To analyse the bone-ligament integration “ligamentisation” of the tendon graft in the reconstruction of anterior cruciate ligament (ACL) performing tunnels of different diameter.
Material and methodsWe performed the same reconstruction procedure using an autologous tendon graft taken from the superficial tendon of the hoof in 41 adult sheep. In Group A the tibial and femoral tunnels were 5mm in diameter and in Group B they were 7mm in diameter. The sheep were sacrificed at 3, 6 and 12 months after the surgery. Histological studies were performed on the graft and the tunnels, as well as a biomechanical analysis of the tibial–femoral complex.
ResultsIn Group A we did not observe direct integration of the bone and the tendon graft or any fibres joining both structures, although there was vascularised fibrous tissue. In Group B we did not observe any direct binding of the bone and the tendon graft either, although there was abundant fibrous tissue. The tendon graft showed a fascicular structure that increased over time in order to create septa for vascular penetration. Macroscopically the ACL graft had a dense appearance, which was very similar to the original tendon graft. The tendon grafts from group B and with a longer follow up period required a higher strength to produce maximum breakage than the tendon grafts from group A.
ConclusionAfter one year follow up, the histological study shows that the tendon graft is not transformed into a ligament, and there is no integration of the tendon graft in tunnels regardless of their diameter. Therefore, fixation techniques are essential to maintain the orientation and tension of the tendon graft.
Estudiar la integración de una plastia tendinosa en la reparación de un ligamento cruzado anterior (LCA) y el proceso de «ligamentización» con túneles de diferente calibre.
Material y métodosEstudiamos la integración del LCA en 41 ovejas adultas, según los siguientes grupos: grupo A: plastia tendinosa autóloga del tendón flexor superficial de la pezuña en túnel femoral y tibial de 5mm de diámetro; grupo B: en túneles de 7mm de diámetro. Sacrificio a los 3, 6 y 12 meses. Efectuamos un estudio histológico de la plastia en el túnel femoral, en el túnel tibial e intraarticular. También analizamos biomecánicamente el complejo fémur-plastia-tibia, con túneles de diferente diámetro y distintos tiempos de evolución.
ResultadosEn los túneles de 5mm no vimos integración directa de la plastia con el hueso. Observamos un tejido fibroso celular y vascularizado. En los túneles de 7mm tampoco observamos unión directa de la plastia con el hueso. El tejido de interfaz era un tejido fibroso con una estructura fascicular desarrollando la formación de septos y penetración de vasos. Macroscópicamente la plastia seguía recordando al tendón original. Las plastias de túnel de 7mm y con mayor tiempo de evolución necesitaban más fuerza de rotura y presentaban mayor elongación que las plastias de túneles de 5mm.
ConclusiónHistológicamente, al año de evolución, el tendón no se transformó en un ligamento y no había integración de la plastia con el hueso de los túneles, independientemente del diámetro de éstos.
Anterior cruciate ligament (ACL) tear is a common injury in sport. Its treatment has changed considerably over the last 3 decades, with there being a preference for replacing it in very active people to reduce the risk of secondary meniscal or cartilage lesions.1,2 The ACL is made up from 2 fascicles, the anteromedial, which tenses during flexion, and the posterolateral, which does so when the knee is fully extended.3 Human knee joint ligaments are structures made up from non-homogeneously distributed collagen. In cruciate ligaments, the central area contains more collagen than the distal and proximal ones and the density is less in the ACL than in the rest of ligaments,4 as it is mainly made up from type I collagen. The main function of the ACL is to prevent anterior displacement of the tibia in relation to the femur and – to a lesser extent – to control, under load, laxity in the varus, valgus and in rotation.5 The arteries from the cruciate ligaments come from the medial genicular artery that sends 4 branches to the posterior cruciate ligament and only 1 to the ACL; however, the insertion of these is free of vessels as they are fed by the synovial vessels that anastomose with the vessels of the periosteum.6 The need for surgical repair when this ligament is injured comes from this.
Methods to reconstruct the ACL have been described using autologous grafts with semitendinosus and gracilis tendons or from both tendons of the anserine, as well as from the iliotibial band and fascia lata or patellar tendon. These techniques usually offer good results, but they always damage the non-injured structure. In addition, they are not exempt from complications, such as perforating branch lesions, infrapatellar branch damage of the saphenous nerve and subcutaneous fat atrophy in the donor area, causing retractile and painful scars. Patellar ligament autograft (H-T-H) also offers good results, but has the disadvantage of sometimes causing pain in the anterior aspect of the knee. Whichever types of autograft we use, they require stable fixation to avoid graft movement inside the tunnel, with the first 12 weeks after surgery being the most crucial.
The graft integration stages start with necrosis in the centre of the graft together with hypocellularity; then there is a stage of proliferation where there is great cell activity with abundant agglomeration of fibroblasts. This is followed by a remodelling stage where there is less resistance to the graft whilst it is being structurally reorganised.7 For its part, ligamentisation consists of the functional adaptation that takes place in the graft as it changes into the ligament that it is replacing.8
The hypothesis of this study was that bone ligament integration of grafts in tunnels with a larger diameter is less than that in tunnels with the same diameter. This is due to the osteolysis produced by the synovial fluid of the joint itself, and that a graft that is well adapted to the femoral and tibial tunnel diameter integrates itself more quickly and offers more guarantees. The aim of this work was to study the integration of a tendon graft in ACL repair with different diameter tunnels, to study the structural transformation of the graft with different size tunnels and examine the biomechanics of the femur–graft–tibia complex with different diameter tunnels and over different periods of development.
Material and methodWe carried out the study on the integration of the anterior cruciate ligament on 41 sheep (merino breed), which were 3 years old and weighed 30kg. The animals were sacrificed at 3, 6 and 12 months and were grouped together as follows:
Group 0: animals to design the methodology or animals that died prematurely.
Group A: autologous bone-graft taken from the superficial flexor tendon of the hoof with tibial and femoral tunnels of 5mm in diameter.
Group B: autologous bone-graft taken from the superficial flexor tendon of the hoof with tibial and femoral tunnels of 7mm in diameter.
General anaesthesia with endotracheal intubation was used. To administer anaesthetic drugs, the cephalic vein in the right forelimb was cannulated and a drip system was attached, through which maintenance fluid therapy was also supplied, with dextrose 5%. Anaesthesia was achieved through intravenous administration of thiobarbital® (Abbot) at a dosage of 12mg/kg, atropine® (B. Braun) 0.5mg/kg and fentanyl® (Kern), at a dosage of 0.015mg/kg; anaesthesia was maintained with thiobarbital® at a dosage of 10mg/kg and fentanyl® at a dosage of 0.015mg/kg. The animal woke up on its own.
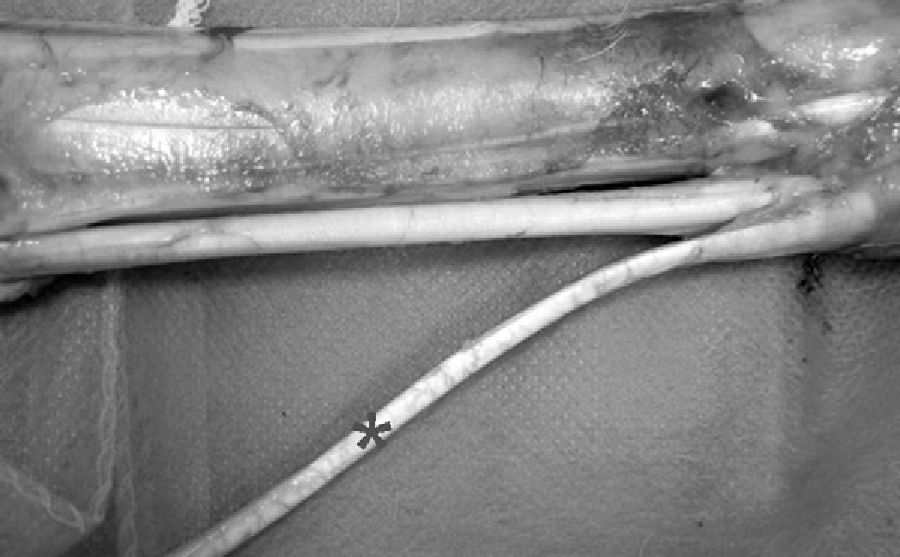
Surgical techniqueThe animal was placed in a supine decubitus position and both front legs, together with the left hind leg, were immobilised. The area of left leg to be operated on was carefully shaved and cleaned. An arthrotomy of the knee was undertaken, with an internal parapatellar incision that allowed us to see the joint and the entry and exit of the femoral and tibial tunnels. We used the superficial flexor tendon of the hoof for the autologous bone-graft as this did not produce great functional changes in the animal, leaving the deep tendon intact (Fig. 1). We performed a medial parapatellar approach, from the distal 3rd of the thigh to 2cm distal to the anterior tuberosity of the tibia. Using an electric scalpel we sectioned the superficial aponeurosis and part of the vastus medialis, which helped us to dislocate the kneecap outwards and completely bend the knee. Once the anterior cruciate ligament had been sectioned by its grafts, we cleaned the rest of the ligament and then perforated the tunnels. First we made the femoral tunnel, trying to go in through the metaphyseal junction of the diaphysis with the external condyle and exiting through the ACL anatomic insertion point. The tunnels were randomly made using 5mm or 7mm diameter drills, according to the groups. Later we performed the tibial tunnel with the same thickness as the femoral tunnel. We drilled from the inside of the proximal end of tibia, trying to exit through the ACL insertion point of the tibia. Once these tunnels were made, we cleaned them with abundant saline so as not to leave any bone debris.
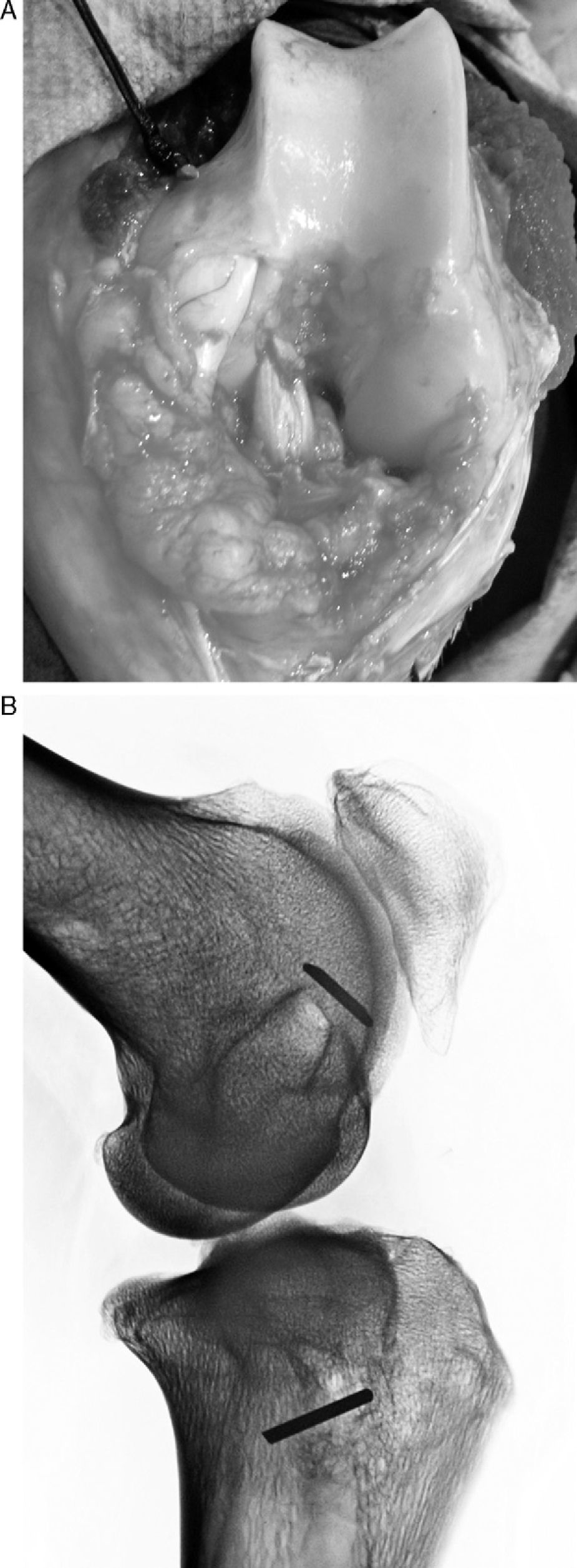
At the same time another team extracted the graft from the superficial flexor muscle of the hoof. After measuring its length (80mm) and thickness (5mm), the graft was made with sutures at its ends using 00 silk thread. We passed the graft using a bone wire guide to occupy the entire tunnel length. Once the graft was in place, we flexed and extended the knee to see if it had been correctly positioned and had the correct tension; next we anchored it with a cortical stop using a Kirschner wire that was 3cm long, on which we tied the suture of the ends, leaving the graft at a tension of 60° with the knee in flexion. We avoided interfering with the integration process of graft in the tunnel. Later we closed by planes, leaving no intra-joint drainage and performing no immobilisation (Fig. 2).
The animals were sacrificed according to their group, extracting pieces that were decalcified and later dehydrated using gradually increased doses of alcohol of (70%, 80%, 96% and 100%), changed every 12h, and constantly shaken. They were later placed into xylene for 4h, and then put into paraffin, at 60°C. Finally, 4μm cuts were obtained using a conventional microtome (Microm®. Model HM 340-E, Germany) and were stained with Masson trichrome (MT). Once the process was finished, we sectioned the femoral and tibial tunnels transversally through the middle and the anterior cruciate ligament was removed to perform a transversal section. The bone portions were cut carefully so as not to cut the tunnel and an X-ray of the tunnels was later taken. The tunnels were transversally cut, trying to make the cuts perpendicular to the tunnel axis; they were placed in formaldehyde and later decalcified. Sagittal cuts were performed on the grafts and they were stained with MT. All the preparations were later viewed using an optic microscope and polarised light. We dissected the knee, leaving only the bone ends and the graft for the biomechanical studies and these were frozen at −20°C. Mechanical tension testing was performed using an Instron Electropuls 3000 machine, leaving the knee at 30° of flexion and obtaining the necessary information on the maximum break load, resistance and break point along the femur–ligament–tibia complex.
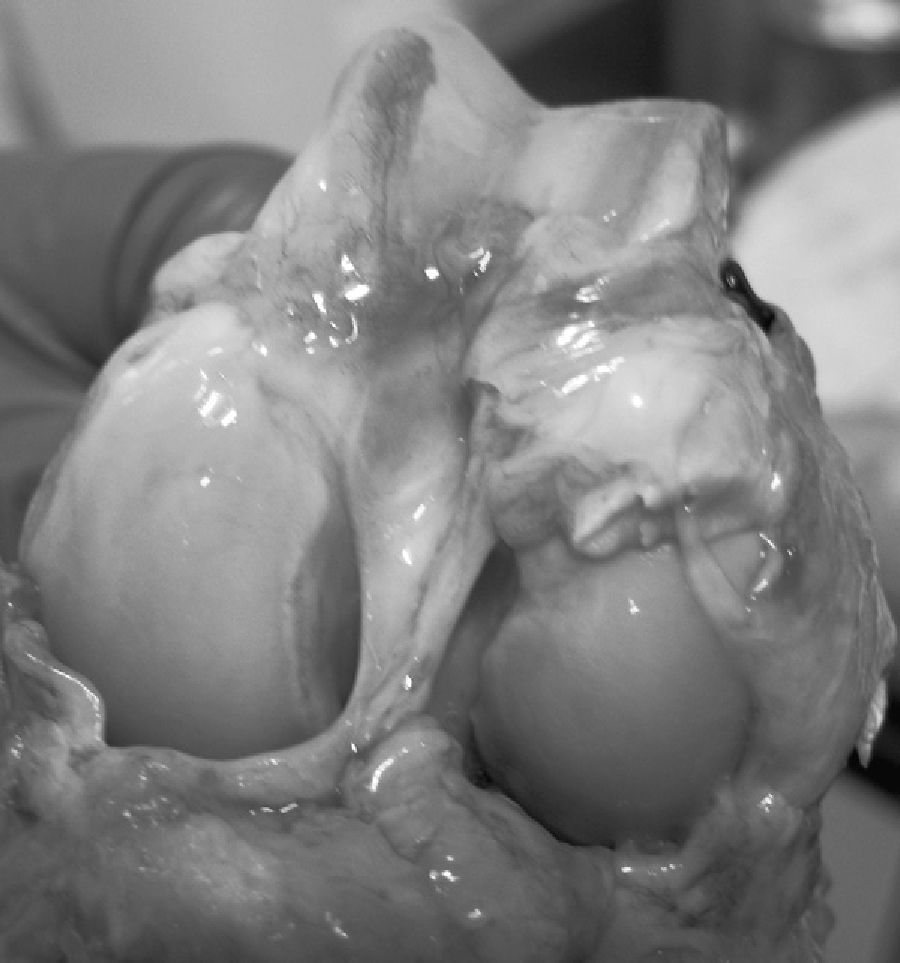
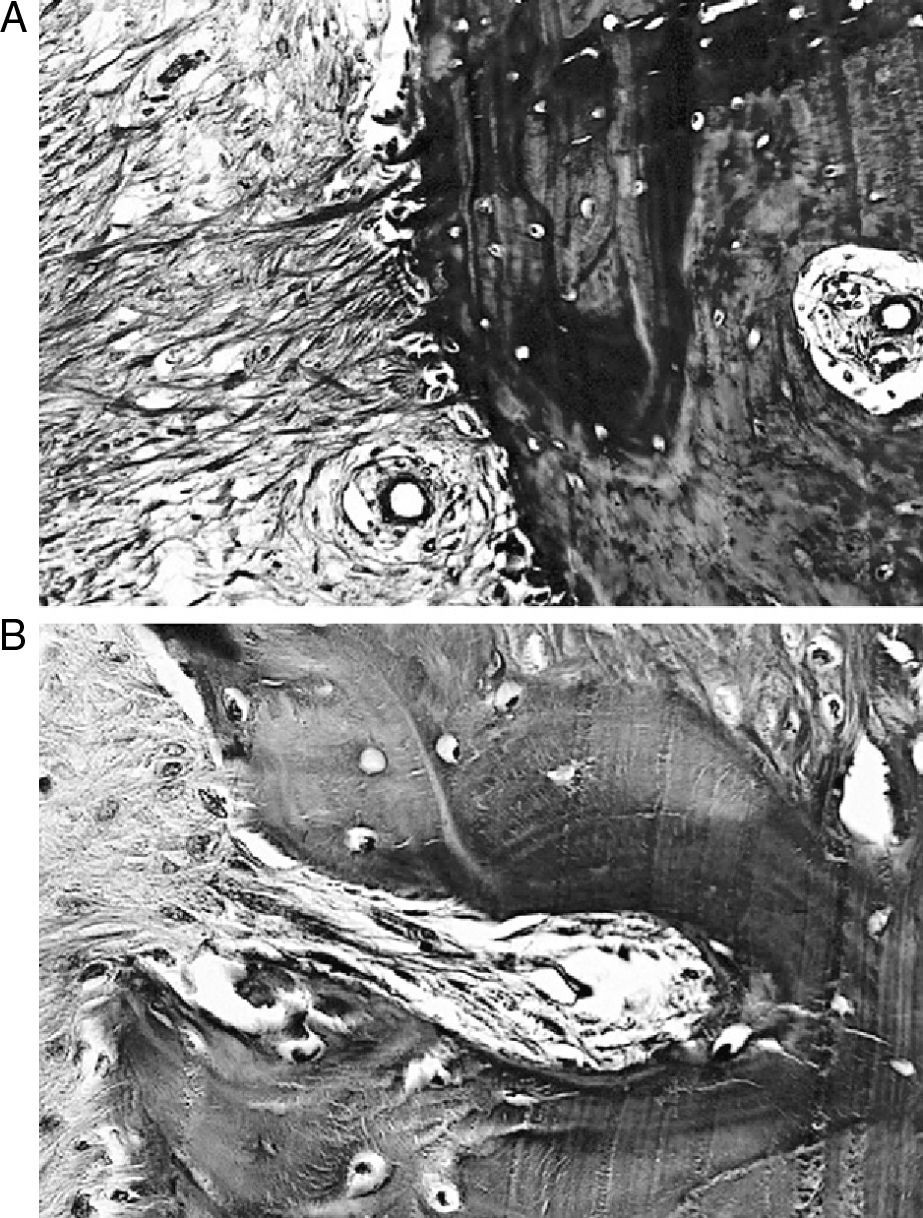
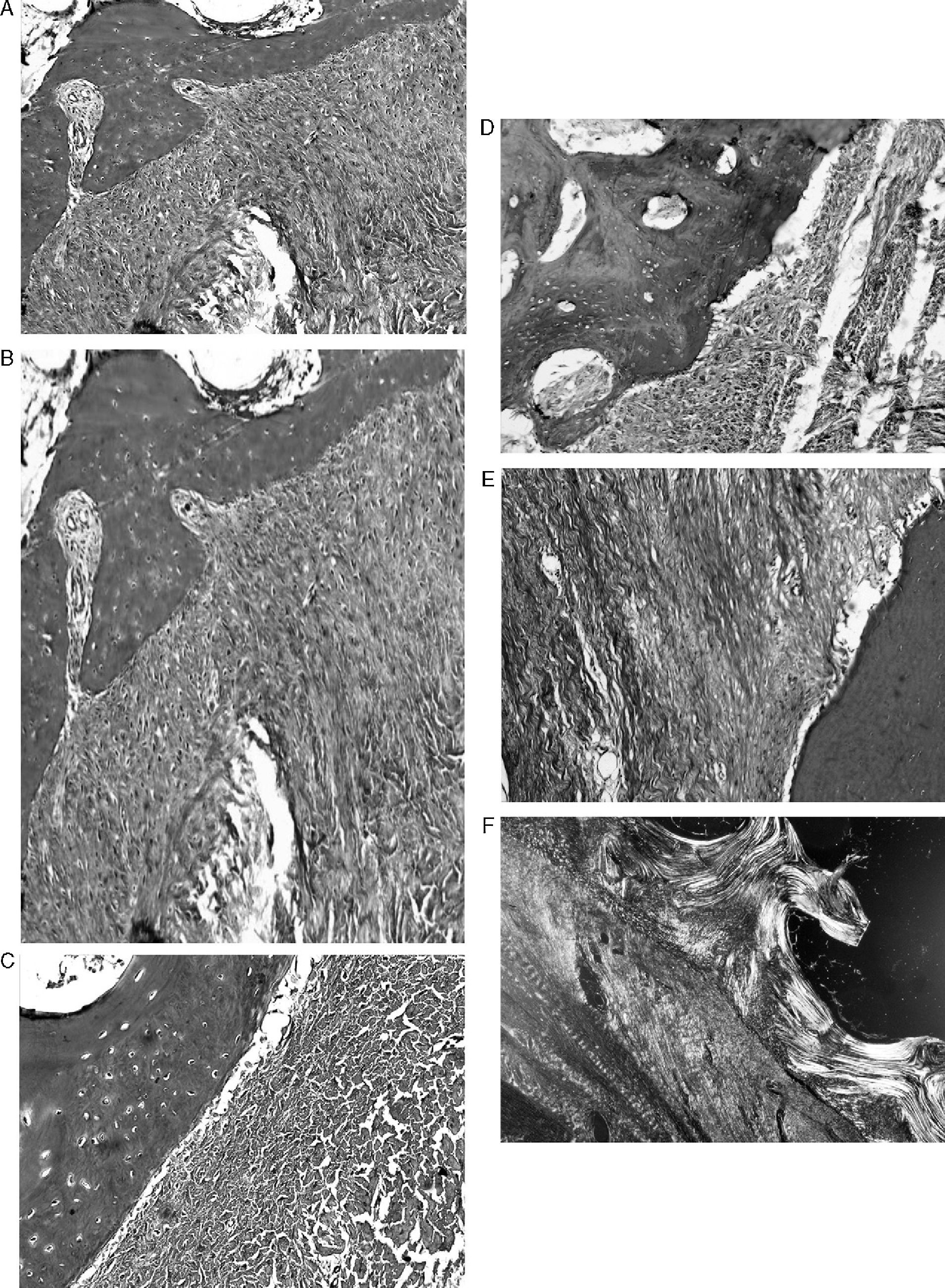
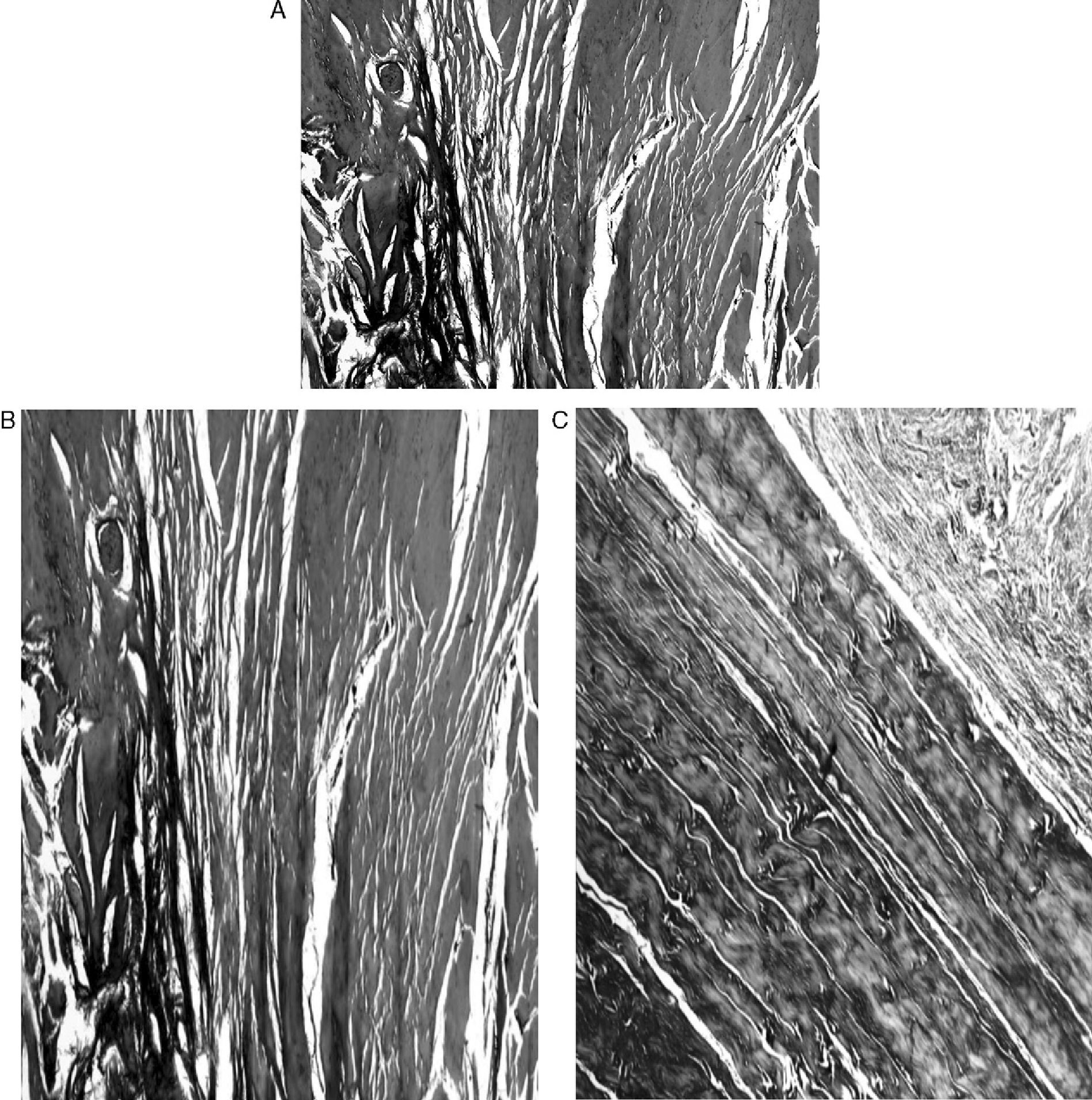
ResultsMacroscopically we observed that the ACL graft reconstruction had a dense appearance, similar to a tendon, and was covered in synovial tissue, which was more conspicuous after the 6-month follow-up period. This was the same in both the 5mm and the 7mm tunnels (Fig. 3). With regards to the histology of the grafts in the 5mm tunnels, despite the tension when introducing the graft into the tunnels and the total occupancy of the graft in the femoral or tibial tunnel, we did not observe direct integration of the graft with the bone, and few Sharpey's fibres were seen (Fig. 4) among both structures; in all cases we saw very cellular, vascular fibrous tissue. Likewise, the histological results of the grafts in the 7mm tunnels did not show a direct union of the graft with the bone tunnel, the fibrous tissue filling was very abundant (Fig. 5) and the graft presented a fascicular structure that formed septa for vascular penetration (Fig. 6).
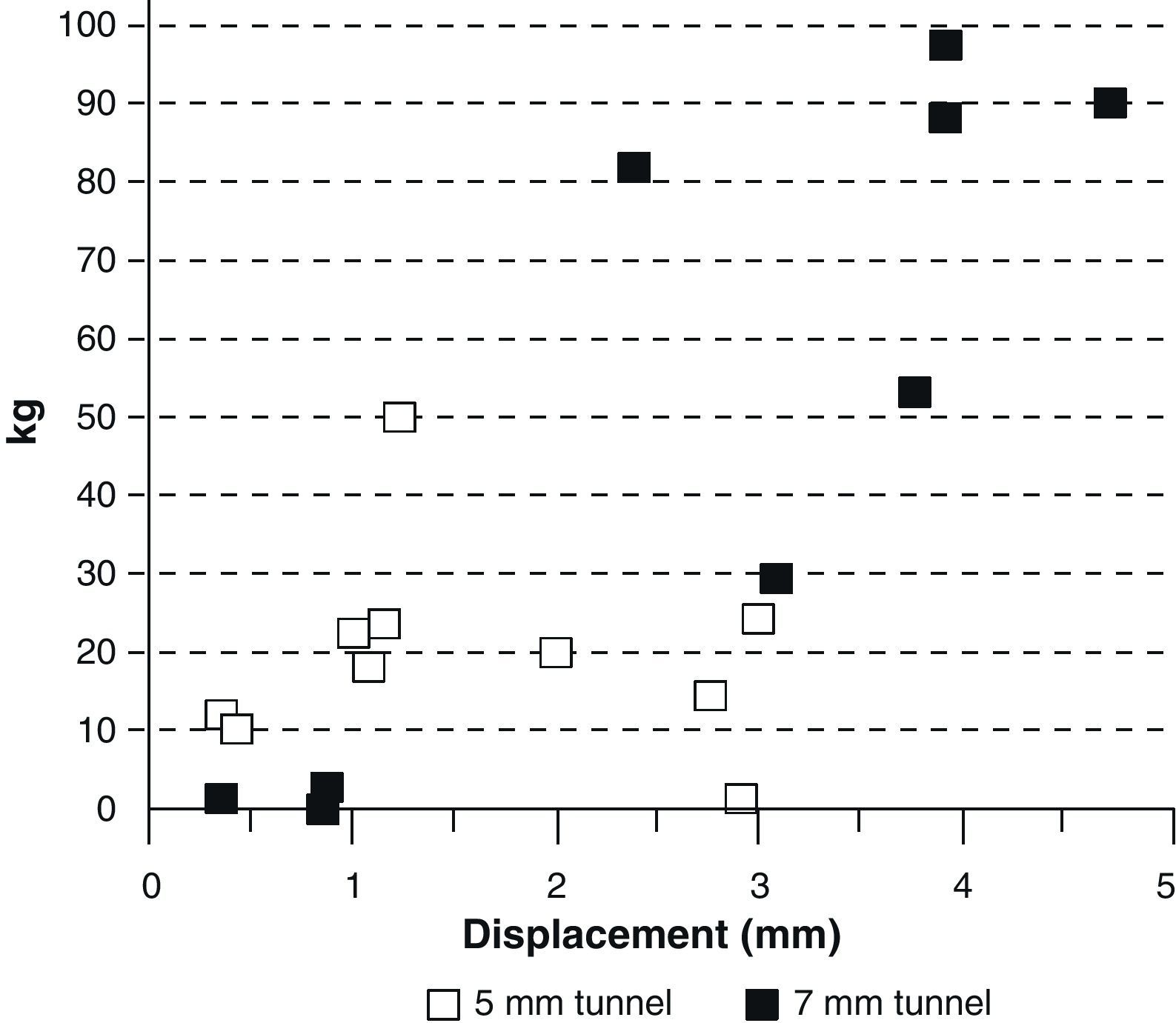
When the biomechanical tests were performed, there were grafts that came out of the tunnel whole, others were frayed, but none broke either in the tibial or femoral insertion. That was the reason why we thought that an elastic anchoring made up by fibrous tissue was produced and there was no complete integration in any of the cases (Fig. 7). We grouped the biomechanical results according to the tunnel diameter (5mm or 7mm), observing that the grafts in the 5mm tunnels bore less than 30kg of tension and lengthened less than 3cm. On the other hand, the grafts in the 7mm tunnels bore tensions of between 80 and 100kg and lengthened over 3cm (Fig. 8). By examining the biomechanical results according to the tunnel diameter and the development period, we found that those grafts in 7mm tunnels that bore less than 30kg tension were the ones that had had a lesser development period.
Different theories regarding graft size in ACL repair are well known. Some authors confirm that there is better integration the more the graft is introduced into the tunnel9; however, others recommend that the graft and the tunnel should be the same diameter10; while some articles say that to obtain the best bone-graft integration, the length of the graft is more important than its thickness.11 There are also some that suggest that a femoral and tibial tunnel should be performed with a tunnel that is 1mm less than the graft, to achieve proper integration.12 Graft repair in the tunnel has been seen to be caused by bone growth in the interface by forming a sclerosis, producing endochondral ossification with a few variable Sharpey's fibres being observed in the first few months.13 In fact, it has even been confirmed that tendon resistance is related to the amount of new bone.14
We used tibial and femoral suspension bindings to see the behaviour of the graft and not impede its integration with the bone, as occurred when using an interference screw. However, we did not see the integration of the graft and bone in any of the cases, not even in those of longer evolution.
A bone sclerosis is formed in the tibial tunnel as well as in the femoral tunnel and this separates the epiphyseal trabecular bone from the graft, leaving a space between both made up from a peritendinous fibrous tissue with there being few Sharpey's fibres joining both structures. In our study we observed sclerosis of the tunnels in all cases, probably also related to perforation speed and temperature and type of drill wear, but this was greater with the longer development period. Sclerosis is a trabecular reaction to graft movements on the inside of the tunnel and this prevents the integration between the bone and the tendon fibres. The widening of the tunnel after an ACL graft in sheep has shown that the widest tunnels did not produce greater anteroposterior transfer. On the contrary, they showed greater rigidity and hypertrophy of the graft and greater sclerosis in the tunnel walls.
The integration process of a graft has very well-documented stages: necrosis, revitalisation, type III collagen formation and remodelling.15 In literature we see that cellularity returns to normal values between 3 and 6 months after surgery16,17 and that the collagen fibres are similar to the ACL ones between 6 and 12 months after surgery18; however, we also argue that the composition of the fibre characteristics of collagen of different diameters of the ACL was never achieved.19
We have also seen that grafts follow an adaptation process more than a repairing one20 and that they acquire a characteristic ligament aspect that is very different to the original tendon. This has been given the name “ligamentisation” and should not be seen as a transformation of a tendon into a ligament, but as an adjustment process of the extra articular tendon, subjected to continuous deformation by muscular contraction, to act as a control structure for the displacement of the bone structures, surrounded by synovial tissue. We discard the term “ligamentisation” as part of the integration process of a graft.
In our study the graft maintained a tendinous structure throughout the follow-up period, made up by large wavy collagen fibres with interstitial spaces occupied by vessels. On the other hand, we saw that the external appearance of the graft was very similar to the ACL, covered by synovial tissue, with even the reconstruction of adipose ligament.
In mechanical trials, the graft never tore at its bone insertions; it frayed as the tension increased. We found a tendency in which the graft resistance increased according to time and due to the thickness of the tunnel performed. The grafts placed in roomy tunnels showed breakage values that were higher than those that were inserted under pressure in tunnels with a lesser diameter. This could be caused by a greater amount of fibrous tissue in the tunnel that improves the anchorage of the graft in the tunnel, and that it is also denser the greater the follow up period. However, we have not found references to this in literature consulted.
Our work is a preliminary study to determine the best experimental model to repair ACL breakage in sheep, which allows us to examine the integration and evolution of a graft at a cellular and molecular level. However, this study presents certain limitations, such as the number of samples available for the different groups and studies; relying on an anchorage that depended possibly on knot type and tension constituted another limitation, although we always attempted to do them in the same way. Another limitation was performing the biomechanical trials with anchorages. However, this trial performed on sheep allowed us to establish a quick homogeneous experimental model, being sure about the follow up period of the animals, without seeing histological differences in the integration of the bone, tibial or femoral tunnels, of a graft that was introduced under pressure or loosely. We saw that it produced bone sclerosis in all the samples, like a bone shield that protects the trabecular bone from the aggression of the joint and of the graft itself with its mechanical reaction, by the movements of the graft on the inside of the tunnel, and biologically, through inflammation and necrosis during the graft remodelling process.
Level of evidenceLevel of evidence III.
FundingFIS PI09/1729.
Ethical DisclosuresProtection of human and animal subjects. The authors will declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of Data. The authors declare that no patient data appears in this article.
Right to privacy and informed consent. The authors declare that no patient data appears in this article.
Conflict of interestThe authors have no conflict of interests to declare.
We thank Isabel Zapero for her histological preparations and Purificación Ripalda for her polarised light pictures.