Prolongation of drug-based thromboembolism prophylaxis after discharge from hospital is clearly recommended following total hip and knee replacement. The aim of this study was to evaluate and compare adherence to and satisfaction with outpatient thromboembolism prophylaxis (by injection and oral) under routine clinical practice conditions.
Material and methodWe analysed two consecutive cohorts of patients (480 and 366, respectively) who had undergone total hip or knee replacement surgery in 120 Spanish hospitals, and were prescribed outpatient thromboembolism prophylaxis, by injection and orally, respectively. Information on adherence to and satisfaction with both treatments, sociodemographic data and treatment compliance was collected using specific questionnaires.
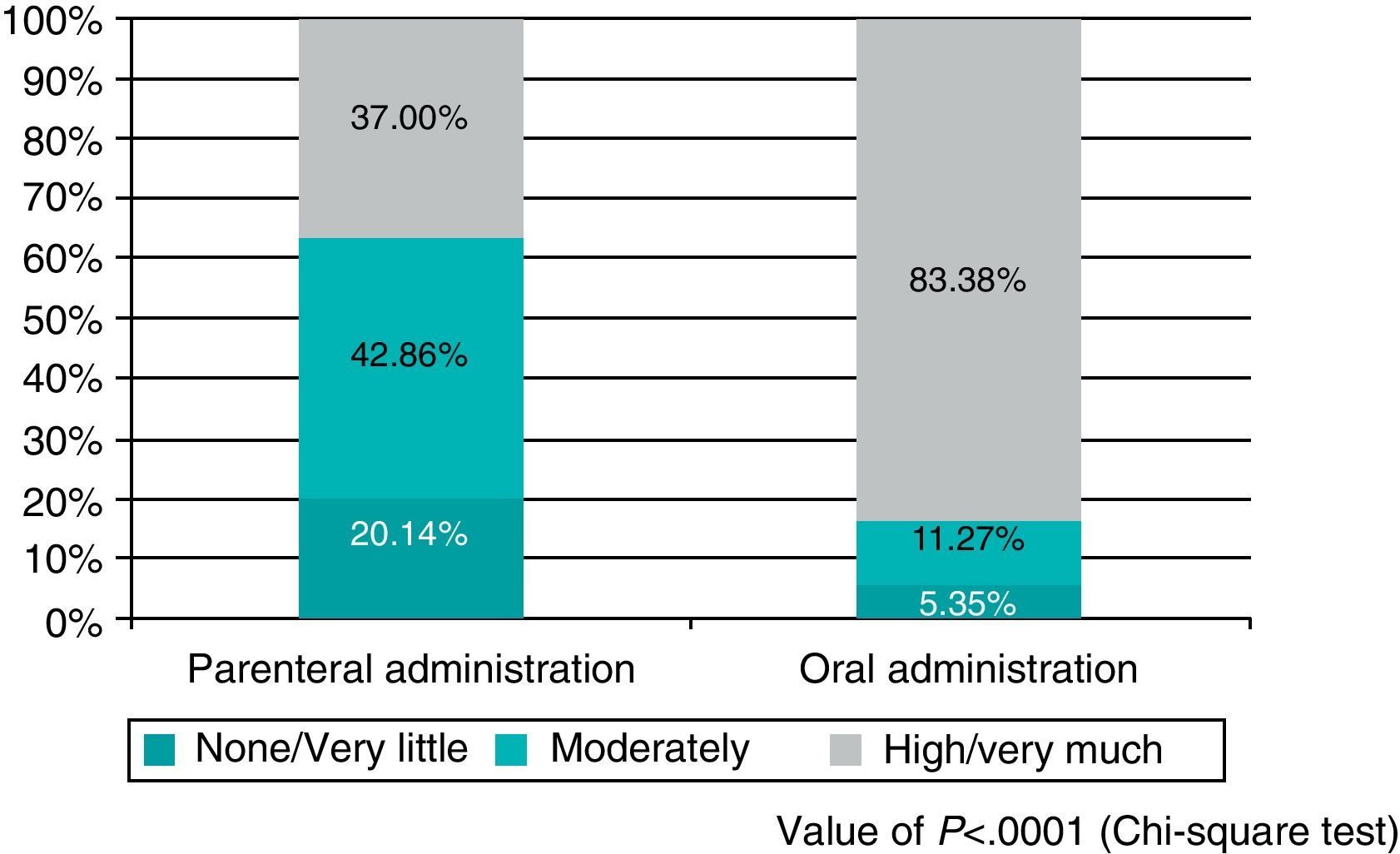
ResultsThe drop-out rate (9.49% vs. 4.14%), general satisfaction (37% vs. 83.38%), and the TSQM satisfaction scale were better in the oral prophylaxis cohort and, although the differences between the two routes of administration were not significant, treatment compliance was also better in the oral cohort (Morisky–Green test: 53.49% vs. 59.05%).
ConclusionsAdherence to and satisfaction with the oral thromboembolism prophylaxis were better than for prophylaxis by injection in the context of outpatient prolongation. Nevertheless, suboptimal treatment compliance was found in both cohorts, which could result in lack of efficacy of the prophylaxis.
Both patients and doctors have to be made aware of the importance of post-discharge extension of thromboprophylaxis in orthopaedic surgery with high thrombotic risk. Moreover, strategies should be developed to encourage compliance.
La profilaxis tromboembólica farmacológica, prolongada tras el alta hospitalaria, está claramente recomendada en el postoperatorio de la artroplastia total de cadera y rodilla. Este estudio pretende valorar y comparar la adherencia y la satisfacción a la profilaxis tromboembólica ambulatoria (inyectable y oral), en condiciones de práctica clínica habitual.
Material y métodoSe analizan 2 cohortes consecutivas de pacientes (480 y 366 respectivamente) intervenidos de artroplastia total de cadera o rodilla en 120 hospitales españoles, a las que se indica la profilaxis tromboembólica ambulatoria inyectable y oral, respectivamente. Mediante cuestionarios específicos, se recoge información sobre la adherencia y satisfacción a ambos tratamientos, datos sociodemográficos y cumplimiento terapéutico.
ResultadosLas tasas de abandono (9,49 vs. 4,14%), la satisfacción general (37 vs. 83,38%) y la escala de satisfacción de TSQM fueron más favorables en la cohorte de la profilaxis oral. El cumplimiento terapéutico, si bien sin diferencias significativas entre ambas pautas (test de Morisky–Green de 53,49 vs. 59,05%), fue también superior en la cohorte de la tromboprofilaxis oral.
ConclusionesLa adherencia y la satisfacción a la profilaxis tromboembólica oral son superiores a la profilaxis inyectable en el contexto de la prolongación ambulatoria. Sin embargo, en ambas cohortes se evidencia un subóptimo cumplimiento terapéutico que podría repercutir en una merma en la eficacia de dicha profilaxis.
Es preciso concienciar al paciente y a los médicos de la importancia de la extensión ambulatoria de la tromboprofilaxis en la cirugía ortopédica de alto riesgo trombótico, así como desarrollar estrategias que favorezcan su cumplimiento.
Deep vein thrombosis (DVT) and its main complication, pulmonary embolism (PE), are the 2 most important manifestations of the same process: venous thromboembolism (VTE). In many cases, the clinical symptoms of VTE are silent and the first manifestation may be a fatal PE.
VTE is a common complication after orthopaedic surgery. All patients undergoing lower limb surgery are susceptible to DVT, associated to PE or not, with operations for hip fractures and prosthetic joint replacements of the hip and knee carrying the highest risk.1–3 According to a study conducted in different European countries, Spain among them, an estimated 4,65,000 episodes of VTE and nearly 3,00,000 cases of non-fatal PE take place each year.4 In a meta-analysis including the evaluation of over 10,000 patients in approximately 50 controlled studies,5 the prevalence of DVT among patients undergoing surgery due to hip fractures or prosthetic hip or knee implants ranged between 48% (according to non-clinical phlebography) among patients not following any pharmacological prophylaxis and 20% among those in whom an appropriate prophylaxis protocol was applied. Furthermore, a 7.9% prevalence of PE was found, which led to a fatal outcome in 1.4% of cases.
In Spain, hospital mortality for PE is around 11% and for DVT it is around 2%.6 The duration of thromboprophylaxis varies depending on the type of surgery. In the case of total hip replacements (THR) or total knee replacements (TKR), the recommendations of international clinical guidelines include extending pharmacological prophylaxis up to 4–6 weeks after surgery,2,3,7 although with a lower grade of recommendation (2B) for knee arthroplasties. Considering that patients usually remain hospitalised for 7 days, it is clear that the majority of postoperative prophylaxis will occur outside of a hospital environment, without direct medical supervision.
Adherence to these guidelines is essential, but possibly far from being fully assured in an outpatient context. Some series of patients7,8 estimated that adequate outpatient thromboprophylaxis could not be assured in up to 28% of patients. The reasons included fear of injection, poor technique when administering the drug, forgetfulness, the appearance of adverse effects caused by low molecular weight heparins (LMWH) and insufficient awareness about the importance of proper prophylaxis, among others. All these reasons could cause a low degree of patient satisfaction regarding subcutaneous administration and poor adherence to the therapy, which in turn could lead to a potentially fatal failure of prophylaxis.
On the other hand, new oral antithrombotic drugs (dabigatran, rivaroxaban, apixaban) have been recently introduced for the therapy and treatment of postoperative DVT prevention in hip and knee arthroplasties. These agents have demonstrated efficacy and safety in clinical trials at least equivalent to that obtained with LMWH (dabigatran) or even higher (rivaroxaban, apixaban), so their use in this indication has been approved.8–12 Moreover, it would be reasonable to expect that an oral therapy may improve patient satisfaction and treatment adherence compared with parenteral treatment, particularly after hospital discharge, but this hypothesis has not been demonstrated in our clinical practice.
The main objective of this study was to compare both types of prophylaxis under conditions of routine clinical practice in Spain, in terms of patient satisfaction and adherence to treatment. Secondary objectives included knowing whether the information received from medical staff was sufficient and whether injectable medication was correctly administered outside a hospital environment. Our hypothesis was that adherence and satisfaction would be higher with an oral treatment protocol than with injection therapy.
Material and methodStudy designThe SALTO study on satisfaction and adherence to treatment in the prophylaxis of thromboembolic disease is an epidemiological, multicentre, cross-sectional study conducted on patients undergoing total hip or knee arthroplasty. The study was conducted in 120 Spanish public and private hospitals of 14 regions and was developed in 2 phases. The study protocol was approved by the Clinical Research Ethics Committee of one of the participating hospitals (Hospital Clínic, in Barcelona), with registration number 2009/5344.
In the first phase, from December 2008 to May 2009, 480 consecutive patients were recruited at 50 centres. These patients received postoperative thromboprophylaxis with subcutaneous injectable drugs (LMWH or fondaparinux, 1 subcutaneous injection every 24h), for at least 1 week after hospital discharge. We excluded patients who had received injectable antithrombotic prophylaxis previously or who suffered insulin-dependent diabetes. Of these 480 patients, 48 were excluded due to stopping prophylaxis early and not having completed a minimum of 1 week of injectable prophylaxis after hospital discharge (inclusion criterion).
In the second phase, from January to December 2010, 366 consecutive patients were recruited at 70 centres, regardless of whether they had followed thromboprophylaxis with injectable drugs (LMWH or fondaparinux) or with oral agents (dabigatran or rivaroxaban) during admission. They received only oral prophylaxis for at least 1 week after they were discharged from hospital. Of these 366 patients, 7 were excluded due to an inability to adequately respond to satisfaction questionnaires.
Data collection instrumentsPatient data were collected in the context of a routine outpatient visit, between 1 week after discharge and up to 8 weeks after surgery: mean period of 33.16 days (95% confidence interval [CI]: 28.36–37.96) between the intervention and the visit in phase I (parenteral antithrombotic prophylaxis); mean period of 45.33 days (95% CI: 36.57–54.10) in phase II (oral antithrombotic prophylaxis).
The data collection questionnaire in the SALTO study consisted of 2 parts. The first part, completed by the surgeon, collected sociodemographic data, of the current illness and patient compliance. Compliance was measured using the Morisky–Green test,13 widely employed to estimate this aspect and with very good psychometric properties. The test considered patients as non-compliant when the answer to any of the 4 closed dichotomous (yes/no) questions posed did not follow the established pattern (no, yes, no, no): “Do you ever forget to take your medication?”, “Do you take your medication everyday at the correct time?”, “When you feel well, do your ever stop taking your medication?” and “Do you stop taking the medication if you believe it is not working well?”.
The second part, completed by the patient, collected all the information on treatment satisfaction using the following questionnaires:
- •
Simplified Treatment Satisfaction Questionnaire for Medication (TSQM) scale.14
- •
Set of questions on general satisfaction.
- •
Simplified Insulin Treatment Satisfaction Questionnaire (ITSQ) (phase I).15
Originally, the TSQM scale consisted of 4 dimensions which assessed satisfaction with the treatment (side effects, effectiveness, convenience and overall satisfaction). In this case, given the indication of the drugs used (prophylaxis), we considered that the efficacy parameter was not applicable in our study and thus the dimension of effectiveness was eliminated. The resulting simplified and adapted questionnaire consisted of 11 items whose response, expressed using a visual analogue scale, ranged between 0 (very dissatisfied) and 100 (very satisfied).
In the series of questions on overall satisfaction, patients were asked about the information they received regarding the medication and its method of application, as well as ease, discomfort and difficulty inherent to antithrombotic medication, in order to assess whether they were not/a little, moderately or very/highly satisfied with the treatment. No ad hoc prospectus was prepared to be delivered to patients included in the study. All patients received information according to everyday clinical practice at their hospitals. Furthermore, in the first phase of the SALTO study (injectable prophylaxis) we used 3 of the 22 questions from the ITSQ questionnaire, validated specifically for diabetic patients treated with insulin, but which may be used to assess satisfaction with subcutaneous injection treatment. Using an analogue scale from 1 to 7, where 1 represented the most favourable option for the patient (without pressure, pain or stress) and 7 the worst, patients were asked about: preparation of medication, pain or physical discomfort and emotional stress or anxiety due to the application of the medication.
The study protocol was reviewed and approved by the Clinical Research Ethics Committee of Hospital Clínic of Barcelona and all patients completed the necessary informed consent form. In both phases, patients who had difficulty understanding and answering the questionnaires were excluded from the study, as were those who suffered cognitive impairment compromising their ability to sign an informed consent form.
Statistical analysisSince no single assessment was established to calculate compliance and satisfaction, an overall, descriptive analysis was performed for each indirect method.
Bivariate analysis allowed us to study the relationship between compliance and satisfaction, as well as between adherence and the information available to patients regarding their medication and how to administer it. Quantitative variables were described using measures of central tendency and dispersion, and qualitative variables using frequency tables. We conducted a point estimate and by 95% CI of proportions and means of the main variables.
Bivariate analysis of categorical variables consisted of the Chi-square test for comparison of contingency tables when the variables were nominal or Fisher's exact test in cases when the aforementioned was not adequate. For continuous variables that met the assumptions of normality we performed analysis of variance or, alternatively, used its nonparametric equivalent, the Kruskal–Wallis test.
Finally, we conducted a hierarchical classification analysis, a type of multivariate analysis which enabled us to classify and group patients according to their compliance profiles.
All statistical analyses were performed using the software package Statistical Analysis System® v.8.2. (SAS Institute Inc., Cary, NC, USA).
Study promoterThe SALTO study was sponsored by Bayer Hispania SL.
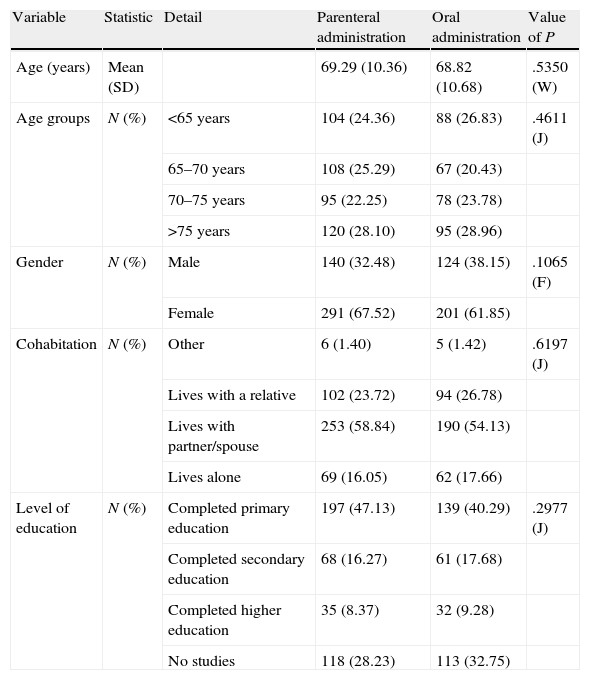
ResultsSociodemographic dataTable 1 shows the sociodemographic characteristics of the patients included in the study, homogeneous between the first and second phases. It is noteworthy that the mean age of the total population was 69.08±10.49 years and that only 34.92% of the participants were male (264 males vs. 492 females). We also noted that over half of those selected lived with a partner (56.72%). In addition, when asked about their completed studies, it was found that only 196 participants (25.96%) had completed secondary or higher education.
Sociodemographic characteristics of patients included in the SALTO study.
| Variable | Statistic | Detail | Parenteral administration | Oral administration | Value of P |
| Age (years) | Mean (SD) | 69.29 (10.36) | 68.82 (10.68) | .5350 (W) | |
| Age groups | N (%) | <65 years | 104 (24.36) | 88 (26.83) | .4611 (J) |
| 65–70 years | 108 (25.29) | 67 (20.43) | |||
| 70–75 years | 95 (22.25) | 78 (23.78) | |||
| >75 years | 120 (28.10) | 95 (28.96) | |||
| Gender | N (%) | Male | 140 (32.48) | 124 (38.15) | .1065 (F) |
| Female | 291 (67.52) | 201 (61.85) | |||
| Cohabitation | N (%) | Other | 6 (1.40) | 5 (1.42) | .6197 (J) |
| Lives with a relative | 102 (23.72) | 94 (26.78) | |||
| Lives with partner/spouse | 253 (58.84) | 190 (54.13) | |||
| Lives alone | 69 (16.05) | 62 (17.66) | |||
| Level of education | N (%) | Completed primary education | 197 (47.13) | 139 (40.29) | .2977 (J) |
| Completed secondary education | 68 (16.27) | 61 (17.68) | |||
| Completed higher education | 35 (8.37) | 32 (9.28) | |||
| No studies | 118 (28.23) | 113 (32.75) |
CI, confidence interval; F, Fisher exact test; J, Chi-square test; N, number of patients; SD, standard deviation; W, Wilcoxon–Mann–Whitney test.
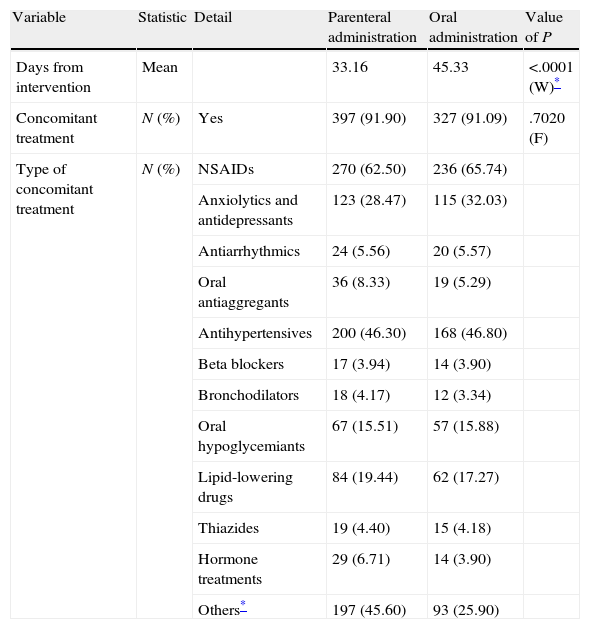
Neither surgical practice nor the type of concomitant treatment received showed statistically significant relevant differences between the first and second phases of the SALTO study, as shown in Table 2 regarding clinical features.
Clinical characteristics of patients included in the SALTO study.
| Variable | Statistic | Detail | Parenteral administration | Oral administration | Value of P |
| Days from intervention | Mean | 33.16 | 45.33 | <.0001 (W)* | |
| Concomitant treatment | N (%) | Yes | 397 (91.90) | 327 (91.09) | .7020 (F) |
| Type of concomitant treatment | N (%) | NSAIDs | 270 (62.50) | 236 (65.74) | |
| Anxiolytics and antidepressants | 123 (28.47) | 115 (32.03) | |||
| Antiarrhythmics | 24 (5.56) | 20 (5.57) | |||
| Oral antiaggregants | 36 (8.33) | 19 (5.29) | |||
| Antihypertensives | 200 (46.30) | 168 (46.80) | |||
| Beta blockers | 17 (3.94) | 14 (3.90) | |||
| Bronchodilators | 18 (4.17) | 12 (3.34) | |||
| Oral hypoglycemiants | 67 (15.51) | 57 (15.88) | |||
| Lipid-lowering drugs | 84 (19.44) | 62 (17.27) | |||
| Thiazides | 19 (4.40) | 15 (4.18) | |||
| Hormone treatments | 29 (6.71) | 14 (3.90) | |||
| Others* | 197 (45.60) | 93 (25.90) |
CI, confidence interval; F, Fisher exact test; N, number of patients; NSAIDS, non-steroidal anti-inflammatory drugs; W, Wilcoxon–Mann–Whitney test.
Among valid patients, 62.50% in phase I and 65.74% in phase II were receiving concomitant therapy with NSAIDs, 46.30% and 46.80%, respectively, with antihypertensives and 28.47% and 32.03%, respectively, with anxiolytics and/or antidepressants. Only the group of “others”, which mainly included gastrointestinal and analgesic treatments, showed a significant difference between both phases (P<.0001) (Table 2).
Adherence dataAdherence to antithrombotic prophylaxis was analysed by studying 3 variables: treatment dropout, completion and satisfaction.
The results showed statistically significant differences in treatment dropout depending on the administration method of antithrombotic prophylaxis (P=.0131). Thus, while 9.49% of patients discontinued therapy when this was administered parenterally, only 4.14% did so when it was administered orally, and past the first week of outpatient treatment.
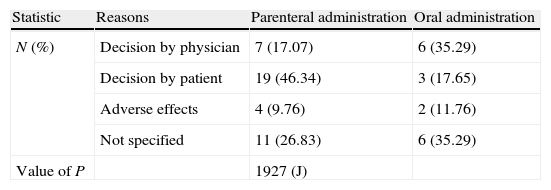
It is noteworthy that, in the case of parenteral administration, the main reason for abandoning treatment was a decision by the patient (46.34% of parenteral cases vs. 17.65% of oral cases), while for oral administration it was mainly a decision by the physician (primary care physician) (35.29% of oral cases vs. 17.07% of parenteral cases), with no statistically significant differences between hip and knee surgeries (Table 3).
Reasons for abandoning treatment among patients included in the SALTO study.
| Statistic | Reasons | Parenteral administration | Oral administration |
| N (%) | Decision by physician | 7 (17.07) | 6 (35.29) |
| Decision by patient | 19 (46.34) | 3 (17.65) | |
| Adverse effects | 4 (9.76) | 2 (11.76) | |
| Not specified | 11 (26.83) | 6 (35.29) | |
| Value of P | 1927 (J) |
J, Chi-square test; N, number of patients.
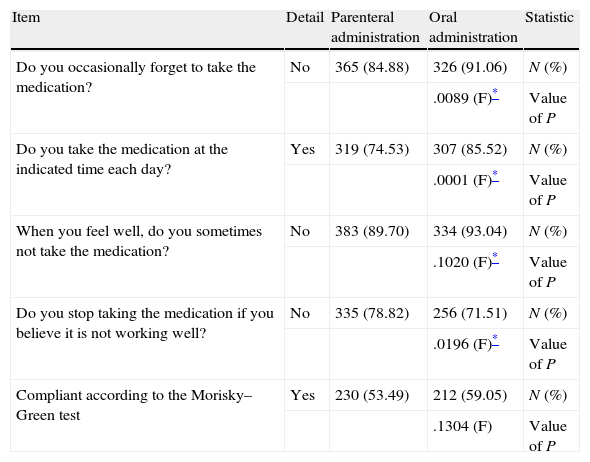
Regarding treatment compliance by type of intervention, no statistically significant differences were observed according to the Morisky–Green test (Table 4). However, it was observed that the compliant percentage was higher when antithrombotic prophylaxis was administered orally (59.05% vs. 53.49%).
Morisky–Green test to evaluate treatment compliance by patients included in the SALTO study.
| Item | Detail | Parenteral administration | Oral administration | Statistic |
| Do you occasionally forget to take the medication? | No | 365 (84.88) | 326 (91.06) | N (%) |
| .0089 (F)* | Value of P | |||
| Do you take the medication at the indicated time each day? | Yes | 319 (74.53) | 307 (85.52) | N (%) |
| .0001 (F)* | Value of P | |||
| When you feel well, do you sometimes not take the medication? | No | 383 (89.70) | 334 (93.04) | N (%) |
| .1020 (F)* | Value of P | |||
| Do you stop taking the medication if you believe it is not working well? | No | 335 (78.82) | 256 (71.51) | N (%) |
| .0196 (F)* | Value of P | |||
| Compliant according to the Morisky–Green test | Yes | 230 (53.49) | 212 (59.05) | N (%) |
| .1304 (F) | Value of P |
F, Fisher exact test; N, number of patients.
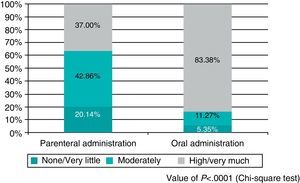
Finally, after analysing patient satisfaction, it was noted that the set of questions about general satisfaction revealed statistically significant differences in satisfaction with the antithrombotic treatment based on the route of administration. Specifically, satisfaction was higher among orally treated patients (83.38% high satisfaction/very satisfied with oral administration vs. 37.00% with the parenteral method; P<.0001) (Fig. 1). Similarly, the question of whether patients would prefer to maintain the same medication in case they required it was responded in the affirmative by 83.09% of patients treated orally vs. 38.18% of patients treated by injections (P<.0001).
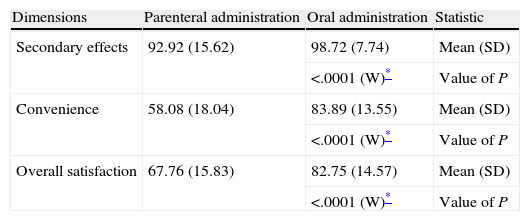
Furthermore, the score of the 3 dimensions evaluated by the simplified TSQM satisfaction scale (side effects, convenience and overall satisfaction) was higher among patients receiving oral antithrombotic prophylaxis (Table 5), with a statistically significant difference.
TSQM satisfaction scale of the patients included in the SALTO study.
| Dimensions | Parenteral administration | Oral administration | Statistic |
| Secondary effects | 92.92 (15.62) | 98.72 (7.74) | Mean (SD) |
| <.0001 (W)* | Value of P | ||
| Convenience | 58.08 (18.04) | 83.89 (13.55) | Mean (SD) |
| <.0001 (W)* | Value of P | ||
| Overall satisfaction | 67.76 (15.83) | 82.75 (14.57) | Mean (SD) |
| <.0001 (W)* | Value of P |
N, number of patients; SD, standard deviation; TSQM, Treatment Satisfaction Questionnaire for Medication; W, Wilcoxon–Mann–Whitney test.
Regarding the simplified ITSQ questionnaire completed by patients after the first phase of the SALTO study, we should note that there were no significant differences in the preparation of medication, pain or physical discomfort and emotional stress or anxiety due to the application of medication among patients undergoing surgery for knee replacement and hip replacement.
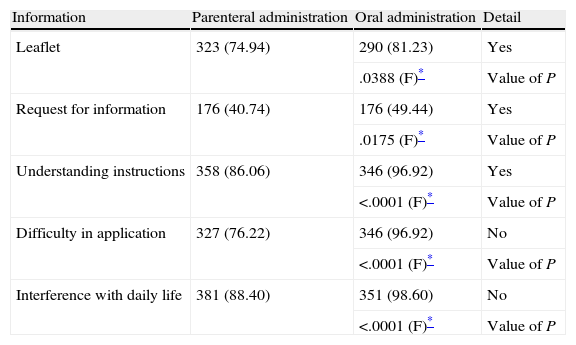
Informative dataFinally, regarding the secondary objectives of the SALTO study, namely information received about the treatment and its mode of administration, those patients who followed oral treatment received and/or requested more information than patients treated parenterally, understood instructions in a greater percentage, had less difficulty in applying the treatment and were inconvenienced to a lesser extent (Table 6), in a statistically significant proportion.
Information on treatment and its type of administration among patients included in the SALTO study.
| Information | Parenteral administration | Oral administration | Detail |
| Leaflet | 323 (74.94) | 290 (81.23) | Yes |
| .0388 (F)* | Value of P | ||
| Request for information | 176 (40.74) | 176 (49.44) | Yes |
| .0175 (F)* | Value of P | ||
| Understanding instructions | 358 (86.06) | 346 (96.92) | Yes |
| <.0001 (F)* | Value of P | ||
| Difficulty in application | 327 (76.22) | 346 (96.92) | No |
| <.0001 (F)* | Value of P | ||
| Interference with daily life | 381 (88.40) | 351 (98.60) | No |
| <.0001 (F)* | Value of P |
F, Fisher exact test.
Patients for whom no data was available have been excluded.
The SALTO study shows that, in practice, everyday outpatient thromboprophylaxis, both injectable and oral, is not performed optimally. In recent decades, LMWHs have become predominant in our environment. However, there are scarce data on adequate compliance with injectable prophylaxis once patients leave the hospital.16 Our study clearly reflects that patients have difficulties to administer subcutaneous injections correctly and that the treatment causes high patient dissatisfaction. Furthermore, prophylaxis with new oral anticoagulants obtained high levels of satisfaction, but little improvement in adherence to the treatment.
We recognise limitations in the comparisons obtained in this study, inherent to the non-randomised design which only allows a direct comparison between the 2 types of prophylaxis with a level of evidence IV. However, both groups were homogeneous and there were a considerable number of participating centres, so we believe that the study provides a very close approximation to the behaviour of our patients under routine clinical practice conditions.
Adherence results obtained solely on the basis of patient responses could be questioned. However, some studies suggest that the questionnaires employed are useful to assess adherence, although they tend to overestimate it in absolute terms.17,18 This means that, while our study showed a compliance of 53–59%, the actual figure could be significantly lower.
In the first phase of the SALTO study we noted how patient satisfaction was only moderate in 42.8% of patients. Complaints about pain of injection (47%) and the resulting haematoma in the injection area (76%), probably due to an incorrect method of drug administration, were considerable. This highlighted the importance of proper training for patients and families on how to administer subcutaneous injections. Along with forgetfulness (45.5%), adverse effects were the main reason for leaving the treatment (12%). Although it was not among our goals to establish a cost-benefit assessment for each type of prophylaxis, it should be noted that only 35% reported self-administering the injectable treatment, in 46% of cases it was the responsibility of a family member and 19% of patients required the aid of a healthcare worker.
In the second phase of the SALTO study, 83.38% of patients reported being very or extremely satisfied with oral antithrombotic medication. The main reason for abandoning the treatment was a decision by the physician (35.29% of cases).
The difference in the time from surgery until the visit observed between both phases (33.16 days in phase I vs. 45.33 days in phase II; P<.0001) could be due to the fact that patients forgot or evaluated the clinical situation experienced in a different manner, thus influencing the results of the satisfaction questionnaire.
The fact that most thromboembolic episodes are asymptomatic does not allow us to calculate the extent to which lack of adherence to prophylaxis or its early discontinuation resulted in a lessened clinical prevention of thromboembolic events. However, we do know from some clinical trials that symptomatic episodes occur after about 3 weeks in knee arthroplasties and after between 12 and 34 days in hip arthroplasties,19 so it is clear that this issue deserves our attention.
Since neither the sociodemographic characteristics nor surgical practice nor the type of concomitant treatment received showed relevant, statistically significant differences among patients in the first and second phases of the SALTO study, it seems logical to assume that differences in satisfaction were related to the type of antithrombotic prophylaxis received. Indeed, the results obtained support this hypothesis, since statistically significant differences were observed in abandonment (9.49% in phase I vs. 4.14% in phase II), overall satisfaction (37.00% vs. 83.38%) and the TSQM satisfaction scale (higher with oral administration than with parenteral).
However, it should be noted that both adherence and satisfaction may be affected by the information on prophylaxis provided by physicians and/or nurses during hospital stay.
In conclusion, our study suggests that, in general, there is an incomplete adherence to outpatient thromboprophylaxis. Therefore, regardless of the type of prophylaxis prescribed, it is very important to specify the duration of treatment after hospital discharge in order to prevent premature discontinuations, to improve information for patients regarding the importance of thromboprophylaxis and to implement strategies which avoid forgetfulness in dose administration insofar as possible.
Conflict of interestThe authors have no conflict of interest to declare in the present work.
The authors wish to thank Adelphi and Dr. Iolanda Miró i Vinaixa, for their help in preparing the manuscript and their editorial support, as well as Bayer Hispania SL.
Please cite this article as: Peidro-Garcés L, et al. Adherencia y satisfacción en la profilaxis antitrombótica ambulatoria oral frente a la parenteral: estudio SALTO. Rev Esp Cir Ortop Traumatol. 2013;57:53–60.