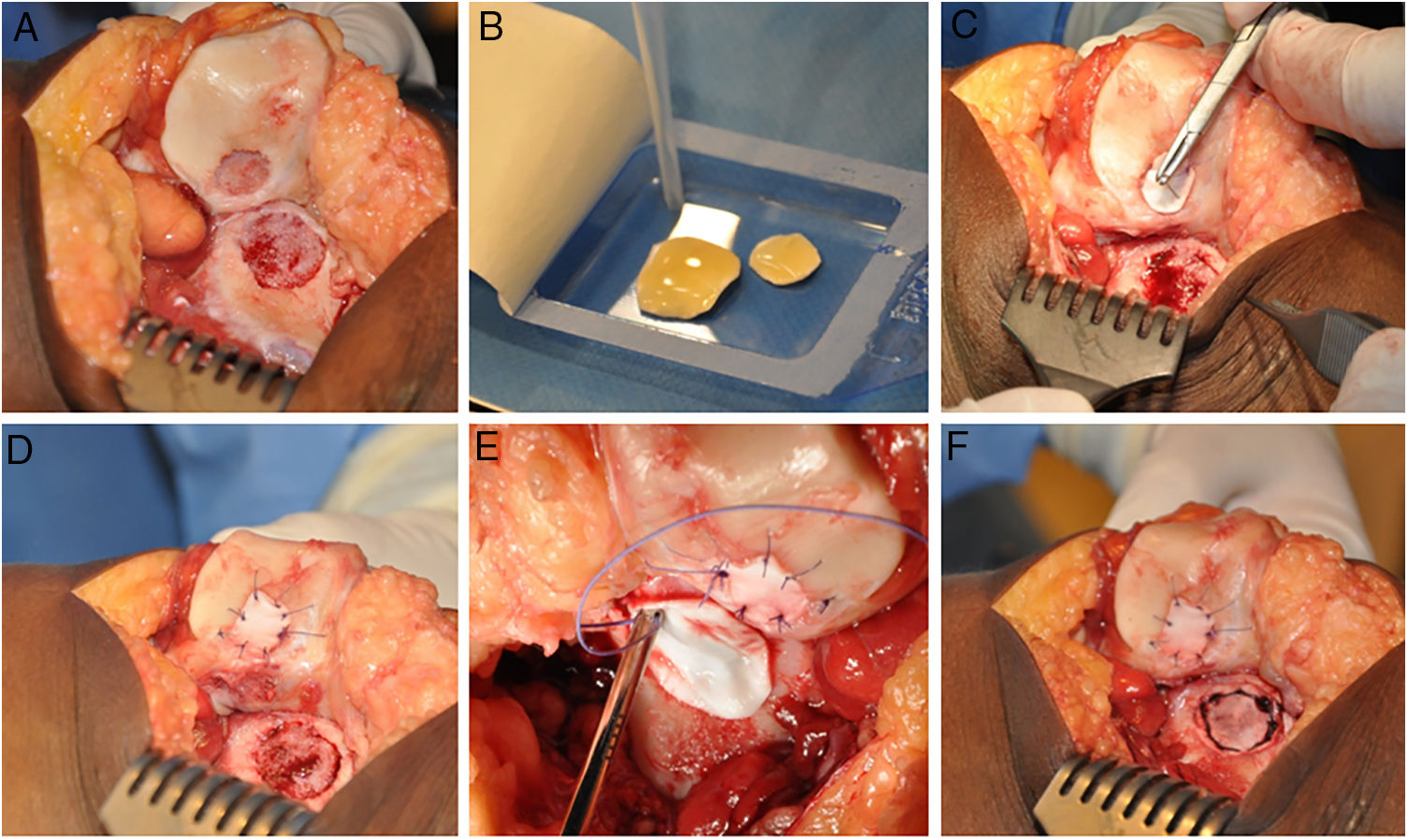
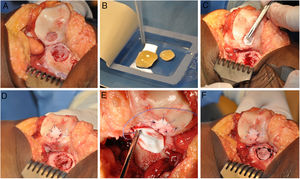
The HD-ACI technique uses a resorbable porcine membrane of collagen I/III (Chondro-Gide, Geistlich Biomaterials, Wolhusen, Switzerland) as a cell transporter. In a second operation (for the cell implantation), each chondral defect is debrided with a curette eliminating the areas of unstable cartilage and leaving the stable cartilage, reaching the subchondral bone but avoiding damaging its surface. The membrane is then trimmed to the size and shape of the lesion. The cultured chondrocytes are sown in the membrane at a density of 5 million cells/cm2 of lesion surface (Fig. 1). After a waiting period of 10min13,14 (time taken for the cells to embed in the membrane) it is placed on the defect and sutured to the adjacent cartilage, and the ends sealed with Tissucol (Baxter, Madrid, Spain). All the interventions were performed by the same team of surgeons.
HD-ACI (high-density autologous chondrocyte implantation) in a patient with 2 knee lesions: patella and external femoral condyle (trochlea). Once the lesions were debrided (A), cultured chondrocytes were sown in the membranes which had previously been trimmed to the size and shape of the lesions, with a 10-min wait time for the cells to be absorbed (B). One of the membranes was placed over the first lesion and sutured to the adjacent cartilage (C and D). The other membrane was then placed and sutured on the second lesion (E and F).
The patient's subjective perception of improvement in symptoms and knee functionality was measured using the IKDC questionnaire at the baseline visit, at 1-year and 2-year follow-up. The presence of bone oedema was assessed at 6, 12 and 24 months of follow-up by MRI. All the patients underwent a baseline MRI. Likewise, pain was assessed with the visual analogue scale at baseline and the same follow-up periods as for bone oedema.
IBM SPSS, version 22.0.0 (New York, U.S.A.) was used for the statistical analysis. The quantitative variables were expressed in means or medians as a measure of central tendency. The dispersion of these variables was expressed as standard deviation or minimum and maximum values. The normality of the quantitative variables was checked using the Kolmogorov–Smirnov test. The values of the quantitative variables at the different follow-up times were compared using Friedman's non-parametric test for related samples. Pairwise comparisons were performed using the Wilcoxon signed- rank test for related samples. The distribution of continuous variables between the patients with or without bone oedema was compared using the Mann–Whitney U test. The categorical variables were expressed in absolute frequency and percentage. The evolution of these variables throughout the follow-up period was studied using cross tables, whose statistical significance was determined using Pearson's χ2 test. All hypothesis contrasts were bilateral, and a p value<.05 was considered statistically significant.
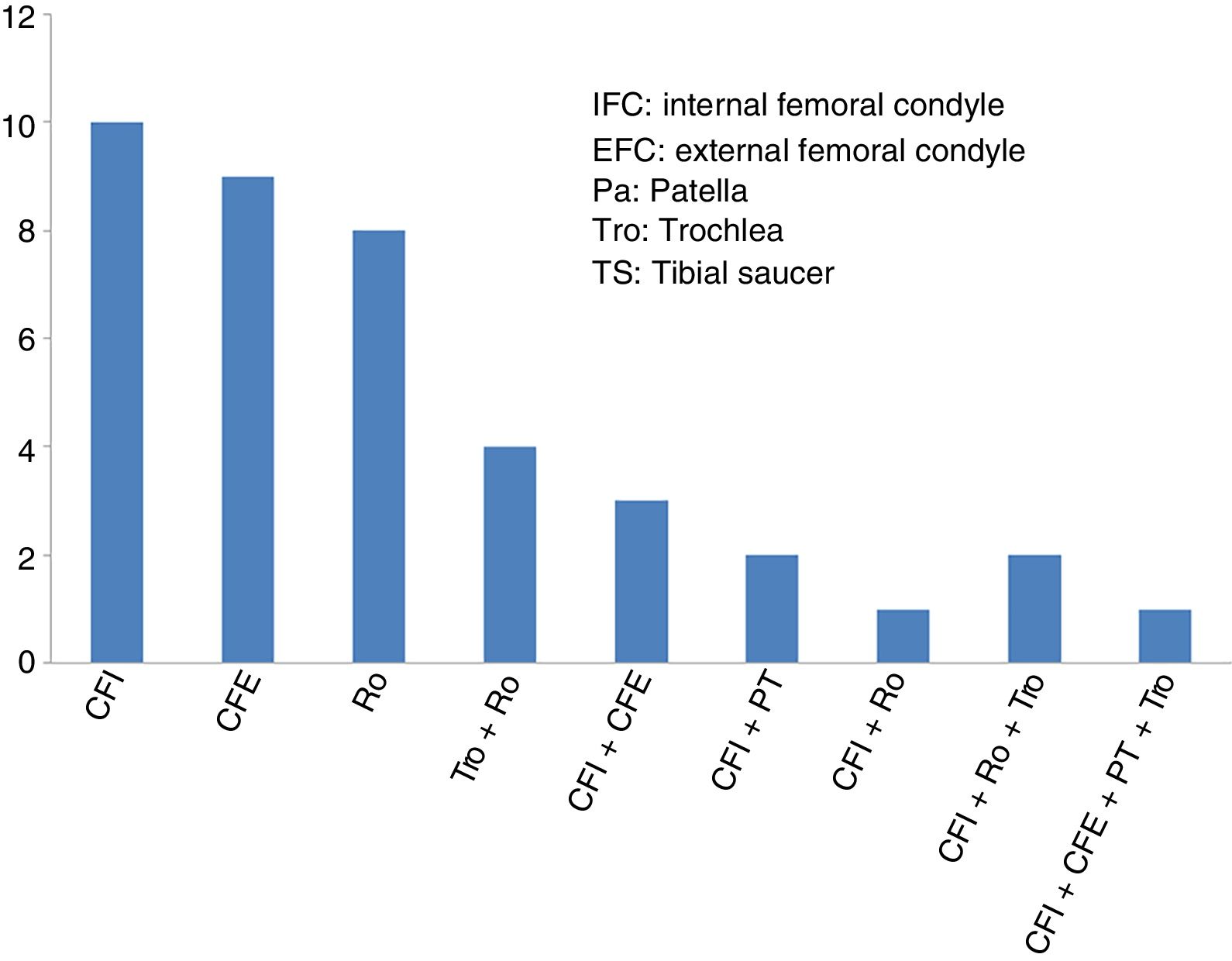
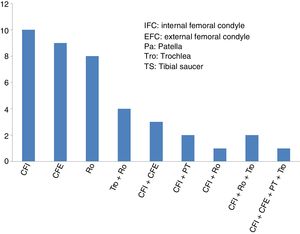
ResultsThe mean age of the 40 patients included in the study (27 men and 13 women) was 34.2±9.6 years (mean±standard deviation), and the most common diagnosis was chondral lesion. Most of the patients (29 of 40 cases: 72.5%) had undergone previous knee surgery before the high-density chondrocyte implantation, HD-ACI. In just over half of these patients (17 of 29 cases: 58.6%) the previous knee surgery was due to cartilage problems. The knee cartilage surgery that these patients had undergone before HD-ACI involved bone marrow stimulation techniques (perforation/microfracture). The median size of the patients’ cartilage lesions was 5cm2 (minimum: 1.4 to maximum: 10.5). Twenty-six patients had only one lesion (68%), 10 patients had 2 (25%), 2 patients had 3 lesions (5%), and only one had 4 (2%). The site of the lesions inside the joint is shown in Fig. 2. In most cases (27 patients) the cell implantation was an open surgery procedure. The time between taking the biopsy and the chondrocyte implantation ranged between 8 and 24 weeks.
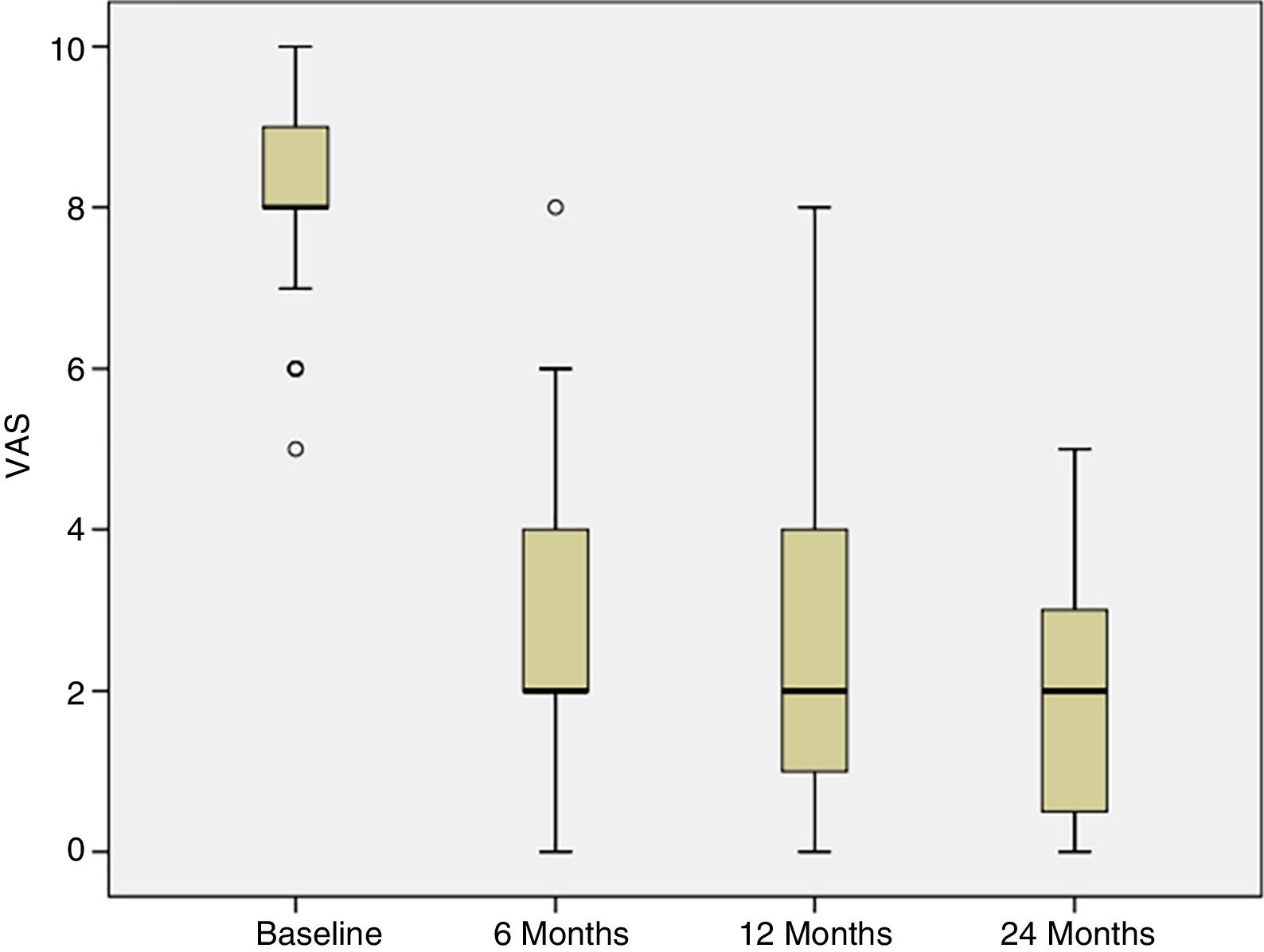
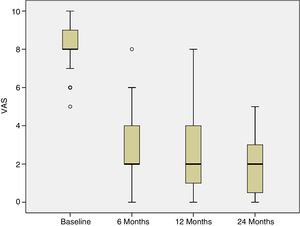
Progress of pain was assessed from the baseline visit to 6, 12 and 24 months following the treatment. At the baseline visit, all the patients had pain. The percentage of patients experiencing pain significantly reduced at 6 weeks following treatment, and this improvement was maintained over the subsequent follow-up visits (p<.001) (Fig. 3).
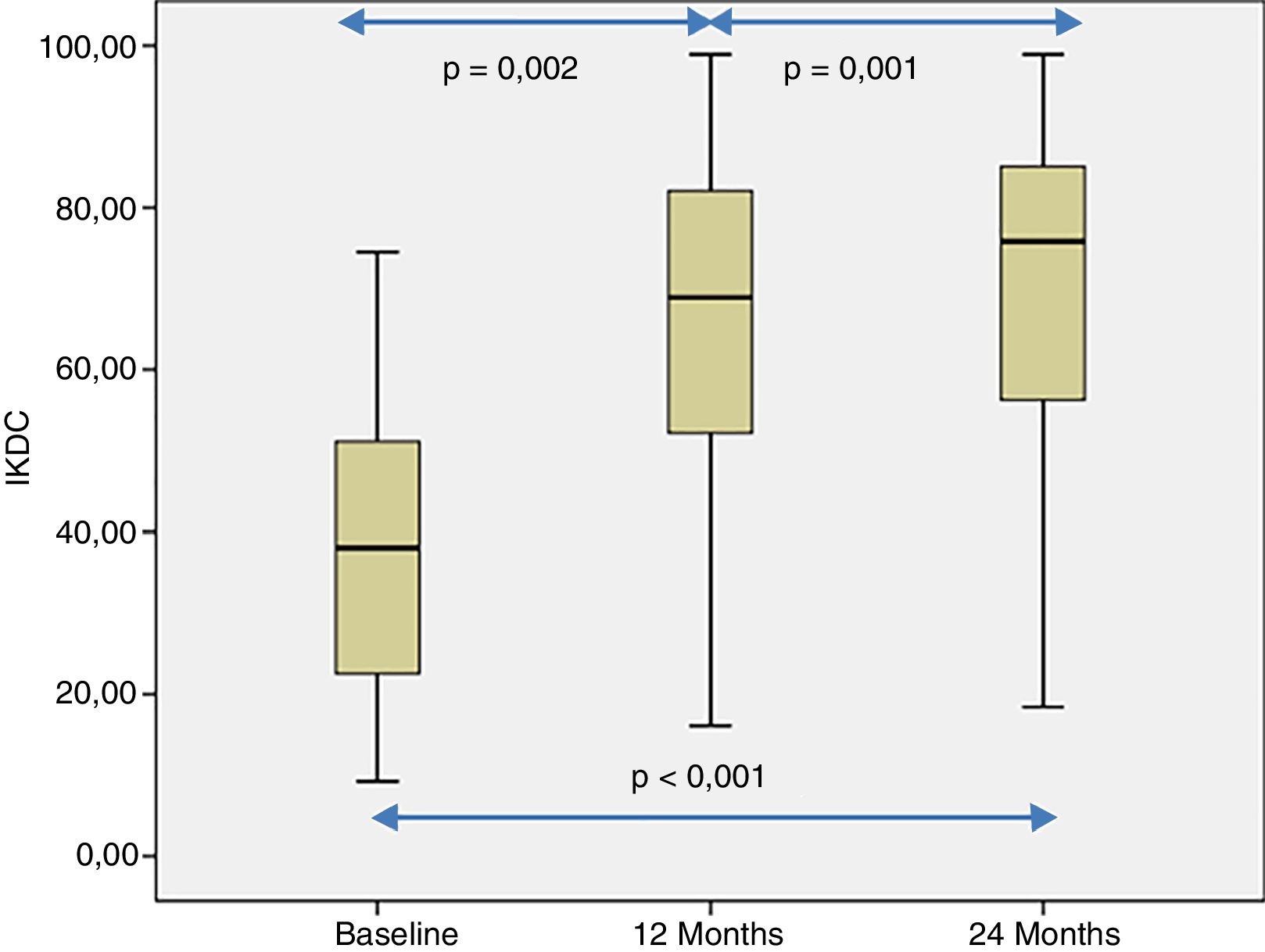
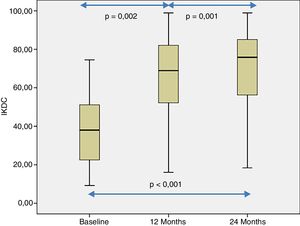
The patient's subjective perception of knee functionality, measured with the IKDC questionnaire, showed significant improvement when the baseline visits and follow-up visits at 12 and 24 months were compared (p<.001) (Fig. 4). The IKDC scores progressively improved from the baseline visit to the 24-month follow-up visit (p<.002) (Fig. 4). In all cases the mean difference in the IKDC score was estimated between the baseline visit and the follow-up periods (12 and 24 months), which the patient perceived as clinically relevant. Therefore, the mean difference in IKDC score between the baseline visit and the 12-month follow-up visit was 26.3 points (95% CI: 17.6–35 points), and 31.6 points (95% CI: 22.8–40.3 points) compared to 24 months following treatment with HD-ACI.
The presence of bone oedema was assessed by MRI in the 40 patients included in the study (Fig. 5). According to the study's inclusion/exclusion criteria, none of the patients had bone oedema on their baseline MRI. The MRI results showed very little variation in the number of patients who showed bone oedema at 6 months (9 patients), 12 months (10 patients) and 24 months (11 patients) following the HD-ACI high-density cell implantation. Likewise, no statistically significant differences were found when the 3 time periods were compared (p=.223). No statistically significant differences were found in the number of previous surgeries between the patients with or without oedema at 6 (p=.406), 12 (p=.701) and 24 months (p=.455) (median [minimum to maximum]: one previous surgery [0–5 surgeries] in the patients with oedema compared to previous surgery [0–4 surgeries] in the patients without oedema at 6, 12 and 24 months). There were no significant differences either in the number of lesions between the patients with and without oedema at 6 (p=.371), 12 (p=.233) and 24 months (p=.139) (one lesion [1–3 lesions]) in the patients with oedema as opposed to one lesion [1–3 lesions] in the patients without oedema in the 3 follow-up times). Finally, no significant differences were found in the size of the lesions among the patients with or without oedema at 6 (p=.503), 12 (p=.939) and 24 months (p=.720) (4cm2 [1.2–10.5cm2] in the patients with oedema compared to 4.6cm2 [1.5–8cm2] in the patients without oedema at 6, 12, and 24 months).
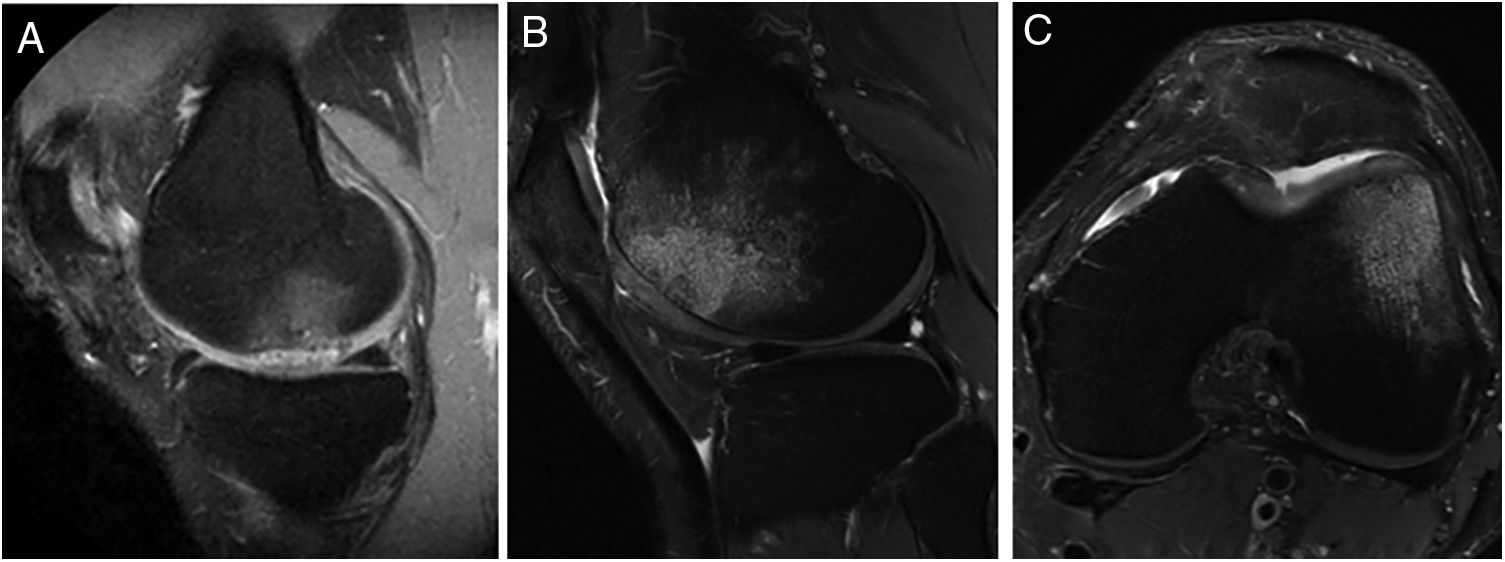
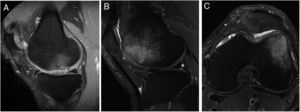
Representative MR images showing the appearance of subcondral bone oedema in 2 patients at 12 months (A) and 24 months (B and C) following high-density autologous chondrocyte implantation (HD-ACI). A) Subchondral oedema in the weight-bearing area of the internal femoral condyle, with intact meniscus and irregularity in subchondral bone (sagittal slice). Sagittal slice (B) and axial slice (C) of MRI of a patient with bone oedema in the external femoral condyle (trochlea) showing good integration of the chondrocyte implant with the subchondral bone.
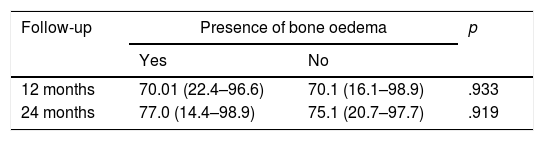
In order to study whether there was a relationship between the presence of bone oedema and the patients’ clinical outcomes, the IKDC scores were compared between the patients who presented bone oedema and those who did not, at 12 and 24 months following treatment with HD-ACI (Table 2). We found no significant differences between the 2 groups in any of the follow-up periods (p=.933 at 12 months; p=.919 at 20 months). Likewise, there was no correlation with the presence of bone oedema and the mean difference in IKDC scores at 12 months (p=.959; Spearman's correlation coefficient=−.009) and 24 months (p=.867; Spearman's correlation coefficient=−.029) compared to the baseline visit. We observed in all the patients included in the study that the area of the implant on the follow-up MRI scans showed a homogeneous and continuous signal, compatible with correct integration of the implant in all cases.
Comparison of IKDC scores expressed as medians (minimum to maximum) between the patients with or without bone oedema at 12 and 24 months following treatment with HD-ACI.
| Follow-up | Presence of bone oedema | p | |
|---|---|---|---|
| Yes | No | ||
| 12 months | 70.01 (22.4–96.6) | 70.1 (16.1–98.9) | .933 |
| 24 months | 77.0 (14.4–98.9) | 75.1 (20.7–97.7) | .919 |
In this study we describe the clinical outcome of 40 patients with cartilage lesions in the knee treated with HD-ACI, and its relationship with the incidence of subchondral bone oedema. Pain was shown to have improved significantly at 6 months (mean: 2.8±1.8) after the intervention compared to the baseline visit (8.2±1.1), which was maintained at 12 (2.3±2.1) and 24 months (2.1±1.8) of follow-up. Similarly, the IKDC scores increased progressively from the baseline visit (median: 39.9 [9.8–77.3]) until the visits at 12 (70.1 [19.3–97.8]) and 24 months (76.1 [17.6–98.3]), indicating that the patient's subjective perception of their knee functionality improved with the treatment. The relationship between clinical improvement perceived by patients and the mean difference in IKDC scores between the baseline visit and 12-month follow-up has been published, estimated at 16.7 points.18 This score, termed clinically relevant minimum difference, means that above it patients perceive real clinical improvement 12 months following autologous chondrocyte treatment. In our patients we observed a mean improvement of IKDC scores of 26.3 points at 12 months and 31.6 points at 24 months, which means that in our case the patients perceived this difference as a real improvement at 12 and 24 months compared to their baseline situation. These outcomes are in line with those found by other authors in patients treated with ACI in suspension or with MACI.19,20
Subchondral bone oedema is a common finding after cartilage treatment, but its interpretation is still under debate. In this study, which included patients without bone oedema at the baseline visit, the percentage who presented bone oedema after treatment with HD-ACI was very similar at 6 (22.5%), 12 (25%) and 24 months (27.5%). Several authors report high and at the same time heterogeneous incidences of bone oedema after chondrocyte implantation. The incidence of bone oedema one year following treatment with ACI in suspension varies between 47% and 56%15,21 whereas it rises to 78%16 with MACI although none of the authors found a correlation with a poorer clinical outcome. Other studies on treatment using MACI with longer follow-up periods found very similar incidences of subchondral bone oedema, from 47% at 5-year22 follow-up to 65.2% at 10 years23 following the intervention.
To assess this aspect, Filardo et al.17 performed a study on 116 patients with knee cartilage lesions. MRI follow-up was undertaken from 6 months to 9 years following treatment with MACI. The subjective IKDC score was taken at each MRI. The authors concluded that oedema following the treatment occurred during the first stages of cartilage maturation until 2 years of follow-up, and then considerably reduced after 2 and 3 years. Subsequently, the level of oedema increased again, and remained constant over medium/long term follow-up. As occurred in shorter follow-up periods,15,16,21 the presence of oedema did not correlate with a poorer clinical outcome. In our study we found a strikingly lower percentage of bone oedema after treatment with HD-ACI (25% at one year; 27.5% at 2 years) than that published by other authors.15–17,21–23 This could be due to the increase in cell density with the HD-ACI technique, which might have resulted in increased density of the extracellular matrix, and therefore to the formation of newly formed tissue with greater similarity to hyaline cartilage that restores the latter's biomechanical properties, protecting the subchondral bone from oedema due to its greater hardness. Regarding the relationship between the incidence of subchondral oedema and the improvement in knee symptoms and functions perceived by our patients, we observed that 12 months following the implantation the patients with oedema had the same IKDC scores (70.1) as those who did not. Very similar scores (75.1 compared to 77) were observed at 2-year follow-up. Likewise, there is no correlation with the presence of bone oedema and the mean difference in IKDC scores at 12 and 24 months. Like the other authors15–17,21 we found no correlation between the presence of bone oedema and the clinical outcomes of our patients.
Although our results do not point to a relationship between the onset of bone oedema and poorer clinical outcome, various publications indicate that this might not be the case for other diseases. There are some diseases where the presence of bone oedema predisposes to poorer outcomes for the underlying pathology,24–27 and could even influence progression to osteoarthritis,24 particularly in women with full-thickness chondral lesions.28 The patients included in this study were of a similar mean age (34.2±9.6) to that of patients in other studies.14,29 This age range coincides with one of the most active periods of life when damage to knee joint cartilage is very frequent due to physical exercise or traumatic injury.2,14 In more than half of our patients, HD-ACI was not the first treatment option to treat their cartilage defects, since they were treated with bone marrow stimulation techniques such as perforations or microfractures. These techniques, along with others such as mosaicplasty, are principally recommended to treat cartilage defects under 1cm2, and in routine practice chondrocyte implantation (in any modality) is considered a rescue procedure when previous techniques have failed. In fact, the defects in the patients included in this study were focal lesions of an average size greater than 1cm2, and therefore they became candidates for chondrocyte implantation following our own criteria and those published by other authors.14,30 More than half of our patients (27 of 40) underwent open surgery. This relatively high number of patients related to the number of patients with more than one lesion or to the site of the cartilage defects, which for us constituted an indication for open surgery.
In our unit, we have been using cell therapy techniques to treat these knee and ankle lesions for 22 years, either with ACI in liquid form (152 patients)9 or MACI (174 patients),12 and we performed our first implantation in 1996.31 López-Alcorocho et al.14 recently published outcomes at 2 years of follow-up in 50 patients treated with the HD-ACI technique on collagen membrane in knee cartilage lesions. The results of their paper indicate that the subjective perception of knee functionality, measured by IKDC score, and the presence of pain and range of motion (degrees of flexion and extension) significantly improved at 12 and 24 months of follow-up. These results together with the absence of adverse events show that HD-ACI is an effective and safe technique.
This study's principal limitation is that it is retrospective, and there was no control group. In this regard, a prospective study that examines the outcome of bone oedema in patients with or without this pathology at their baseline visit could reveal the role played by the condition more clearly. However, the aim of our study was to determine the incidence of bone oedema following treatment considered standard in our hospital for these types of lesions and its relationship with clinical outcomes. The design of this study enables an answer to this question, although we do not rule out a comparative design in the future.
Taking all our results as a whole, we can conclude that the incidence of bone oedema was 22.5–27.5% in our patients treated with the HD-ACI technique for joint cartilage lesions greater than 1cm2. We found no correlation between the presence of bone oedema and the outcomes of clinical parameters such as pain, subjective knee functionality and mobility.
Level of evidenceIII.
FinancingThis paper was financed by the Fundación Dr. Pedro Guillén.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: López-Alcorocho JM, Aboli L, Rodríguez-Iñigo E, Guillén-Vicente I, Guillén-Vicente M, Caballero R, et al. Evolución clínica y presencia de edema óseo subcondral a los dos años de tratamiento con implante de condrocitos autólogos de alta de densidad en la rodilla. Rev Esp Cir Ortop Traumatol. 2019;63:253–260.