The Airway Management section of the Spanish Society of Anesthesiology, Resuscitation, and Pain Therapy (SEDAR), the Spanish Society of Emergency Medicine (SEMES), and the Spanish Society of Otorhinolaryngology and Head and Neck Surgery (SEORL-CCC) present the Guide for the comprehensive management of difficult airway in adult patients. Its principles are focused on the human factor, cognitive processes for decision-making in critical situations, and optimization in the progression of strategies application to preserve adequate alveolar oxygenation in order to enhance safety and the quality of care. The document provides evidence-based recommendations, theoretical-educational tools, and implementation tools, mainly cognitive aids, applicable to airway management in the fields of anesthesiology, critical care, emergencies, and prehospital medicine. For this purpose, an extensive literature search was conducted following PRISMA-R guidelines and was analyzed using the GRADE methodology. Recommendations were formulated according to the GRADE methodology. Recommendations for sections with low-quality evidence were based on expert opinion through consensus reached via a Delphi questionnaire.
La sección de Vía Aérea de la Sociedad Española De Anestesiología, Reanimación y Terapéutica del Dolor (SEDAR), la Sociedad Española de Medicina de Urgencias y Emergencias (SEMES) y la Sociedad Española de Otorrinolaringología y Cirugía de Cabeza y Cuello (SEORL-CCC) presentan la Guía para el manejo integral de la vía aérea difícil en el paciente adulto. Sus principios están focalizados en el factor humano, los procesos cognitivos para la toma de decisiones en situaciones críticas y la optimización en la progresión de la aplicación de estrategias para preservar una adecuada oxigenación alveolar con el objeto de mejorar la seguridad y la calidad asistencial. El documento proporciona recomendaciones basadas en la evidencia científica actual, herramientas teórico-educativas y herramientas de implementación, fundamentalmente ayudas cognitivas, aplicables al tratamiento de la vía aérea en el campo de la anestesiología, cuidados críticos, urgencias y medicina prehospitalaria. Para ello se realizó una amplia búsqueda bibliográfica según las directrices PRISMA-R y se analizó utilizando la metodología GRADE. Las recomendaciones se formularon de acuerdo con la metodología GRADE. Las recomendaciones de aquellas secciones con evidencia de baja calidad se basaron en la opinión de expertos mediante consenso alcanzado a través de un cuestionario Delphi.
Airway management (AWM) is a cornerstone of multiple care procedures in medicine.1 Recent series indicate that the incidence of difficult airway (DAW) and failed airway has decreased to 1.6 and .06 per 1000 cases, respectively,2 although it continues to be an important cause of morbidity and mortality.3,4
A large proportion of complications derived from clinical care are avoidable.5 Analysis of incidents in national registries, as well as medical/legal data, plays a relevant role in detecting failures in clinical practice and implementing new strategies to alleviate them.5–7 Spain does not have a registry of adverse events associated with AWM. However, global data could be extrapolated to our area of influence. Of particular importance is the 4th National Audit Project (NAP4), of the United Kingdom.8,9 The 168 recommendations it contains made it possible to optimise safety.10 Since then, societies from different specialties have developed new guides, algorithms11–16 and cognitive aids,17 to provide updated strategies. Despite this, almost a decade later, many of the deficiencies detected persist,4,18 as evidenced by recent studies that provide practically superimposable data.3,7,19–21 All have reported recurring errors: inadequate assessment and planning, lack of anticipation of a DAW, insufficient preparation and availability of specific equipment, perseverance in a failed strategy, omission of the use of a supraglottic airway device (SAD) in the face of difficulty in ventilation, and no timely progression to surgical airway.5,18 Thus, human factors (HF) and ergonomics play a key contributing role.22,23 These findings support the importance of anticipating, preparing and following the guidelines, and emphasize the need to redouble efforts and continue implementing improvements.4,24
The strategies for addressing DAW are conditioned by the environment, technology and the experience of the professionals involved. The implementation of guidelines adapted to the national and institutional healthcare environments4,18,25 is therefore recommended, as indicated in the Declaration of Helsinki on patient safety in anaesthesiology.26 Current decision-making tools are not entirely satisfactory since they omit the influence of HF and contextual specificity, giving rise to interventions that may be ineffective and lead to errors.23,27 Most algorithms invariably assume tracheal intubation (TI) as the initial objective.28 Their designs are more effective for education and training in a theoretical context than for their execution in real dynamic and stressful clinical situations.23,24,29,30 Some studies have even shown a negative effect on decision-making.31,32 Likewise, they have irregular implementation and generally limited adherence.18,30,33 The reason for these findings has been attributed to their complex and inflexible designs, sometimes being perceived as a barrier to workflow rather than as help in emerging situations.34 Therefore, effective cognitive aids are needed to simplify the transition from one technique to another,35 providing continuity to airway management.
The objective of this document is to provide professionals with a set of evidence-based recommendations, as well as rational and implementation tools for decision-making in the management of DAW.
ObjectivesProvide recommendations from the Spanish Society of Anaesthesiology, Resuscitation and Pain Therapy (SEDAR), the Spanish Society of Emergency and Emergency Medicine (SEMES) and Spanish Society of Otolaryngology, Head and Neck Surgery (SEORL-CCC) based on the scientific evidence for the comprehensive management of DAW in adult patients.
Provide rational and implementation tools, fundamentally cognitive aids with a design based on HF and ergonomics, specific context, focused on cognitive processes in critical situations related to DAW. They can facilitate decision-making and optimise the progression in the application of strategies to preserve adequate oxygenation throughout the procedure and reduce the incidence of complications, thus contributing to the improvement of safety and quality of care.
The assumptions described should in no case be considered mandatory standards and, given the contextual diversity and complexity, their application does not guarantee success in any situation. The recommendations are flexible in nature, with the good clinical judgment of the professional after the pertinent analysis of the risk-benefit balance in each specific case always prevailing.
Validity and applicabilityThe contents of this guide are general recommendations based on current evidence. They could therefore be applicable to any circumstance and procedure that requires airway control, whether that be face mask ventilation (FMV), supraglottic airway device ventilation (SADV) or TI, and by any professional responsible for AW management.
Given the constant increase in knowledge and technological development, these recommendations will be subject to periodic review subsequent to publication.
MethodologyThe development process of this guide adhered to the Appraisal of Guidelines, Research and Evaluation II (AGREE II) directives.36 To ensure evidence-based support of the recommendations, a rapid systematic review was performed following the PRISMA Rapid reviews (PRISMA-R) recommendations.
A steering committee comprising 27 airway experts selected the sections to be treated and formed the "Spanish Airway Management Group", a group formed by physicians from all over Spain, members of SEDAR, SEMES and SEORL-CCC with teaching and research experience in the matter and whose care activities include anaesthesia, critical care and hospital emergencies.
The bibliographic search was carried out in MEDLINE, Embase, Scopus, Web ofScience, PubMed, ScienceCitationIndex and The Cochrane Library in the period between June 1, 2000 and December 1, 2022. The keywords used were “airway”, “airway management", "difficult airway", "tracheal intubation", "guideline", "algorithm", "cognitive aid", "checklist", "awake tracheal intubation", "fiberoptic intubation", "videolaryngoscopy", "supraglottic airway", "face mask", "oxygenation", "preoxygenation", "apneic oxygenation", "ventilation failure", "rapid sequence induction", "can't intubate can't ventilate", "airway complications", "emergency airway", "front of neck access", "cricothyrotomy", "extubation", "teaching", "training" and "competence". The search was limited to literature published in English and Spanish in the last 22 years, and focused exclusively on adult patients. Search terms were used individually and in combination. Randomised controlled clinical trials, case series, surveys, review articles and editorials were included.
Analysis of the literature and recommendations was performed in accordance with the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology.37 One reviewer (MAGR) performed pre-screening of duplicated titles and abstracts using Rayyan software, followed by a full text review by 3 reviewers (JAS, TL and AAG) independently, documenting reasons for exclusion. The literature cited within the identified articles was considered, as well as subsequent relevant publications. The different studies were incorporated into a summary table of findings with an assessment of the quality of the evidence for each result.37 The data obtained were edited and synthesised for the formulation of recommendations and level of evidence.
The recommendations were formulated and classified according to the GRADE system. Recommendations and justifications were initially drafted and critically reviewed by 4 authors. They were subsequently reviewed by the committee before the consensus process. The authors participated in virtual consensus conferences in February and March 2023, during which the formulation and classification of each recommendation was confirmed.
The sections with low quality evidence or practically non-existent literature were used for the preparation of a Delphi questionnaire (Appendix A supplementary material) from which a statement of experts was extracted on those issues in which sufficient consensus was reached.
The final text was sent to all group members and external consultants for review. Their enriching insights were incorporated into the document.
The entire process was entirely independent of the industry and any type of funding.
Appendix A Supplementary material 1 shows the GRADE Evidence scales.
DefinitionsCurrent literature does not provide standard definitions in DAW as there is no universal consensus in this regard.38,39 The use of clear, concise and precise terminology is key to improving team situational awareness and communication, the development of cognitive processes and a shared mental model. This allows for the generation of coordinated actions, adequate progression in an algorithm of strategies, avoidance of errors, and standardisation of criteria for research and documentation in the airway field.38,40–42 Appendix A Supplementary material 2 includes risk factors for the different entities.
Difficult airwayClinical situation in which an operator with conventional training has difficulty performing FMV, SADV, or performing TI, which may result in inadequate alveolar oxygenation.
Difficult face mask ventilation (DFMV) or difficult supraglottic airway device ventilation (DSADV)Situation in which adequate ventilation cannot be provided despite having established an intense neuromuscular blockade (NMB) with the presence of one or more of the following problems: absence of exhaled carbon dioxide or absence of phases II and/or Capnography wave III, decreased oxygen saturation or inadequate saturation, absence or inadequacy of spirometric measurements of expired gas flow, improper sealing, excessive leakage or resistance during gas entry or exit. Signs of inadequate ventilation include (but are not limited to): absence or inadequate movement of the chest, absence or inadequate auscultation of breath sounds, signs of severe obstruction, cyanosis, gastric dilation, and haemodynamic changes associated with hypoxaemia and hypercapnia. (e.g., hypertension, tachycardia, arrhythmias).
Difficult laryngoscopyDue to the wide diffusion of videolaryngoscopy it is appropriate to differentiate between43,44:
Difficult direct or conventional laryngoscopySituation in which it is not possible to visualise the glottic structures with the best possible laryngoscopic exposure and with optimal conditions (patient position, adequate blade, complete NMB, external laryngeal manipulation or BURP), and is defined by a Cormack-Lehane (C-L) grade 3 or 4.
Difficult indirect videolaryngoscopy or laryngoscopySituation in which through videolaryngoscopy it is not possible to obtain any percentage of glottic visualization with the best possible videolaryngoscopic exposure and with optimal conditions (patient position, adequate blade, complete NMB, external laryngeal manipulation or BURP), and is defined by a Percentage Of Glottis Opening (POGO) at 0%, equivalent to a C–L grades 3 or 4 with direct laryngoscopy (DL).45
Difficult tracheal intubationThat which requires multiple attempts, additional operator (s), devices and/or adjuvant techniques or manoeuvres to advance the tube at the endotracheal level.
To quantify and document the difficulty, the Intubation Difficulty Scale (IDS) proposed by Adnet et al.,46 or the Fremantle45,47 can be considered as a scoring system, which includes the degree of laryngeal vision, the ease of passage of the endotracheal tube (ETT), the type of device used and any adjuvant.
Failed tracheal intubationInability to advance a tube at the endotracheal level despite several attempts, with one or more devices and adjuvants.
Can’t-intubate-can’t oxygenate situation (CICO)Impossibility of achieving alveolar oxygenation through non-invasive oxygenation methods (TI, FMV or SADV) given the impossibility of keeping the upper airway patent. Restoration of alveolar oxygenation requires front-of-neck access (FONA) to the airway.
Difficult front-of-neck access (DFONA)Difficulty in identifying cervical spine anatomical structures (cricothyroid membrane, CTM) or achieving an FONA to the airway.
Difficult airway to access and controlClinical situation in which a trained operator is not able to perform FMV, SADV or TI due to a complex interaction between patient, pathology, environment, operator, equipment, experience and circumstances.
Failed attemptAttempt within a specific AW management plan that is unsuccessful.
Failed planThat which does not achieve success after three attempts.
Difficult tracheal extubationExtraction of the ETT of a patient with known or anticipated DAW.
Failed tracheal extubationLoss of AW patency and adequate ventilation after ETT removal.
Reduced tolerance to the apnoea periodPathophysiological state, usually caused by shunt, ventilation/perfusion mismatch or reduced functional residual capacity (FRC), which determines the presence of hypoxaemia, little or no effectiveness of peri-procedural oxygenation techniques and/or a safe apnoea time (period from cessation of ventilation until shorted arterial oxyhaemoglobin saturation ≤ 90%).
Human and ergonomic factorsThe clinical environment is a complex and dynamic socio-technical system, where multiple factors interact, resulting in variability in operational processes and, consequently, in their performance.23 HF refer to individual, group, environmental and organisational factors that affect both decision-making and the general performance of the system.34 The discipline that studies them tries to understand their influence to optimise interactions between humans and other elements of the system so as to increase the human contribution to success (efficiency), and also limit its errors (safety).48
The role of HF in the occurrence of adverse events in AM is as important as the technical limitations.49 The NAP4 concluded that they contributed to 40% of major complications, with an average of 4 contributing human factors per case.50 Accidents usually occur due to an "error of action", such as the omission of a critical task, and are fundamentally due to a lack of awareness of the situation.50 An emergency like the CICO situation requires an immediate coordinated team response.23 However, excess information and pressure generates cognitive and sensory overload,30 as well as changes in mental and behavioural processes secondary to the stress response that nullify systematic thinking, promote cognitive biases and increase the risk of errors.22,23,51,52 The multiplicity of algorithms and their limited applicability in a crisis paralyse the workflow. Factors such as fatigue, caused, for example, by long shifts, further hinder performance.22 Thus, errors are not caused by a deficiency in individual skills, but by the very nature of cognitive processes and their articulation in challenging situations.52 Up to 93% of difficult TIs are unanticipated.53 Therefore, effective tools that do not cause greater perceptual saturation, and that facilitate complex processes such as planning, situational awareness, decision-making, team coordination or task management are required.48,54 The linear algorithmic approach to crisis does not correspond to the flexible and intuitive cognitive processes that are activated to resolve stressful dynamic situations.29,55,56
For the reasons stated, this guide provides cognitive aid applicable to any emergency situation associated with a DAW, the standardisation of the DAW trolley as an extension of this, a pre-procedure checklist, as well as ergonomic principles. Appendix A supplementary material 3 details the purpose of each element in keeping with the HF principles.
Cognitive assistanceIt is recommended to have visual cognitive aids for the management of emerging crises (expert statement [S.D.] 97.1%).
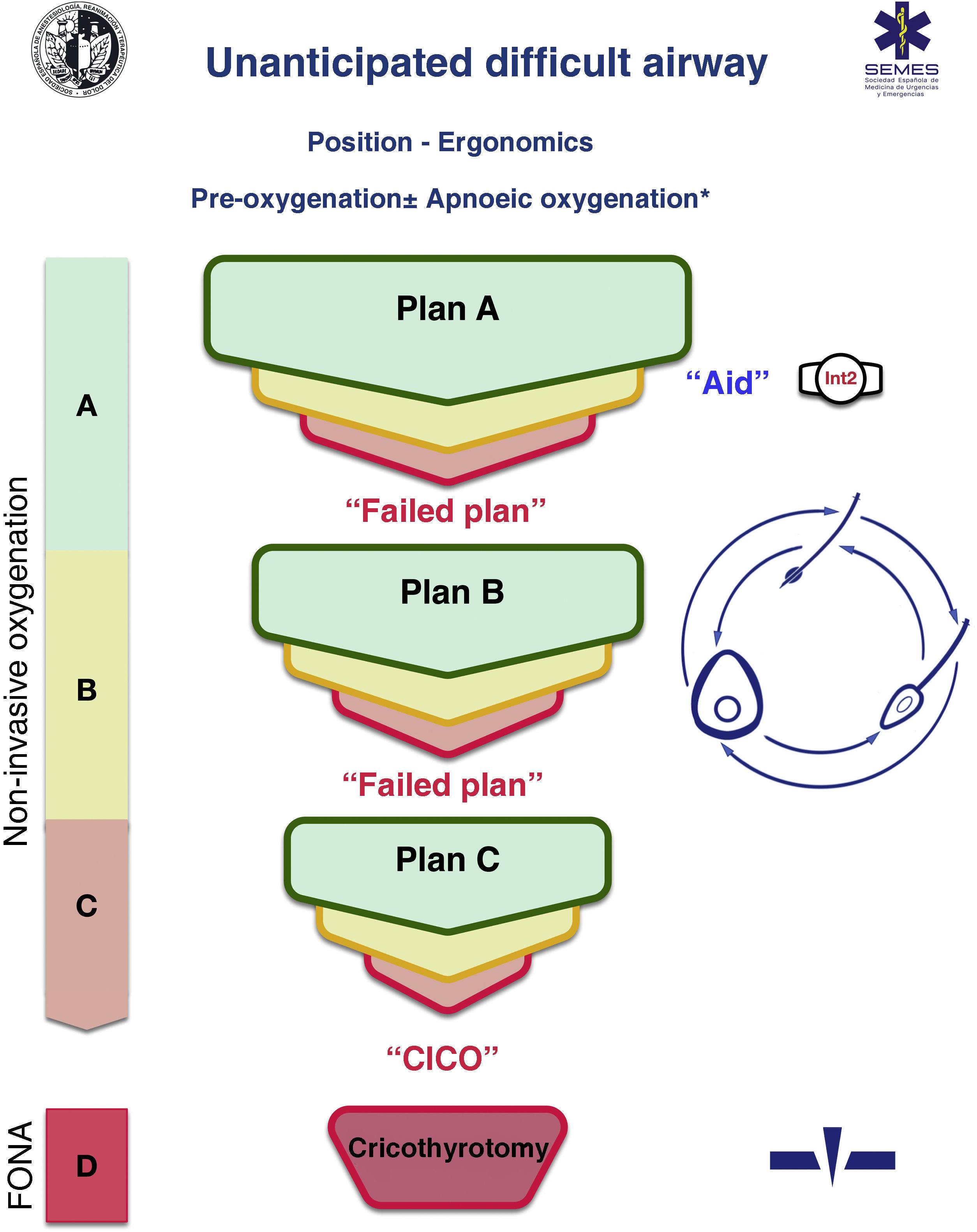
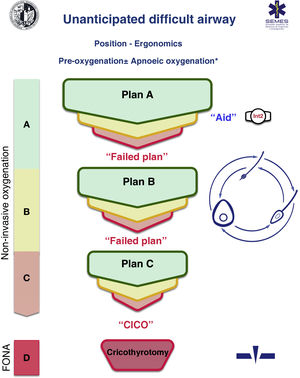
A cognitive aid is a tool aimed at improving cognitive functioning (memory, perception, attention, concentration, language) to improve executive functions such as problem solving, planning, reasoning and control.48Fig. 1 shows the aid proposed by SEDAR, SEMES and SEORL-CCC to treat an unanticipated DAW. Its main objective is to reduce the instrumentalisation of the AW using the smallest possible number of attempts. Its design is context/specific and focused on decision-making and HF to manage an emergent crisis. It consists of a simple visual representation in accordance with the available evidence of the sequence of steps to follow to ensure alveolar oxygenation of a patient with an unanticipated DAW.
Assistance is in keeping with the Vortex philosophy proposed by Chrimes17 to which is added the universal symbology of traffic light colours.
There are 4 categories of techniques to preserve or restore alveolar oxygenation. Three non-invasive: TI, FMV and SADV, and one invasive: FONA, which is necessary when the three non-invasive strategies fail.
The number of attempts of each non-invasive management plan should be limited to 3 (S.D. 88.6%). The first attempt must be carried out under optimal conditions to maximise the chances of success.57–59 Each new attempt requires the use of a new device or new methods or adjuvants that allow the previous technique to be optimised. If success is not achieved in any of them, the "Failure of the Plan" must be verbally declared and a new Plan begun. If the three non-invasive technique plans fail, the "CICO situation" must be declared without delay and an FONA performed as the last resort to safeguard alveolar oxygenation. To ensure a quick transition, it is recommended to open the FONA equipment after a first failed FMV or SADV attempt.
The approach to an AW can begin by selecting any of the 3 non-invasive oxygenation strategies. Selection of first-line technique as well as backup techniques is sensitive to context (patient condition, operator skill, availability of qualified assistance, site and available equipment, or time of day). The one selected as first-line will be called "Plan A." Failure on the first attempt requires declaring an “Unanticipated Difficult Airway” and requesting immediate help. If success is not achieved after the 3 attempts of "Plan A", "Plan B" should be executed and if unsuccessful then "Plan C", using the circular layout of non-invasive techniques, following a clockwise or counterclockwise rotation from the first-line plan. Alternation of plans without exhausting the attempts of each one is optional.
The alerts or sentinel signs that force the transition between techniques are poor or absent ventilation, time-sensitive desaturation and/or clinical signs of hypoxaemia, as well as the failure of a plan after three failed attempts.
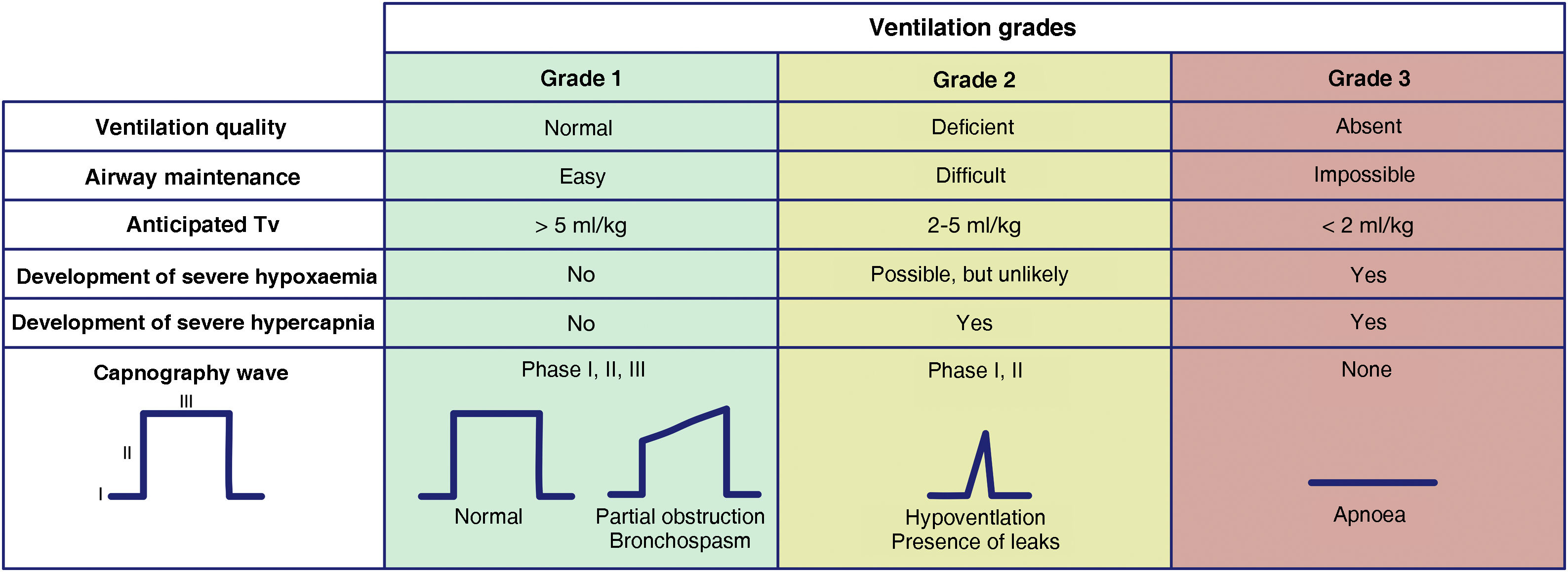
The capnography waveform is gold standard for confirming alveolar ventilation. It must be available in all AM management sites to test the success of any of the 4 plans used.60 For this purpose, we recommend the use of the classification proposed by the Japanese Society of Anaesthesiologists to evaluate the effectiveness of ventilation.61Fig. 2 shows an adaptation of it. This classification allows for a precise and almost instantaneous diagnosis of the ventilation status, for all team members to share a mental model, for a timely transition between techniques or plans, and to avoid attachment errors. Capnography wave patterns are applicable in each respiratory cycle to patients on spontaneous or mechanical ventilation through FM, SAD, ETT or infraglottic cannula and allow predicting severe hypoxaemia and hypercapnia. Grade 2 or 3 ventilation requires changing technique or starting a new, more effective plan to maintain oxygenation. SEDAR, SEMES and SEORL-CCC recommend the declaration of “absent” or “present” capnography to promote team situational awareness and generate coordinated actions.
Ventilation grades according to the capnography waveform and its clinical interpretation. Tv: tidal volume.
Clinical signs such as inspection of chest movements or auscultation can be assessed together, although they are less reliable. Tidal volume measurements can be more precise and objective, although monitoring is not available in all sites.
Changes in peripheral oxygen saturation (SpO2) provide later feedback because there is a relatively long “silent” period until desaturation.
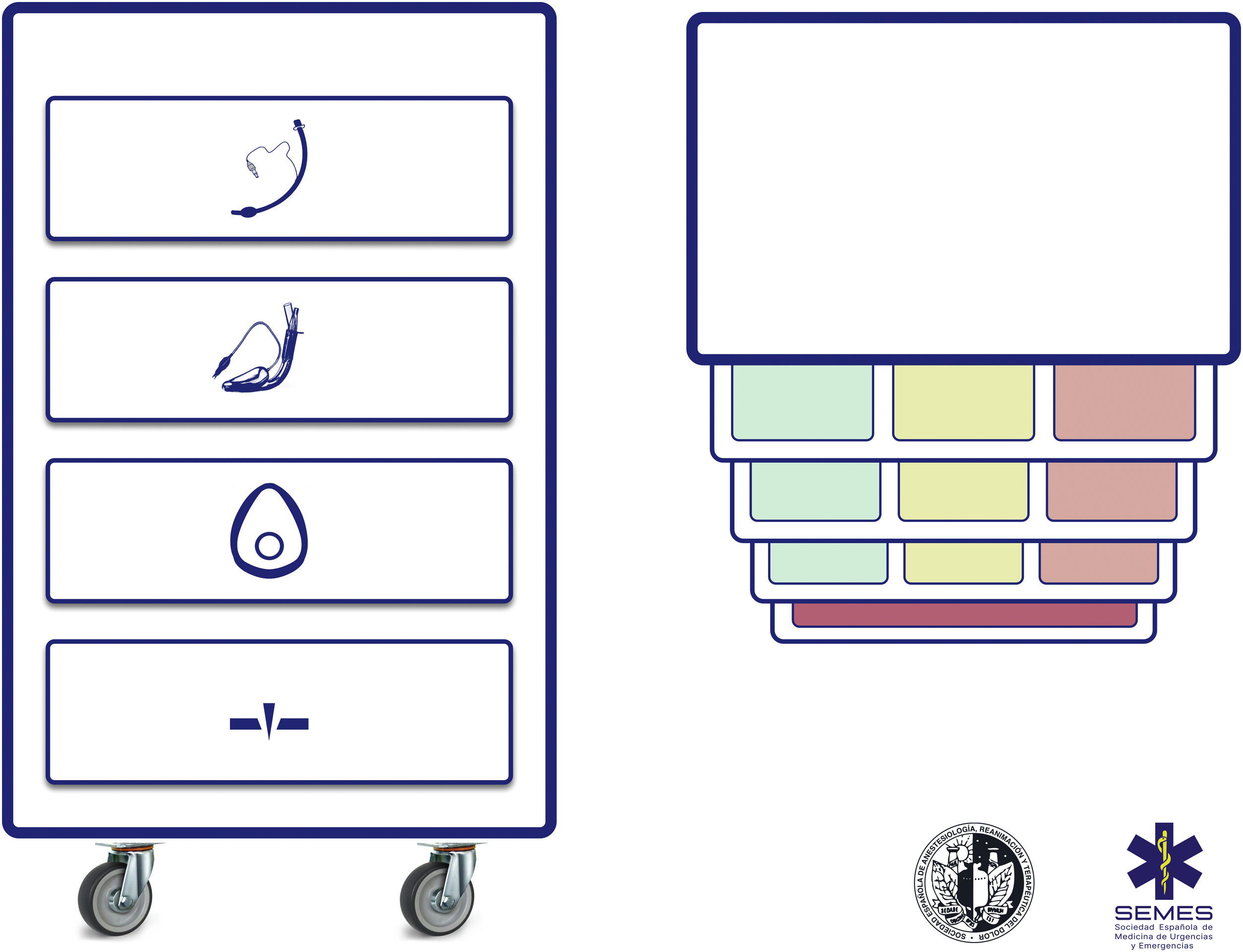
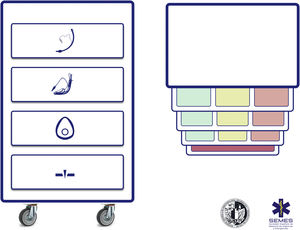
Difficult airway trolleyA standardized DAW trolley in AW management sites is recommended (S.D. 100%). Fig. 3 shows the DAW trolley proposed to complement the cognitive aid.
The NAP4 described multiple incidents caused by the absence of basic material to treat airways.8,9 The rapid availability and presentation of the devices necessary to execute the different plans is a key contextual component.62 Such devices are often included in easily transportable portable units.63 Standardisation aids adherence to algorithms, promotes situational awareness and sequential progression, thereby reducing the risk of delays in decision-making and cognitive overload.63
The layout of the proposed trolley with the integrated cognitive aid consists of 4 compartments labelled with easily recognizable pictogrammes. Each of the first three houses a category of non-invasive alveolar oxygenation techniques of the three which are possible. Each compartment, in turn, is subdivided into 3 sub-compartments (green, amber and red) intended to house the different devices and alternative techniques for each category, as well as optimisation strategies, ordered according to whether they are the first (green), second (amber) or third option (red), categorised by colour similarly to the cognitive aid. Trolleys based on integrated cognitive aids can improve efficiency in the management of DAW.64 The selection of the priority of each alternative within a category can be standardised in each institution according to existing devices. If planning to treat a specific planned DAW makes it advisable to change the order of priority of the technique within each category, the change will be made before starting the procedure, restoring the standard order after completing the case. The fourth compartment is reserved to house the AII sets to rescue a CICO situation.
This arrangement of AW material allows nurses to more effectively develop their crucial role as assistants in preparing alternative equipment when the operator is still executing the preceding option and to offer it immediately in case of failure. This allows anticipation, transition without delay between techniques and prevention of fixation on a given technique.64
Ideally, the trolley should be accessible in less than 1 min from any AW management site in the event of a possible crisis.60,62 In addition to immediate access to the equipment, it is crucial that all professionals have the necessary training in the use of each of the devices included.60,63 A minimum weekly inspection of the contents is recommended, adhering to a checklist permanently attached to the cart, and an additional one after each use.60
Pre-procedural checklistChecklists are recommended to reduce the incidence of human error, improve task execution time and reinforce the safety culture in AW management (S.D.100%).
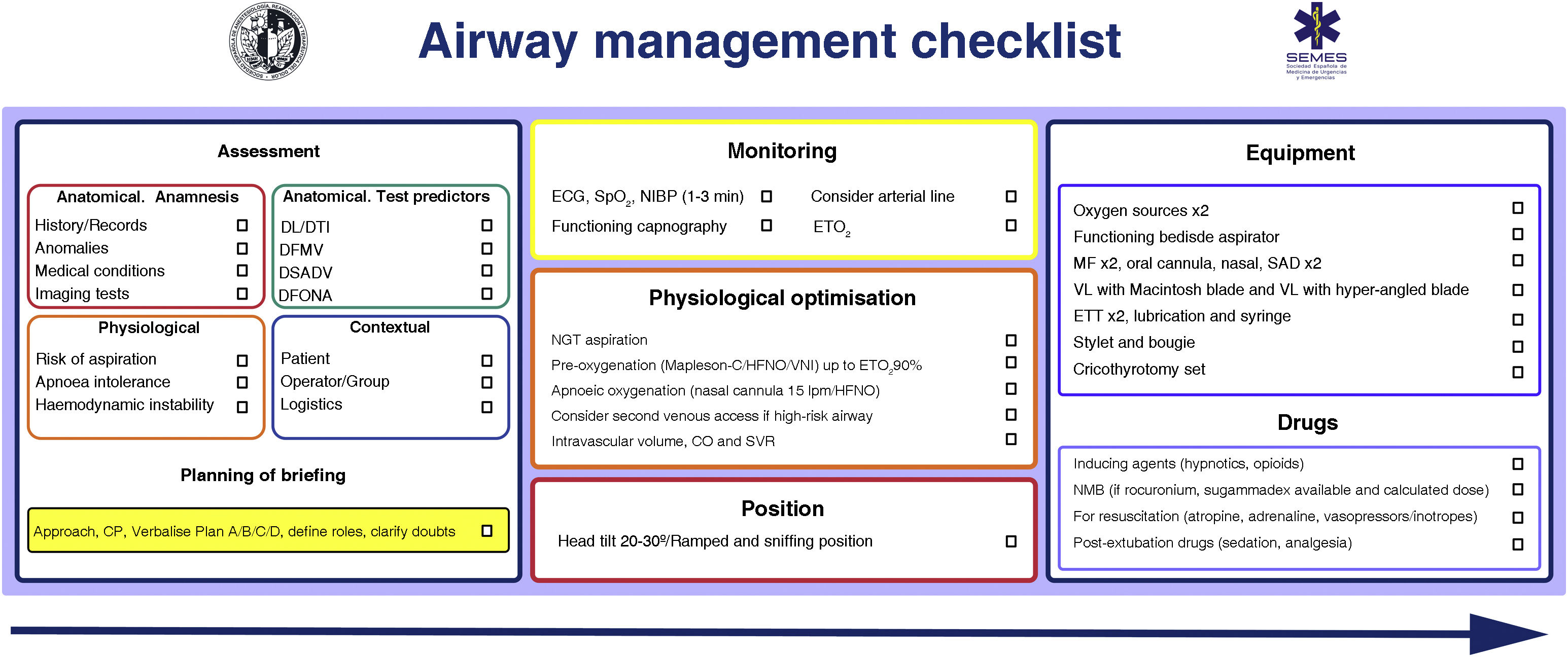
Patient safety is often a product of good communication, teamwork, and anticipation. Verification is the link between them.65,66 Checklists reduce the incidence of human error, improve the time in which tasks are performed and reinforce a culture of safety and control.29,48,67 They are especially useful in demanding, high-workload situations, where one is likely to develop "tunnel vision" (fixation errors) and omit crucial steps, in addition to giving routine, repetitive tasks poor attention which can foster lack of focus, complacency and deviations from standard protocols.65 Systematic reviews of checklists in the operating theatre demonstrate a reduction in complications and morbidity and mortality, but only when teams participate and when compliance with the elements is high68,69 Likewise, they optimise anticipation, proactive debate, teamwork and effective communication,65 mechanisms that can justify improved results.70 Although the use of a TI checklist does not seem to consistently improve some clinical outcomes,71,72 there is evidence of its association with a lower number of hypoxic events.71 More evidence is needed to define its benefit.71 Despite this, they are widely recommended for AW management73,74 as a vital cognitive tool within a comprehensive AW safety programme.65Fig. 4 shows the AW pre-management “read and do” checklist proposed by SEDAR, SEMES and SEORL-CCC.
SEDAR and SEMES airway pre-management checklist.
CP: cricoid pressure; DFONA: difficult front-of-neck access; DL: difficult laryngoscopy; DTI: difficult tracheal intubation; ECG: electrocardiogram; ETT: endotracheal tube; EtO2: end-tidal O2 concentration; FM: face mask; HFNO: high flow nasal oxygen therapy; NIBP: non-invasive blood pressure; NIV: non-invasive ventilation. NMB: neuromuscular blockade; SAD: supraglottic device; SpO2: peripheral oxygen saturation; VDDEG: difficult ventilation with supraglottic device; VDMF: difficult ventilation with face mask; VL: video laryngoscopy.
The use of ergonomic and communication models is recommended (S.D. 91.4%).
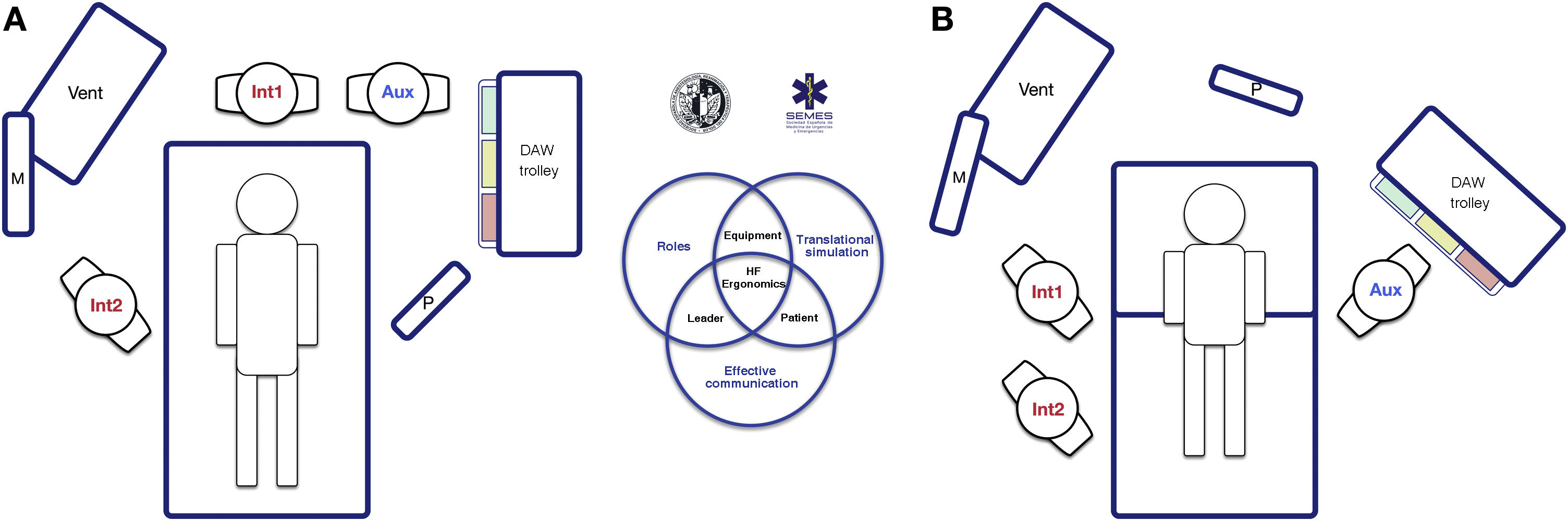
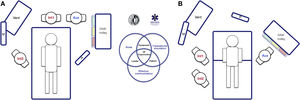
The socio-technical environment has a significant impact on the effectiveness, safety and quality of care.75 Systems with inappropriate designs have been linked to errors, inadequate care and operational loss.76 Therefore, pre-intervention planning of the space and the arrangement of human and material resources is essential to enhance situational awareness, range of movement and rapid response.77Fig. 5 presents 2 ergonomic options to treat DAW that optimise these aspects.
Ergonomics of hospital tracheal intubation (A) in an unplanned DAW after anaesthetic induction (supine position) and (B) in a known or anticipated DAW in an awake patient (sitting position). In a routine intubation, two roles are usually established, operator (Int1) and assistant (Aux). Both must be standing in a line for effective communication and collaboration, and with the screen (P) of the devices used, the patient monitoring (M) and the respirator (Vent). In the case of an unanticipated DAW (A), help should be requested immediately. It is recommended that a DAW expert assumes the role of second operator (Int2), and to bring the DAW trolley closer to the assistant so that they can provide the necessary devices to the operator. The role of leader can be interchangeable between them both. In the case of a planned or known DAW, the awake patient will preferably be placed in a sitting position (benefit of the gravity effect on AW) with a second operator already present at the beginning. Panel B shows the suggested layout for an FOB-guided TI. The early assignment of team roles improves attention and effective communication between members, which allows optimising the results of the intervention. The subsequent debriefing and analysis of the case will allow the application of simulation concepts that will improve the subsequent care provided by the team.
Teamwork improves results and enhances a safety culture.78–80 Professionals must function as a unit through the effective articulation of individual actions to achieve a common goal.81 The figure of the leader is key in uniting the elements.25,80 To do this, the team must be previously informed about what is anticipated to happen and the selected plans. Roles should be assigned to simplify the workflow, and the entire procedure should be clearly and explicitly directed with the creation of shared models in mind.22,80,82 Effective and dynamic communication is essential.22,83 It should be based on clarity, brevity and empathy, reinforce non-verbal communication84 and allow participation and feedback,80,85 avoiding noise and unnecessary information since, otherwise, it causes distraction and errors.22,86
A critical event must be treated by a qualified operator who is expert in handling these situations, not necessarily the most senior specialist, but one with extensive knowledge of a certain advanced procedure. They must be notified as far in advance as possible, and always after the first failure of the first-line plan. Upon arrival, and after being briefly informed of the situation and the plans executed, they must be decisive in avoiding delays.
Availability of the equipment and its strategic location is one of the main facilitators for success.34 Devices with a screen allow the evolution of the procedure to be shared with the entire team, which is why they are recommended to facilitate coordinated work and provide targeted support in anticipation of the operator's needs.87
Ergonomics are highly context sensitive. The COVID-19 pandemic demonstrated the importance of teamwork, communication and adaptation of guidelines in the face of the emergence of new barriers88–90 such as personal protective equipment (PPE) or “intubation boxes”91,92
The ARACHNID mnemonic tool simplifies all the components of ergonomics (Algorithm, Resilience—adaptation and prevention of critical incidents—, Cognitive aids, Checklist, Technical tools, Non-technical aids, Incident communication and Operating room design).93
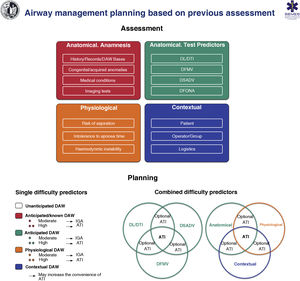
Pre-procedural assessment and planningGeneral assessmentPre-procedural assessment is recommended in all patients who require AW management (S.D. 100%).
Anticipation and planning are fundamental principles in managing a crisis. Pre-procedural AW assessment is a common clinical practice.39 Current DAW prediction tests have a limited and inconsistent diagnostic value,39,94–100 since the vast majority are aimed at predicting difficult DL exclusively,99,101 and all of them have low sensitivity and low negative predictive value, so none are suitable for detecting unanticipated DAW.39,96 The bite test has the highest sensitivity .67 (95% CI: .45–.83) to predict difficult DL, while for difficult TI it is the modified Mallampati (.51 [.40–.61])39,96,98 The combination of the Mallampati score and the thyromental distance provides the highest accuracy for predicting difficult TI.94 Most studies have focused on individual tests, unlike clinical practice in which combined tests are used.97 Multivariate models could have a greater predictive capacity (S.D. 97.1%),44,102–108 but they have been little investigated, with the Wilson test being the most analysed.98 The MACOCHA106 test, which combines anatomy, physiology and operator characteristics, is the only one validated for critical patients. However, up to 93% of difficult ITs are unanticipated53 and cause up to 17% of adverse events related to AW management.109 Despite this, routine AW assessment is recommended as a standard of care, even in emergent situations.51,96,110 Its importance lies in the fact that: 1) risk is stratified and planning is adjusted accordingly,39 with efficient transitions and the rational use of resources96,110 and 2) it promotes a culture of safety by forcing the cognitive process that requires preparation for a possible unanticipated DAW.97,99,110 AW morbidity studies indicate the dangers of omitting assessment or ignoring its findings.7–9 The lack of a documented assessment has been characterized in medical/legal cases as below the standard of care.3
Pre-procedural AW assessment should be multifactorial, structured and oriented towards the detection of anatomical, physiological and contextual DAW (S.D. 97.1%).25,97,111
If possible, creating a medical history and performing a pre-procedural physical examination is recommended.51 A complete history begins with a review of records of previous TI and the presence of factors that may alter the cervical spine or AW anatomy such as radiotherapy, surgeries or previous medical conditions.112 The diagnosis of SAHS is a predictor of difficult VMF (1C) and difficult TI (1B). A history of difficult TI is the risk factor with the greatest predictive value for a new difficult TI.98,113 The review of any imaging test (CT, MRI) is recommended. In the case of stenosis or obstruction, valuable information about their level and severity is provided.51,97,114 In the case of known or suspected glottic or supraglottic obstructive disease, preoperative examination of the AW using fibronasolaryngoscopy (FNL) or flexible videonasolaryngoscopy by an ENT specialist is especially useful for decision-making.115–117
Airway exploration can begin by detecting predictors of difficulty or failure for the first-line plan and subsequently for the 3 alternative plans (S.D. 97.1%).97,118,119 Some experts advocate evaluating a possible difficult FONA only in the case of DAW.51,97,120,121 In this case, CTM must be identified preventively by palpation120,122 and ultrasound-51,121 The latter allows cricothyrotomies to be performed with higher success rates and fewer complications.123
Ultrasound plays a promising role for the rapid detection of DAW,124 with a diagnostic accuracy of difficult TI comparable to CT and radiography, and much higher than that of the modified Mallampati test.125,126 It is particularly useful in assessing aspiration risk121,127–130 and DAW in unconscious or uncooperative patients.99
The existence of a physiologically difficult airway (PDAW)1,111,131,132 due to the presence of pathophysiological changes that increase the risk of complications during TI such as reduced tolerance to the period of apnoea, haemodynamic instability, severe metabolic acidosis or full stomach, as well as a contextual DAW due to a low degree of patient cooperation, emergency situations, limited experience and/or skills of the operator, or the absence of qualified assistance or the most indicated device, can modify the approach, so they must be taken into account in planning.1,25,97
The final result of the assessment should be the establishment of a defined plan for the AW, which should be discussed and shared with the entire team before starting the procedure. This should include preparation to treat an unanticipated DAW for all patients, even in the absence of difficulty predictors.97
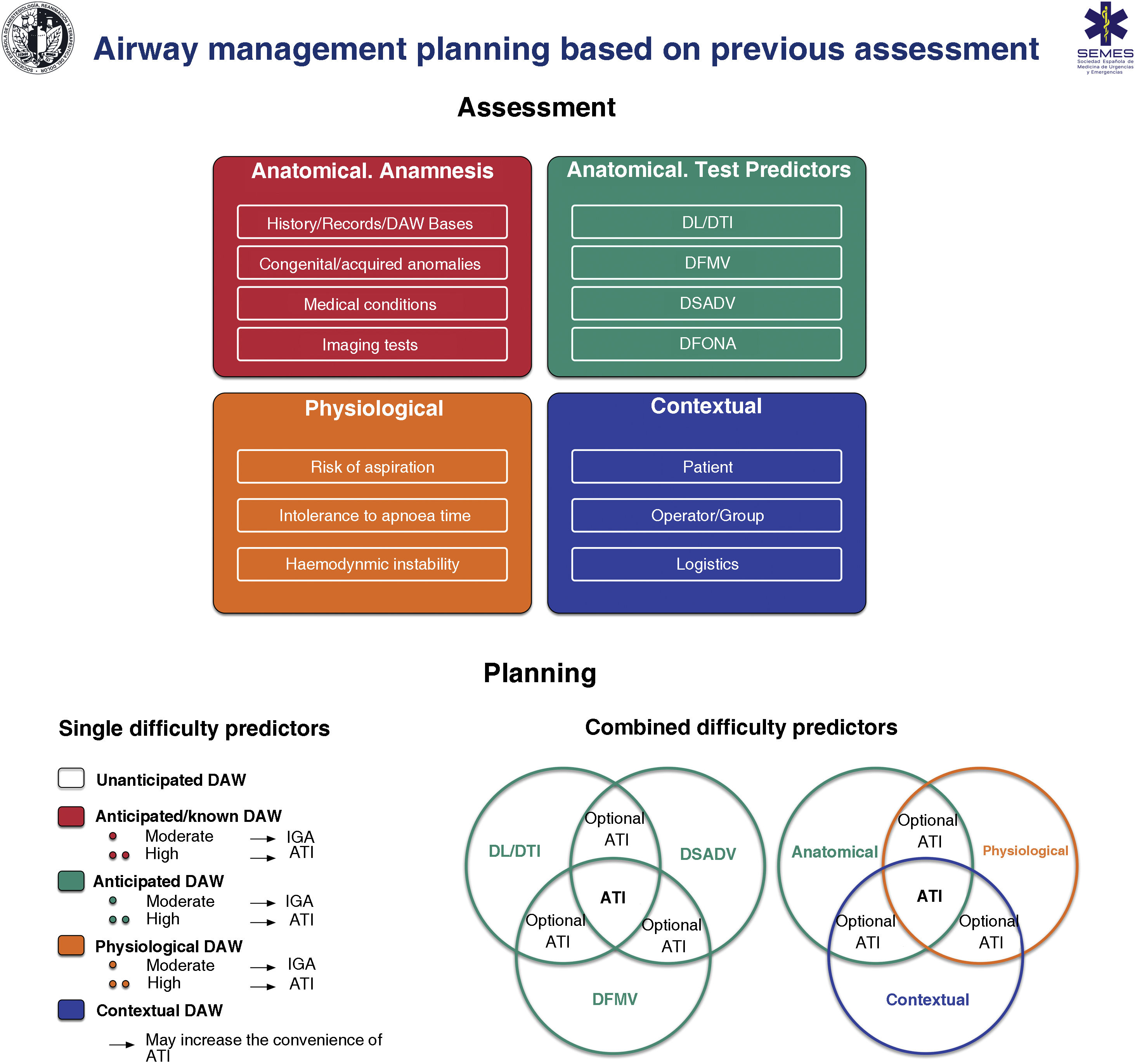
Fig. 6 shows an implementation tool for AW assessment and the planning of its management based on this.
Implementation tool for airway assessment and management planning.
ATI: awake tracheal intubation; DAW: difficult airway; DFONA: difficult front-of-neck access; DL: difficult laryngoscopy; DTI: difficult tracheal intubation; DSADV: difficult supraglottic access device ventilation; DFMV: difficult Face mask ventilation; IGA: induction general anaesthesia.
Decision-making must be individualised according to patient, operator, context and time (S.D. 97.1%).119,133
RecommendationThe diagnosis of SAHS is a predictor of difficulty of Face mask ventilation.
Strong recommendation; low level of evidence (⊕⊕⊝⊝)
The diagnosis of SAHS is a predictor of difficult tracheal intubation.
Strong recommendation; moderate level of evidence (⊕⊕⊕⊝)
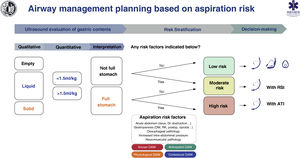
Aspiration risk assessmentAspiration is the main cause of mortality in airways.134,135 It causes up to 50% of deaths8 so its prevention is vital. Poor risk assessment and poor planning are the root cause of these events.8 Adherence to guides and cognitive aids could prevent most cases.136 A full stomach is the main risk factor.135,137 To avoid this, it is necessary to restrict food and liquid intake following the preoperative fasting guidelines (S.D. 97.1%).138 However, these have limited reliability in certain situations, including134,137,139–141: 1) non-compliance with fasting guidelines or uncertain prandial status (e.g., emergency, language barrier, cognitive dysfunction); 2) diseases causing delayed symptomatic gastric emptying (e.g., diabetes mellitus, advanced hepatic or renal dysfunction, Parkinson's disease, critically ill patient, sympathetic activation, pain, chronic opioid administration), and 3) raised intra-abdominal blood pressure (morbid obesity with truncal predominance, ascites, masses, obstruction). It is therefore advisable to complete these guidelines with an objective tool to increase the safety margin.141,142 Gastric ultrasound provides individual risk stratification with greater precision by verifying the nature and volume of the gastric contents in a simple,139,141 non-invasive and immediate way, with high sensitivity (1.0) and specificity (.975).139,143–145
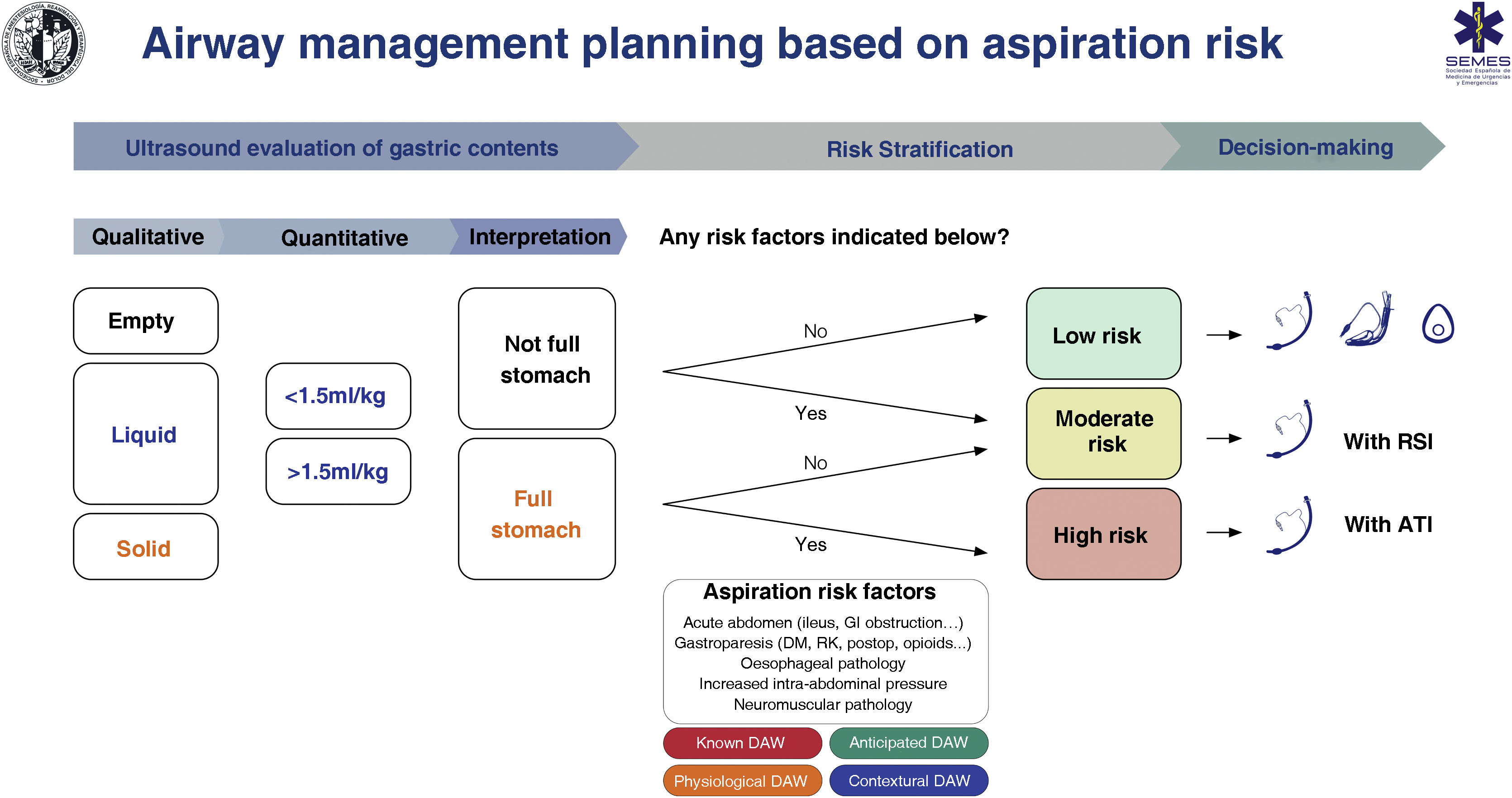
Despite limited evidence of its cost/effectiveness, gastric ultrasound has been shown to lead to changes in decision-making.135,146 The absence of a full stomach and other risk factors indicates that no special precautions are required. On the contrary, the presence of a full stomach with or without additional risk factors indicates that the AW should be protected with a TI (S.D. 88.6 %). The individual clinical context and the rest of the specific risk factors for aspiration must be taken into account when making decisions.119,147 SEDAR, SEMES and SEORL−CCC recommend gastric ultrasound examination to evaluate the risk of aspiration in risk situations (1C).
Fig. 7 shows a cognitive aid for AW management based on the risk of aspiration.
Cognitive help for planning, risk stratification and decision-making for AW management based on risk of aspiration.
ATI: awake tracheal intubation; DAW: difficult airway; DM: diabetes mellitus; GI: gastrointestinal; KF: kidney failure; postop: postoperative; RSI: rapid sequence induction.
Gastric ultrasound examination is recommended to evaluate the risk of aspiration in risk situations.
Strong recommendation; low level of evidence (⊕⊕⊝⊝)
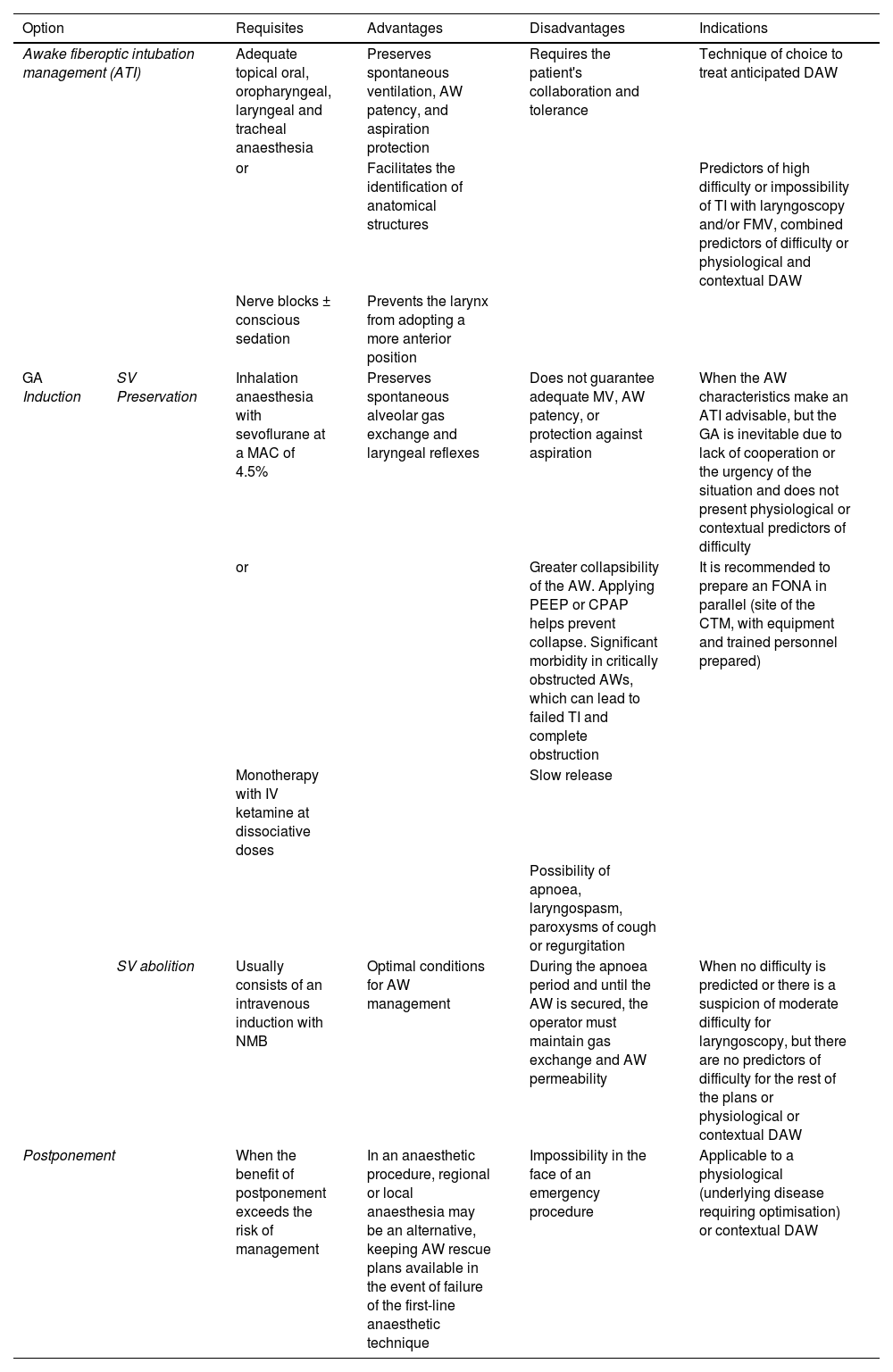
Basic options for difficult airway managementAirway management carries risks.6,148,149 Most techniques involve suppression of spontaneous ventilation and protection against aspiration.134,137 Laryngeal injuries frequently occur after simple instrumentation, in healthy low-risk patients, and after elective procedures.3,19,150,151 Therefore, before each procedure, the appropriateness of the management must be evaluated and a risk-benefit balance analysis performed (S.D. 97.1%). Once the indication is confirmed, the best approach must be decided to guarantee the fundamental principles of management: maintaining alveolar oxygenation, maintaining AW patency, and minimising the risk of aspiration. Patient preference and operator skill should be considered in this decision. Options include110:
| Option | Requisites | Advantages | Disadvantages | Indications | |
|---|---|---|---|---|---|
| Awake fiberoptic intubation management (ATI) | Adequate topical oral, oropharyngeal, laryngeal and tracheal anaesthesia | Preserves spontaneous ventilation, AW patency, and aspiration protection | Requires the patient's collaboration and tolerance | Technique of choice to treat anticipated DAW | |
| or | Facilitates the identification of anatomical structures | Predictors of high difficulty or impossibility of TI with laryngoscopy and/or FMV, combined predictors of difficulty or physiological and contextual DAW | |||
| Nerve blocks ± conscious sedation | Prevents the larynx from adopting a more anterior position | ||||
| GA Induction | SV Preservation | Inhalation anaesthesia with sevoflurane at a MAC of 4.5% | Preserves spontaneous alveolar gas exchange and laryngeal reflexes | Does not guarantee adequate MV, AW patency, or protection against aspiration | When the AW characteristics make an ATI advisable, but the GA is inevitable due to lack of cooperation or the urgency of the situation and does not present physiological or contextual predictors of difficulty |
| or | Greater collapsibility of the AW. Applying PEEP or CPAP helps prevent collapse. Significant morbidity in critically obstructed AWs, which can lead to failed TI and complete obstruction | It is recommended to prepare an FONA in parallel (site of the CTM, with equipment and trained personnel prepared) | |||
| Monotherapy with IV ketamine at dissociative doses | Slow release | ||||
| Possibility of apnoea, laryngospasm, paroxysms of cough or regurgitation | |||||
| SV abolition | Usually consists of an intravenous induction with NMB | Optimal conditions for AW management | During the apnoea period and until the AW is secured, the operator must maintain gas exchange and AW permeability | When no difficulty is predicted or there is a suspicion of moderate difficulty for laryngoscopy, but there are no predictors of difficulty for the rest of the plans or physiological or contextual DAW | |
| Postponement | When the benefit of postponement exceeds the risk of management | In an anaesthetic procedure, regional or local anaesthesia may be an alternative, keeping AW rescue plans available in the event of failure of the first-line anaesthetic technique | Impossibility in the face of an emergency procedure | Applicable to a physiological (underlying disease requiring optimisation) or contextual DAW | |
Awake management is recommended when there is a high degree of difficulty or impossibility of TI, combined predictors of difficulty or physiological alterations and negative contextual conditions (S.D. 82.9%).
Induction of general anaesthesia with preservation of spontaneous ventilation is suggested in those situations that make management with an awake patient advisable, but general anaesthesia is inevitable due to lack of cooperation or urgency, and does not present physiological or contextual predictors of difficulty or obstructive pathology. (S.D. 91.4%).
When there are physiological or contextual predictors of AW difficulty, the benefit of postponement can be assessed if it outweighs the risk of proceeding with management, or the possibility of establishing alternative anaesthetic strategies can be assessed (S.D. 85.7%).
PreparationInformed consentInformed consent is an essential presupposition of the lex artis ad hoc. As a general rule, it is collected in writing in invasive procedures and, in general, in those that pose health risks, such as those used to treat AWs. However, procedures such as TI form part of other procedures such as general anaesthesia or an informed consent critical care protocol.152,153 A specific document will therefore not be necessary, although documentary evidence of all the elements of the discussion and the informed consent process will be necessary, particularly for “non-routine” procedures, such as AW management with an awake patient.154
In cases of exemption to obtaining informed consent,153 a reasoned record of the circumstances will be left in the medical record and the decision will be communicated to family members or close friends.155 It is often possible to have a short discussion.
MonitoringFor AW management, the monitoring standards for an anaesthetic procedure are applicable.156,157
The capnography waveform must be available in all AW management sites to test the success of any of the 4 plans used (S.D. 97.1%) to provide alveolar oxygenation158 and early detection of the displacement of any artificial AW, as well as inadvertent hyper- or hypoventilation.1,6,9,159 It is also recommended for use during moderate or deep sedation for awake fiberoptic intubation.
Monitoring end-tidal oxygen concentration (EtO2) is the gold standard for assessing the effectiveness of Pre-oxygenation.160
Neuromuscular monitoring is recommended if a neuromuscular relaxant is administered to determine optimal conditions for TI, NMB recovery, and the need for reversal during eduction.161,162
Monitoring the end-tidal concentration of volatile anaesthetic agents is useful for performing inhalation induction.
Advanced invasive haemodynamic monitoring may be necessary to perform pre-procedural goal-guided optimisation in case of haemodynamic instability.111,163
PositionEnsuring the best positioning before any intervention provides optimal anatomical and physiological conditions.164 Thus, a correct position maximises the possibilities of laryngoscopy and TI, improves upper AW patency, optimising pre-oxygenation, apnoeic oxygenation and FMV,165,166 access to it (e.g., access to CTM) or respiratory mechanics. Ramped position or elevated head to 30 ° is recommended in the obese population to improve TI conditions (1C). The ramped position prolongs safe apnoea time in this population (1B).
The sitting or semi-sitting position (Fowler’s) or the head tilt 25−30° or reverse Trendelenburg position at 30°, is desirable in patients with a high risk of desaturation or aspiration, if the haemodynamic status allows it,1,159,167,168 since it increases FRC, reduces the formation of atelectasis,169,170 reduces the risk of aspiration,159 and could be associated with better laryngeal exposure,171 better rates of TI on the first attempt,172 and fewer complications.173 The sitting or semi-sitting position is optimal for ATI by providing anatomical and physiological advantages.174,175
The external auditory canal should be aligned with the suprasternal notch in the horizontal axis to facilitate AW management.1,176 In the case of obese patients, this requires a "ramped" position using a pile of sheets or a wedge on the upper part of the torso and head40,177 The “sniffing” position (lower cervical spine flexion and upper cervical spine extension) is optimal for DL.1,178,179 TI with both positions does not offer differences,180,181 although laryngeal exposure could be greater with the "ramp" position in the surgical population.181 The head in hyperextension could be the most appropriate position for FOB guided OTI with the patient awake, as it is associated with better glottal vision.182
RecommendationThe use of a ramped position or elevated head to 30° is recommended in the obese population to improve tracheal intubation conditions.
Strong recommendation; low level of evidence (⊕⊕⊝⊝),
The ramped position prolongs the time of safe apnoea in the obese population.
Strong recommendation; moderate level of evidence (⊕⊕⊕⊝)
Pre-procedural oxygenationGiven the potential difficulty in treating AWs, peri-procedural oxygenation should be universal183 to increase pulmonary oxygen reserve primarily through FRC, and extend the apnoea time without desaturation.184,185 To do this, it is necessary to choose the most appropriate technique based on the physiology, cooperation and clinical situation of the patient.184
Pre-oxygenationPre-oxygenation is a standard of care as it extends the safe apnoea time (period from cessation of ventilation to arterial oxyhaemoglobin saturation ≤ 90%).186 It should therefore be applied to all patients, and especially meticulously in AWs with predictors of difficulty; patients at high risk of hypoxaemia, or if manual ventilation is contraindicated.187 It is therefore an essential component of rapid sequence induction (RSI).184
The objective is to achieve an EtO2 > 90% before starting anaesthetic induction.184
The conventional pre-oxygenation method consists of spontaneous ventilation with FM and 100% oxygen and basically includes 2 techniques: tidal volume (Tv) for 3 min and 8 vital capacities (8 VC) for 1 min for emergency TI.160,188 The oxygen flow must be appropriate to eliminate reinhalation; 5 l/min for 3 to 5 min for Tv, and 10 l/min for 1 min for 8 VC.188 The presence of leaks under the FM and the reinhalation of exhaled gases reduces effectiveness as a FiO2 of 1.0 is not obtained. The presence of a normal capnography trace (grade 1 ventilation), a clear measurement of the inspiratory and expiratory CO2 (EtCO2) values, and correct movement of the reservoir bag are indicative of an appropriate seal.184 In the presence of a leak, it is recommended to add a nasal cannula with a flow greater than 10 l/min.189,190
Apnoeic oxygenationNasal oxygen therapy during efforts to secure an ETT (NOT DESETT), pharyngeal oxygen insufflation, and high-flow nasal oxygen therapy (HFNO) 40−70 l/m186 can prolong apnoea time up to 100 min, but does not prevent progressive respiratory acidosis due to hypercapnia.160,186,191,192 Standard nasal cannulae at 10−15 l/min allow well-tolerated apnoeic oxygenation, with low cost and risk.193
Apnoeic oxygenation has been shown to be useful in reducing desaturations in emergency TI.60,194–198
Apnoeic oxygenation with high-flow nasal cannula (NOT DESETT/HFNO) is recommended (1C).
RecommendationApnoeic oxygenation with high-flow nasal cannula (NO DESAT/HFNO) is recommended.
Strong recommendation; low level of evidence (⊕⊕⊝⊝)
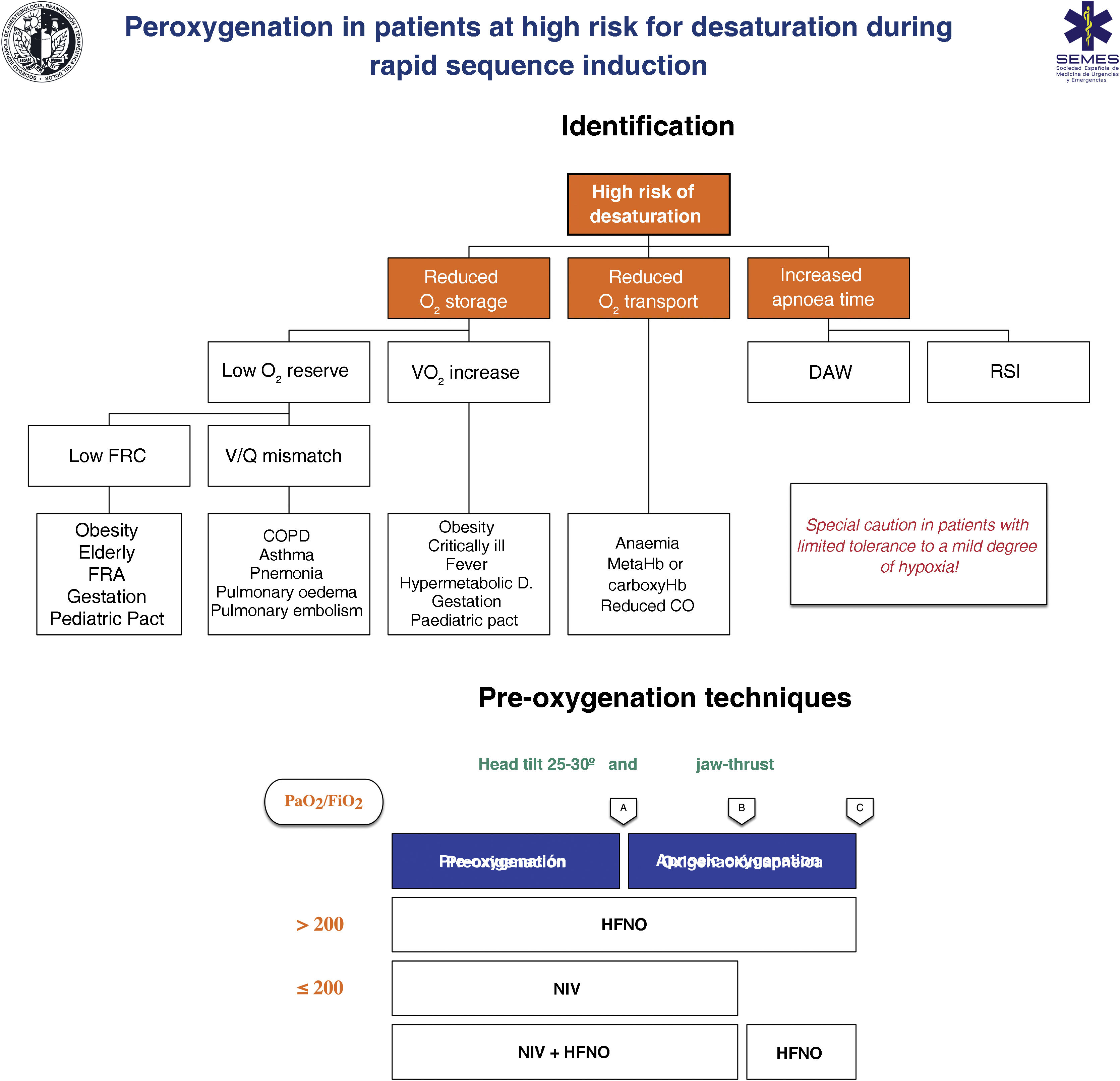
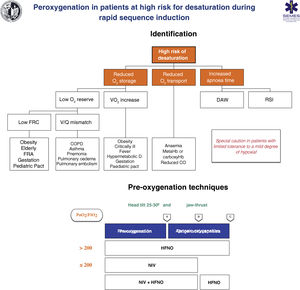
Techniques for patients at high risk or poor tolerance to hypoxaemiaThe effectiveness of conventional techniques is limited in patients at high risk of hypoxaemia (due to shunt, V/Q mismatch, low FRC, or increased oxygen consumption) and reduced tolerance to hypoxaemia (e.g., cerebrovascular disease, epilepsy or coronary artery disease).199 Attempts made to compensate for this deficiency by increasing the pre-oxygenation time may even exacerbate hypoxaemia, probably due to resorption atelectasis.200 Likewise, RSI is associated with desaturations in 10%–30% of cases. To plan pre-oxygenation, it is advisable to ask the following questions before starting management201: Are there likely to be difficulties with ventilation and/or TI? How quickly will desaturation occur? What is the safe level of desaturation? Fig. 8 shows the main entities associated with a high risk of desaturation and the peri-procedural oxygenation techniques recommended for this population.
Theoretical/educational tool for the detection of patients at high risk of desaturation and recommended pre-oxygenation and apnoeic oxygenation techniques during rapid sequence induction. A: aesthetic induction; B: laryngoscopy; C: tracheal intubation; ARF: acute respiratory failure; CO: cardiac output; COPD: chronic obstructive pulmonary disease; FRC: functional residual capacity; HFNO: high flow nasal oxygen therapy; NIV: non-invasive ventilation; RSI: rapid sequence induction; VO2: oxygen consumption; V/Q mismatch: ventilation/perfusion mismatch.
This figure illustrates the 2 methods used to increase pulmonary oxygen reserves: pre-oxygenation and apnoeic oxygenation. Pre-oxygenation refers to oxygen applied before anaesthetic induction, while apnoeic oxygenation refers to the delivery of oxygen after loss of spontaneous ventilation.
The greater the risk of desaturation, the more options that should be combined.202 The use of pre-apnoea adjuvants such as upright head position, jaw thrust, PEEP and apnoeic oxygenation provides optimisation of the O2160,167 safety reservoir. HFNO, NIV or a combination of both are more effective than conventional methods,203 since they reduce shunt and improve V/Q mismatch through alveolar recruitment. HFNO is recommended as a first-line pre-oxygenation technique for patients with mild hypoxaemia (PaO2/FiO2 > 200 mmHg) (1C). NIV would be the technique of choice in those with severe hypoxaemia (PaO2/FiO2≤ 200 mmHg) (S.D. 87.15%)204–209 since it generates greater PEEP and allows pressure support to be applied to increase FRC.210,211
High-flow nasal oxygen therapy (HFNO)Pre-oxygenation with HFNO showed mixed results.212–214 A recent meta-analysis demonstrated that in adults with hypoxaemia it reduced the risk of complications related to TI compared to conventional oxygen therapy.215 Thus, HFNO could be superior to this,216–220 but inferior to NIV215,221 although it is a good alternative when the latter is not well tolerated.168
For pre-oxygenation, patients should perform VC nasal breaths at an initial O2 flow of 30 L/min and 100% FiO2 with the mouth tightly closed for 3 min and with the cannula tightly fitted to the nostrils to avoid pollution. After induction, the flow is increased to 70 l/min and maintained until TI. AW patency must be maintained by jaw-thrust.160,189
HFNO allows effective apnoeic oxygenation during laryngoscopy. This could be its main mechanism for reducing desaturation194,222,223
HFNO makes EtO2192 monitoring difficult. Also, it could worsen TI conditions224 and potentially cause gastric insufflation.225 Recent research contradicts this last possibility,226,227 although it is uncertain whether these data can be extrapolated to patients with a full stomach.225
RecommendationHFNO is recommended as a first-line pre-oxygenation technique for patients with mild hypoxaemia.
Strong recommendation; low level of evidence (⊕⊕⊝⊝)
Non-invasive ventilation (NIV)NIV is especially beneficial in patients with reduced FRC.212,228 It maximises pre-oxygenation in obese and/or critically ill patients.160,186,202 The beneficial effect on PaO2 is still observed 30 min after TI due to alveolar recruitment and increased lung volume.229 NIV is recommended over conventional oxygen therapy for the induction of anaesthesia in obese patients (1B).
CPAP (5–10 cmH2O) with assisted respirations (VC 7−10 ml/kg) has shown better oxygenation in clinical practice.230 NIV must be interrupted during laryngoscopy,228 so it may be superior to HFNO during the spontaneous ventilation phase,207 and HFNO may be more beneficial during apnoeic oxygenation-204,205,210 pre-oxygenation with NIV plus HFNO and apnoeic oxygenation with HFNO should be a priority option for critically ill patients (S.D. 85.7%) as they are associated with significantly lower desaturation.214,231,232
For patients who do not tolerate the interface or with delirium, analgosedation with dexmedetomidine or induction of a dissociative state with ketamine (iv boluses of 10−20 mg) can be considered to facilitate pre-oxygenation ("delayed sequence intubation").111,168,211,233
It is recommended to consider NIV before and after general anaesthesia (GA) in obese patients.199,234
Pressures > 20 cmH2O can cause gastric distension, which requires a risk-benefit analysis in patients at risk of aspiration. Likewise, another method of pre-oxygenation is desirable in patients with facial fractures, after laryngeal, oesophageal or gastric surgery, and those with hemodynamic instability, pulmonary arterial hypertension, pulmonary embolism or right ventricular failure.209
RecommendationNIV is recommended over conventional oxygen therapy for the induction of anaesthesia in obese patients.
Strong recommendation; moderate level of evidence (⊕⊕⊕⊝)
Physiological difficult airwayThe considerations in this section refer to the patient with previously defined PDAW or the critically ill patient.235 Management of an emergency AW is a high-risk procedure.113,167,235–237 The incidence of DAW in this context is up to 20 times higher compared to elective TI238 and events that cause death or brain damage are approximately 30–60 times more frequent.10,239 Underlying pathophysiological disorders such as hypoxaemia and haemodynamic instability are responsible for peri-intubation decompensations that cause cardiovascular collapse in up to 30% of critically ill patients240,241 due to myocardial depression caused by hypoxia or low perfusión.241–243 Thus, up to 50% of critically ill patients may suffer a major adverse event peri-intubation.243 This risk is exacerbated when TI requires more than one attempt.111,243,244 Difficult TI is an independent predictor of death. Thus, complication rates multiply by 5 after the second TI attempt,245,246 so the objective of ensuring an AW on the first attempt is especially important in critically ill patients.58,111,243,247,248
Peri-intubation desaturation is the greatest risk factor for cardiorespiratory arrest and occurs in 19%–70% of TI in critically ill patients.168 It is the most important reason for aborting TI on the first attempt.168 Pre-oxygenation and apnoeic oxygenation are the most important interventions to prevent it.248,249 which is why they should be performed in all patients in the upright position.168,235
Haemodynamic instability is an independent predictor of mortality after TI.235,250,251 Peri-intubation hypotension168 affects up to 46% of PDAW cases252,253 and is associated with longer ICU stays, target organ damage, and higher in-hospital mortality.241,252,254 Pre-intubation risk factors include MAP ≤ 65 mmHg and shock index (SI, heart rate/systolic blood pressure) >.7168.235. During TI of the critically ill patient, the risk of cardiovascular collapse increases due to hypovolemia, altered systemic vascular resistance, vasodilatation and myocardial depression due to anaesthetic agents, sympathetic stimulation due to hypoxia and/or hypercapnia, and reduced venous return due to conversion to positive pressure ventilation (PPV).167,240,242,255
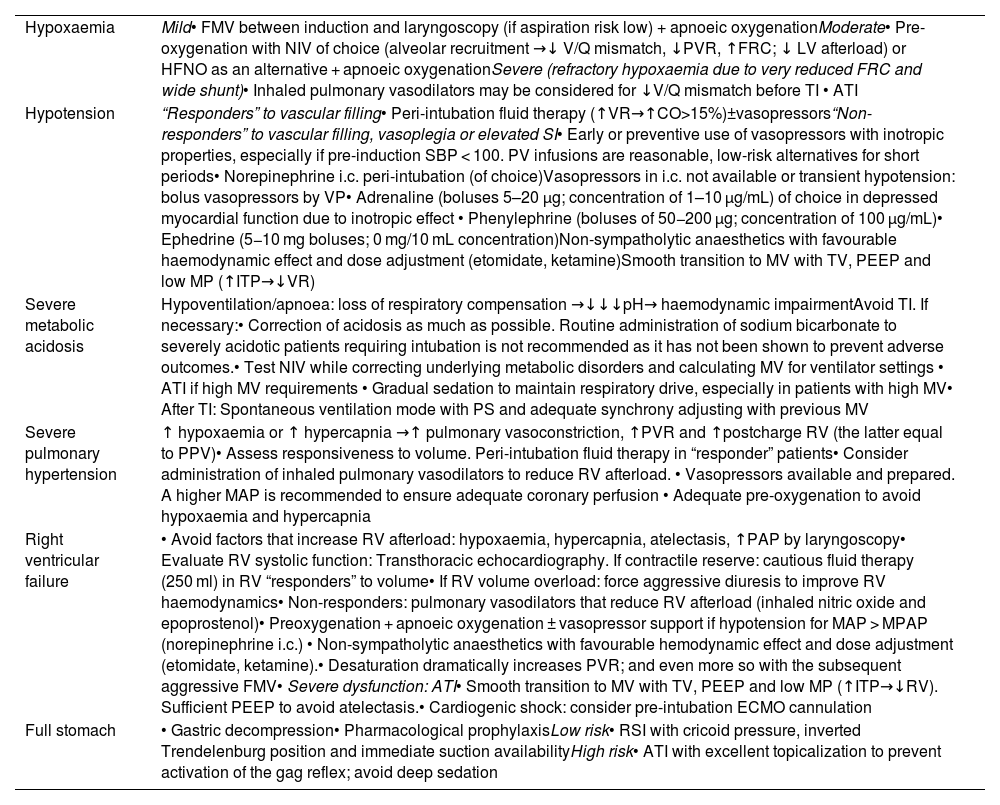
Physiological threats are, therefore, as dangerous as technical difficulties and equally require anticipation, planning and pre-instrumentalisation physiological optimisation if the specific situation allows it.1,256 The evidence for interventions aimed at achieving physiological stability before TI is limited,167,236,243 and it thus seems prudent to plan individualised therapy.236 If time permits, a point-of-care ultrasound examination may be useful for targeted optimisation.257Table 1 shows the main predictors of PDAW and the methods proposed to reduce peri-intubation complications.1,111,113,159,167,168,236,248,258
Main predictors of difficult physiological airway and proposed methods to reduce peri-intubation complications derived from.
| Hypoxaemia | Mild• FMV between induction and laryngoscopy (if aspiration risk low) + apnoeic oxygenationModerate• Pre-oxygenation with NIV of choice (alveolar recruitment →↓ V/Q mismatch, ↓PVR, ↑FRC; ↓ LV afterload) or HFNO as an alternative + apnoeic oxygenationSevere (refractory hypoxaemia due to very reduced FRC and wide shunt)• Inhaled pulmonary vasodilators may be considered for ↓V/Q mismatch before TI • ATI |
| Hypotension | “Responders” to vascular filling• Peri-intubation fluid therapy (↑VR→↑CO>15%)±vasopressors“Non-responders” to vascular filling, vasoplegia or elevated SI• Early or preventive use of vasopressors with inotropic properties, especially if pre-induction SBP < 100. PV infusions are reasonable, low-risk alternatives for short periods• Norepinephrine i.c. peri-intubation (of choice)Vasopressors in i.c. not available or transient hypotension: bolus vasopressors by VP• Adrenaline (boluses 5–20 μg; concentration of 1–10 μg/mL) of choice in depressed myocardial function due to inotropic effect • Phenylephrine (boluses of 50−200 μg; concentration of 100 μg/mL)• Ephedrine (5−10 mg boluses; 0 mg/10 mL concentration)Non-sympatholytic anaesthetics with favourable haemodynamic effect and dose adjustment (etomidate, ketamine)Smooth transition to MV with TV, PEEP and low MP (↑ITP→↓VR) |
| Severe metabolic acidosis | Hypoventilation/apnoea: loss of respiratory compensation →↓↓↓pH→ haemodynamic impairmentAvoid TI. If necessary:• Correction of acidosis as much as possible. Routine administration of sodium bicarbonate to severely acidotic patients requiring intubation is not recommended as it has not been shown to prevent adverse outcomes.• Test NIV while correcting underlying metabolic disorders and calculating MV for ventilator settings • ATI if high MV requirements • Gradual sedation to maintain respiratory drive, especially in patients with high MV• After TI: Spontaneous ventilation mode with PS and adequate synchrony adjusting with previous MV |
| Severe pulmonary hypertension | ↑ hypoxaemia or ↑ hypercapnia →↑ pulmonary vasoconstriction, ↑PVR and ↑postcharge RV (the latter equal to PPV)• Assess responsiveness to volume. Peri-intubation fluid therapy in “responder” patients• Consider administration of inhaled pulmonary vasodilators to reduce RV afterload. • Vasopressors available and prepared. A higher MAP is recommended to ensure adequate coronary perfusion • Adequate pre-oxygenation to avoid hypoxaemia and hypercapnia |
| Right ventricular failure | • Avoid factors that increase RV afterload: hypoxaemia, hypercapnia, atelectasis, ↑PAP by laryngoscopy• Evaluate RV systolic function: Transthoracic echocardiography. If contractile reserve: cautious fluid therapy (250 ml) in RV “responders” to volume• If RV volume overload: force aggressive diuresis to improve RV haemodynamics• Non-responders: pulmonary vasodilators that reduce RV afterload (inhaled nitric oxide and epoprostenol)• Preoxygenation + apnoeic oxygenation ± vasopressor support if hypotension for MAP > MPAP (norepinephrine i.c.) • Non-sympatholytic anaesthetics with favourable hemodynamic effect and dose adjustment (etomidate, ketamine).• Desaturation dramatically increases PVR; and even more so with the subsequent aggressive FMV• Severe dysfunction: ATI• Smooth transition to MV with TV, PEEP and low MP (↑ITP→↓RV). Sufficient PEEP to avoid atelectasis.• Cardiogenic shock: consider pre-intubation ECMO cannulation |
| Full stomach | • Gastric decompression• Pharmacological prophylaxisLow risk• RSI with cricoid pressure, inverted Trendelenburg position and immediate suction availabilityHigh risk• ATI with excellent topicalization to prevent activation of the gag reflex; avoid deep sedation |
ATI: awake tracheal intubation; c.i: continuous infusion; CO: cardiac output; ECMO: extracorporeal membrane oxygenation; FRC: functional residual capacity; HFNO: high flow nasal oxygen therapy; ITP: intrathoracic pressure; LV: left ventricle; MAP: mean arterial pressure; MP: medium pressure; MPAP: mean pulmonary arterial pressure; MV: mechanical ventilation; MV: minute volume; NIV: non-invasive ventilation; PAP: pulmonary arterial pressure; PEEP: positive end-expiratory pressure; PS: pressure support; PVR: pulmonary vascular resistance; PPV: positive pressure ventilation; PV: peripheral venous line; RV: right ventricle; SBP: systolic blood pressure; SI: shock index; TI: tracheal intubation; Tv: tidal volume; VMF: ventilation with face mask; V/Q mismatch: ventilation/perfusion mismatch; VR: venous return.
Fluid therapy in the form of a pre-intubation bolus has minimal benefit,240,259 although administered as part of a package of measures for TI. These include pre-oxygenation with NIV; pre-induction administration of 500 ml of isotonic crystalloids in patients without cardiogenic pulmonary oedema, and early administration of norepinephrine in cases of diastolic blood pressure < 35 mmHg after TI with this being associated with a 50% relative reduction in cardiovascular collapse and severe hypoxaemia,260 that could prevent peri-procedural hypotension. However, routine administration of a pre-induction crystalloid bolus in patients not receiving PPV may not be justified as it only showed benefit in the subgroup of patients who received NIV for pre-oxygenation or FMV between induction and laryngoscopy, while it could be harmful in the rest of the population that does not respond to the volume.240 The implementation of a TI protocol could reduce these complications.260–262
Although its effectiveness in avoiding peri-intubation hypotension has not yet been established,248,255 preventive administration or early initiation of vasopressors is suggested211 and that an expert operator be in charge of treating the AW while another member of the team leads the management of the haemodynamic status.16,168,243 Norepinephrine infusion would be the first-line vasoactive therapy.168,235 Initial administration through peripheral venous cannulae is safe,263,264 so initiation of vasopressors does not require central venous access.235
Rapid sequence inductionTI is the gold standard to ensure the AW and RSI is the recommended technique when there is a considerable risk of aspiration in an AW without predictors of difficulty (S.D. 97.1%).265,266 Its components (gastric decompression, pre-preparation, adequate positioning, peri-procedural oxygenation, aesthetic induction and cricoid pressure in selected cases) are designed to223,267–269: 1) shorten the time interval between the loss of protective reflexes and tracheal sealing by air—of the ETT; 2) achieve optimal conditions for a successful TI on the first attempt with an adequate anaesthetic depth and NMB to avoid cough, active vomiting or increased intra-abdominal pressure,265 and 3) minimise the risks secondary to its use, fundamentally hypoxia, hypotension and difficult TI. Its practice is supported by little evidence.266,268,270–273 and can be associated with harmful results,266,274,275 so it must be justified with clear indications.22,268 The key point is to identify patients at risk of aspiration (Fig. 7). In case of doubt or if a gastric ultrasound is not feasible, the highest risk should be assumed.268 Likewise, it is recommended to use RSI with or without Sellick manoeuvre in all emergency TI (S.D. 84.4%) given the characteristic poor gastric emptying and the high risk of aspiration in the fragile critical patient.223,268,276
For the safe preparation of RSI, the use of a checklist is suggested (S.D. 97.1%). The use of a checklist (Fig. 4) could reduce the complication rate71,277–279 by minimising cognitive load and errors, and improve safety through a standardised approach.223,235,266,280
For high-risk patients, premedication with a nonparticulate antacid (e.g., sodium citrate) is suggested immediately before induction and an H2 receptor antagonist or proton pump inhibitor 40−60 min before to increase the pH and reduce the volume of gastric contents (S.D. 82.9%).265,281
Management with a nasogastric tube must be individualised (S.D. 88.6%) since there is no scientific basis for it.265,282 It is usually inserted if the anticipated or ultrasonographically evaluated residual gastric volume exceeds 200−300 ml.265,268 Gastric emptying with a double-lumen Salem-type tube is mandatory during the preoperative management of patients with ileum or intestinal obstruction.265,283,284 Gastric decompression should begin as soon as possible in the surgical ward and continue in the pre-induction and pre-eduction period.267,284 The probe should be kept in continuous suction during RSI.265,284,285
Preparation for RSI includes evaluating potential anatomical, physiological, or situational challenges, developing a first-line and rescue plan with clear instructions, and assembling the personnel, equipment, and medications necessary to perform an emergency TI.223,266,286 In the event of possible regurgitation, the availability of high-efficiency suction devices with large-calibre multi-hole probes (S.D. 100%) such as Yankauer or DuCanto223,287 must be guaranteed.
A position with the head elevated 20−30º (sitting or semi-sitting position or reverse Trendelenburg) is recommended to prevent passive regurgitation and, if it occurs, the Trendelenburg position, turning the head to one side and suctioning the oropharynx and trachea before of starting PPV (S.D. 94.3%).267,288
It is essential that optimal pre-oxygenation and apnoeic oxygenation, as well as individualised haemodynamic optimisation, precede induction.223,286 The selection of the hypnotic anaesthetic has been described as the only factor independently associated with cardiovascular instability and/or collapse,289 which is why it is particularly important.255 The choice of the hypnotic agent, as well as the dose and speed of administration, must be individualised (S.D. 91.4 %), according to the comorbidity profile, haemodynamic status of the patient and the speed with which AW needs to be ensured.223,266 Propofol (2−3 mg kg−1) is the agent of choice in the haemodynamically stable euvolemic patient since it provides the best intubation conditions.265,274,276 In unstable patients, it can increase haemodynamic complications and the risk of death,243 and has been identified as an independent risk factor for peri-intubation haemodynamic collapse.289 These data suggest that it should be avoided in critically ill patients with potential haemodynamic instability.255 Etomidate (.2–.3 mg kg−1) and ketamine (1−2 iv mg kg−1) are alternatives for haemodynamic instability.275,286 Ketamine may produce haemodynamic collapse in the patient with depleted sympathetic reserve (e.g., severe hypovolemic shock) as a result of its mild direct myocardial depressant effect.290 It should be avoided in patients with acute myocardial ischaemia.223,291 The use of etomidate may be associated with a lower risk of postinduction hypotension compared to ketamine.290 In agitated and non-cooperative patients, a delayed sequence induction can be performed, which consists of the administration of ketamine in boluses of .25–.5 mg kg−1 until a dissociative state, after which pre-oxygenation is carried out and the subsequent administration of the neuromuscular relaxant233,292–294
Although the classic RSI did not include the administration of an opioid, currently the use of alfentanil (15−40 μg kg−1), remifentanyl (1 μg kg−1) and fentanyl (2−5 μg kg−1) is common practice since it reduces the necessary dose of the hypnotic, promotes hemodynamic stability by attenuating the cardiovascular response to laryngoscopy and improves intubation conditions,265,271,283,285 without causing excessive hypotension and bradicardia.275,283,295
The administration of a neuromuscular relaxant is essential,286 since it improves TI conditions, suppresses cough and laryngospasm, reduces complications and optimises the compliance of the chest wall.296,297 Neuromuscular blocking is recommended to improve TI conditions and reduce the incidence of AW-associated adverse events in the general population (1B).
Rocuronium 1.0–1.2 mg kg−1 is comparable to succinylcholine 1.0–1.5 mg kg−1 for RSI,269,298–300 has a safer clinical profile, offers a longer-lasting blockade266 and can be reversed more quickly than succinylcholine with sugammadex (16 mg kg−1 ).301 The rescue dose must be precalculated and immediately available for emergency reversal.266,302 Succinylcholine can cause malignant hyperthermia, hyperkalemia, and the muscle fasciculations caused increase intragastric pressure and shorten apnoea time.303,304 Overall, the use of rocuronium is increasingly favored.235,303–305 The combination rocuronium + sugammadex is not inferior to succinylcholine for RSI (1B). The precurisation or priming technique is not recommended due to its questionable efficacy and safety given the risk of loss of protective reflexes.265,306
The use of cricoid pressure is controversial.265,268,295 The manoeuvre has not been shown to prevent aspiration,307–309 it is biomechanically impossible to maintain the recommended pressure310 and its use produces a reduction in the tone of the lower oesophageal sphincter.311 It can also contribute to obstruction of the AW,270 make laryngoscopy and TI309 FMV,312 insertion, ventilation and TI difficult through a SAD313 and make visualization of the glottis difficult with FOB,314 potentially prolonging TI times.309,315 For all these reasons, the routine use of cricoid pressure (S.D. 81.3%) cannot be recommended.223,260,286,316,317 It must be planned individually and applied when FMV is necessary during the apnoea period,286 since it prevents gastric insufflation.318 In the indicated cases, it should: 1) be applied correctly: 1 kg (10 N) until loss of consciousness and subsequently 3 kg (30 N) until the ETT cuff pressure is established,265,317 and 2) be released if laryngoscopy, TI or ventilation is made difficult, before inserting an EDG or in case of active vomiting.
Apnoeic oxygenation is associated with a lower prevalence of desaturation and greater success of TI on the first attempt.196,319–321 A “modified RSI” can be applied in patients at high risk of hypoxia who are not candidates for ATI (S.D. 85.7%)322 consisting of bimanual or mechanical FMV with a limited inspiratory pressure (<15 cmH2O without cricoid pressure or a <20 cmH2 with cricoid pressure) weighing individualised potential risks/benefits.132,159,223,266,268,318,323,324 This practice of excluding patients at high risk for aspiration was associated with a significantly lower prevalence of desaturation without negatively affecting aspiration rates.325,326
For TI, it is recommended to use the laryngoscope and blade with a greater chance of success on the first attempt. There is no evidence to support a specific device. The choice will depend on the clinical situation and the operator's preference.266 VL with stylet could be the best option.211,327–330
RecommendationNeuromuscular blockade is recommended to improve TI conditions and reduce the incidence of AW-associated adverse events in the general population.
Strong recommendation; Moderate level of evidence (⊕⊕⊕⊝)
The combination of rocuronium + sugammadex is not inferior to succinylcholine for RSI
Strong recommendation; moderate level of evidence (⊕⊕⊕⊝)
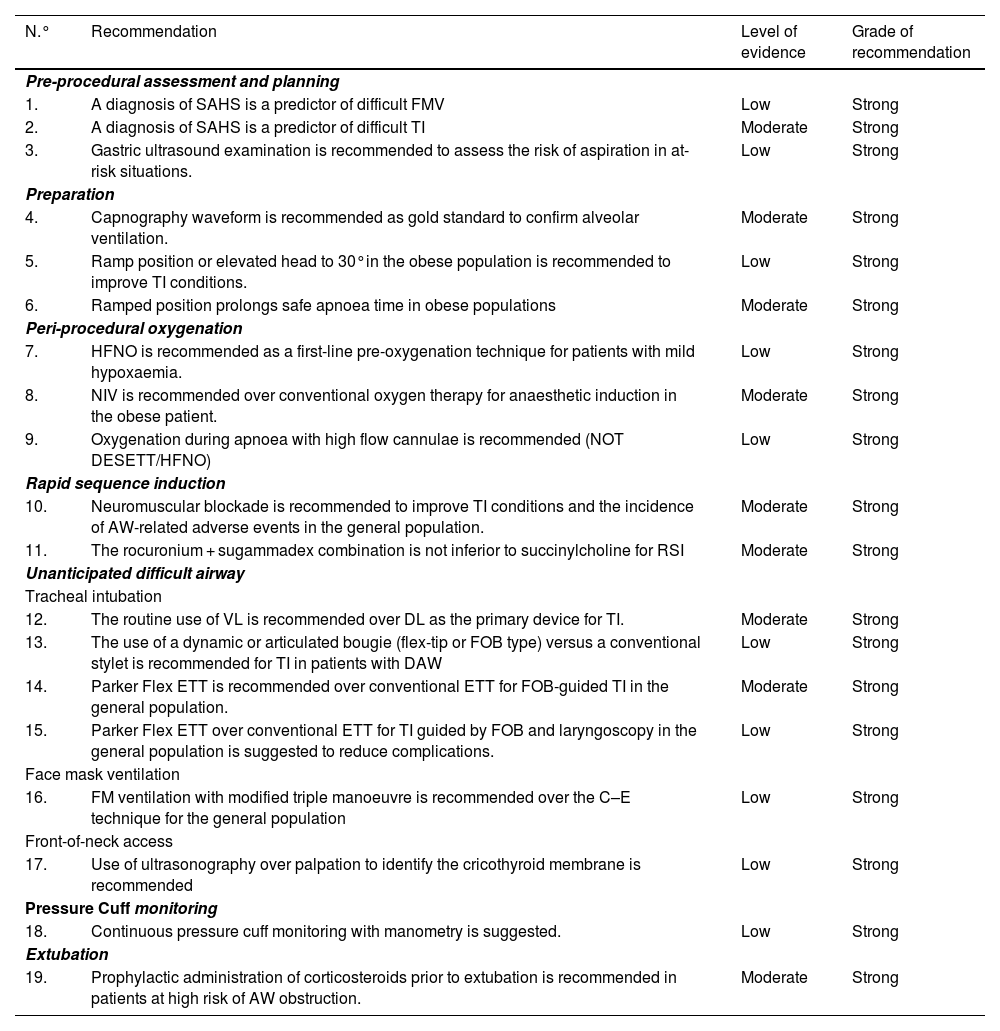
Summary of recommendations from the systematic search of the literatureSearch strategies and GRADE tables are shown in Appendix A Supplementary material.
AW: airway; DL: direct laryngoscopy; ETT: endotracheal tube; FOB: fiberoptic bronchoscopy; HFNO: high flow nasal oxygen therapy; NIV: non-invasive ventilation NOT DESETT: Nasal oxygen therapy during efforts to secure an ETT; RSI: Rapid sequence induction; SAHS: Sleep apnoea-hypopnoea syndrome; TI: tracheal intubation; VL: videolaryngoscopy.
| N.° | Recommendation | Level of evidence | Grade of recommendation |
|---|---|---|---|
| Pre-procedural assessment and planning | |||
| 1. | A diagnosis of SAHS is a predictor of difficult FMV | Low | Strong |
| 2. | A diagnosis of SAHS is a predictor of difficult TI | Moderate | Strong |
| 3. | Gastric ultrasound examination is recommended to assess the risk of aspiration in at-risk situations. | Low | Strong |
| Preparation | |||
| 4. | Capnography waveform is recommended as gold standard to confirm alveolar ventilation. | Moderate | Strong |
| 5. | Ramp position or elevated head to 30°in the obese population is recommended to improve TI conditions. | Low | Strong |
| 6. | Ramped position prolongs safe apnoea time in obese populations | Moderate | Strong |
| Peri-procedural oxygenation | |||
| 7. | HFNO is recommended as a first-line pre-oxygenation technique for patients with mild hypoxaemia. | Low | Strong |
| 8. | NIV is recommended over conventional oxygen therapy for anaesthetic induction in the obese patient. | Moderate | Strong |
| 9. | Oxygenation during apnoea with high flow cannulae is recommended (NOT DESETT/HFNO) | Low | Strong |
| Rapid sequence induction | |||
| 10. | Neuromuscular blockade is recommended to improve TI conditions and the incidence of AW-related adverse events in the general population. | Moderate | Strong |
| 11. | The rocuronium + sugammadex combination is not inferior to succinylcholine for RSI | Moderate | Strong |
| Unanticipated difficult airway | |||
| Tracheal intubation | |||
| 12. | The routine use of VL is recommended over DL as the primary device for TI. | Moderate | Strong |
| 13. | The use of a dynamic or articulated bougie (flex-tip or FOB type) versus a conventional stylet is recommended for TI in patients with DAW | Low | Strong |
| 14. | Parker Flex ETT is recommended over conventional ETT for FOB-guided TI in the general population. | Moderate | Strong |
| 15. | Parker Flex ETT over conventional ETT for TI guided by FOB and laryngoscopy in the general population is suggested to reduce complications. | Low | Strong |
| Face mask ventilation | |||
| 16. | FM ventilation with modified triple manoeuvre is recommended over the C–E technique for the general population | Low | Strong |
| Front-of-neck access | |||
| 17. | Use of ultrasonography over palpation to identify the cricothyroid membrane is recommended | Low | Strong |
| Pressure Cuff monitoring | |||
| 18. | Continuous pressure cuff monitoring with manometry is suggested. | Low | Strong |
| Extubation | |||
| 19. | Prophylactic administration of corticosteroids prior to extubation is recommended in patients at high risk of AW obstruction. | Moderate | Strong |
ATI: awake tracheal intubation; AW: Airway; DAW: difficult airway; DL: direct laryngoscopy; ECMO: extracorporeal membrane oxygenation; ETT: endotracheal tube; EtCO2: end-tidal carbon dioxide concentration; EtO2: end-tidal oxygen concentration; FOB: fiberoptic bronchoscopy; FiO2: inspiratory fraction of oxygen; FMV: Face mask ventilation.
FONA: front-of-neck access; 2GSAD: second generation supraglottic device; HFNO: high flow nasal oxygen therapy; IC: informed consent; NIV: non-invasive ventilation; NMB: neuromuscular blockade; PaO2: arterial partial pressure of oxygen; RSI: rapid sequence induction; SAD: supraglottic device; SADV: with supraglottic device ventilation; SpO2: peripheral oxygen saturation; TI: tracheal intubation; VL: videolaryngoscopy.
| N.° | Question | Percentage in favour [in favour; neutral; against] |
|---|---|---|
| Human factors | ||
| 1. | The number of attempts for each non-invasive treatment plan should be limited to three | 88.6 [31; 2; 2] |
| 2. | The first attempt should be made under optimal conditions. | 100 [35; 0; 0] |
| 3. | The most appropriate primary technique should be the one that offers the best guarantee of first-attempt success | 94.3 [33; 1; 1] |
| 4. | Visual cognitive aids are recommended for the management of emerging crises | 97.1 [34; 1; 0] |
| 5. | A standardised difficult AW trolley is recommended in areas with AW management | 100 [35; 0; 0] |
| 6. | Use of checklists is recommended to reduce the incidence of human error, improve task completion time, and reinforce a culture of safety in the AW management | 100 [35; 0; 0] |
| 7. | The use of ergonomic and communication models is recommended | 91.4 [32; 3; 0] |
| Pre-procedural assessment and planning | ||
| 8. | Pre-procedural assessment is recommended for all patients requiring AW management | 100 [35; 0; 0] |
| 9. | Pre-procedural assessment of AW should be multifactorial, structured and aimed at detecting anatomical, physiological, and contextual DAW | 97.1 [34; 1; 0] |
| 10. | AW exploration can start by detecting predictors of difficulty or failure for the primary plan and subsequently for the 3 alternative plans | 97.1 [34; 1; 0] |
| 11. | Multivariate models may have a higher predictive capacity | 97.1 [34; 1; 0] |
| 12. | Decision-making should be individualised according to patient, operator, context and time | 97.1 [34; 1; 0] |
| 13. | Restriction of food and liquid intake following preoperative fasting guidelines is necessary | 97.1 [34; 1; 0] |
| 14. | The presence of a full stomach indicates that the AW must be protected with TI | 88.6 [31; 2; 2] |
| Preparation | ||
| 15. | Capnography waveform should be available in all AW management sites to assess the success of any of the 4 plans used | 97.1 [34; 1; 0] |
| Basic options for difficult airway management | ||
| 16. | Before each procedure, the appropriateness of the management must be assessed and a risk-benefit analysis performed | 97.1 [34; 1; 0] |
| 17. | Awake management is recommended when there is a high degree of difficulty or impossibility of TI, combined predictors of difficulty or physiological disturbances and negative contextual conditions | 82.9 [29; 5; 1] |
| 18. | Induction of general anaesthesia with preservation of spontaneous ventilation is suggested in situations where awake management is recommended but general anaesthesia is unavoidable due to uncooperativeness or urgency, and there are no physiological or contextual predictors of difficulty or obstructive pathology | 91.4 [32; 2; 1] |
| 19. | When physiological or contextual predictors of difficult AW exist, the benefit of deferral may be assessed if it outweighs the risk of proceeding to AW management, or alternative anaesthetic strategies may be considered | 85.7 [30; 5; 0] |
| Known or anticipated difficult airway | ||
| 20. | Awake management is the option of choice to ensure a known or anticipated DAW | 85.7 [30; 4; 1] |
| 21. | High-flow nasal oxygen therapy is recommended over conventional low-flow cannulae | 91.4 [32; 3; 0] |
| 22. | NIV with endoscopic mask may have a role in TI of critically ill patients with hypoxaemia | 82.9 [29; 6; 0] |
| 23. | Premedication with an antisialogogue is recommended to optimise the effectiveness of the local aesthetic and field of vision, glycopyrrolate being the preferred option | 80.[28; 5; 2] |
| 24. | Sedation is an optional adjunct to adequate topical anaesthesia in awake airway management | 88.6 [31; 2; 2] |
| 25. | The goals of conscious sedation for awake AW management are effective amnesia, patient satisfaction, and analgesia to reduce cough, gag, and haemodynamic reflexes, while preserving AW patency, spontaneous ventilation, and protective laryngeal reflexes | 94.3 [33; 2; 0] |
| 26. | If the selected primary technique (FOB or VL) fails, the alternative technique should be used | 80 [28; 6; 1] |
| 27. | A third attempt may benefit from a multimodal approach (VL + FOB) | 100 [35; 0; 0] |
| 28. | The combination of a TI SAD and FOB can be useful as a rescue technique to maintain oxygenation, maintain airway patency and to perform a TI through it. | 100 [35; 0; 0] |
| 29. | A smaller than usual ETT is recommended with VL and FOB | 85.7 [30; 4; 1] |
| 30. | Decreasing the difference between the outer diameter of the FOB and the inner diameter of the ETT is recommended to facilitate FOB-guided TI | 85.7 [30; 3; 2] |
| 31. | Standard PVC ETTs are not recommended for FOB-guided TI as they are more likely to impact glottic structures | 71.9 [23; 4; 5] |
| 32. | After visual confirmation of TI, it is recommended to induce general anaesthesia after establishing cuff pressure and capnographic confirmation of TI | 94.3 [33; 2; 0] |
| 33. | Alternative techniques and approaches should be planned in advance and applied without delay after failure of the primary approaches | 100 [35; 0; 0] |
| 34. | With a high probability of awake TI failure, it is recommended to prepare a FONA in parallel to the invasive treatment plan in case of total obstruction | 88.6 [31; 4; 0] |
| 35. | Awake tracheostomy under local anaesthesia is recommended in the presence of pre-existing critical AW compromise. | 82.9 [29; 6; 0] |
| 36. | Awake cricothyrotomy would be the most indicated technique in the event of an emergent critical compromise | 91.4 [32; 3; 0] |
| 37. | Awake ECMO under local anaesthesia may be the safest option when all 4 conventional plans are expected to be impossible, unsuccessful, or ineffective, with risk of full AW obstruction | 90.6 [29; 1; 2] |
| Unanticipated difficult airway | ||
| Peri-proceduraloxygenation | ||
| 38. | HFNO should be considered as a first-line pre-oxygenation technique for patients with mild hypoxaemial (PaO2/FiO2>200 mmHg), while NIV is the technique of choice in those with severe hypoxaemia (PaO2/FiO2 ≤ 200 mmHg) | 87.5 [28; 3; 1] |
| 39. | Pre-oxygenation with NIV + HFNO and apnoeic oxygenation with HFNO should be a priority option for critically ill patients during TI | 85.7 [30; 4; 1] |
| Rapid sequence induction | ||
| 40. | RSI is the recommended technique when there is a significant risk of aspiration in an AW without predictors of difficulty | 97.1 [34; 1; 0] |
| 41. | RSI with or without Sellick manoeuvre is recommended for all emergency TIs | 84.4 [27; 1; 4] |
| 42. | The use of checklists is suggested for safe preparation of RSI | 97.1 [34; 1; 0] |
| 43. | Premedication with non-particulate antacid immediately before induction and an H2 receptor antagonist 40−60 min before or a proton pump inhibitor to increase pH and reduce the volume of gastric contents is suggested in patients at high risk of aspiration | 82.9 [29; 5; 1] |
| 44. | Nasogastric tube treatment should be individualised | 88.6 [31; 4; 0] |
| 45. | Highly efficient suction devices with large multi-hole suction tubes should be available in case of potential regurgitation | 100 [35; 0; 0] |
| 46. | An elevated head position to 20°–30° is recommended to prevent passive regurgitation and, if regurgitation occurs, the Trendelenburg position, turning the head to one side and suctioning the oropharynx and trachea before initiating positive pressure ventilation | 94.3 [33; 2; 0] |
| 47. | The choice of hypnotic, as well as the dose and speed of administration, must be individualised | 91.4 [32; 3; 0] |
| 48. | Delayed sequence induction is suggested in agitated and uncooperative patients for adequate pre-oxygenation | 71.9 [23; 3; 6] |
| 49. | Routine use of cricothyroid pressure cannot be recommended | 81.3 [26; 2; 4] |
| 50. | A “modified RSI” can be applied in patients at high risk of hypoxia who are not candidates for ATI | 85.7 [30; 5; 0] |
| Tracheal intubation | ||
| 51. | Devices with a standard Macintosh-type blade (allows direct and indirect laryngoscopy) are appropriate for AW management without predictors of difficulty, while those with a hyperangulated blade (without or with a guide channel) are indicated for known or anticipated DAW | 94.3 [33; 1; 1] |
| 52. | It is recommended that a bougie be available in all AW management sites | 97.1 [34; 1; 0] |
| 53. | Absence of capnography trace (ventilation grade 3) indicates failed TI until proven otherwise | 80 [28; 6; 1] |
| 54. | Capnography waveform monitoring during maintenance of mechanical ventilation is highly recommended in all sites | 100 [35; 0; 0] |
| Ventilation with supraglottic airway device | ||
| 55. | A SAD should be inserted without delay to preserve alveolar oxygenation in the event of a difficult or failed TI | 85.7 [30; 3; 2] |
| 56. | Immediate availability of a 2GSAD is recommended, as well as competence in its use in all AW management sites | 100 [35; 0; 0] |
| 57. | Cricoid pressure should be released during insertion of a SAD if it is being used | 80 [28; 5; 2] |
| 58. | 90 ° rotation, jaw-thrust, and DL or VL (of choice) with the "insert-detect-correct-as-you-go" technique increase the efficacy and safety of the SAD by facilitating insertion, increase first-attempt success rate, and reduce pharyngeal trauma | 82.9 [29; 4; 2] |
| 59. | FOB-guided TI through the SAD may be chosen if the situation is stable, under adequate NMB and if the operator is competent in the technique | 97.1 [34; 1; 0] |
| Ventilation with face mask | ||
| 60. | For FMV it is recommended at the beginning to use the optimal technique (triple manoeuvre of full neck extension, anterior jaw thrust, and mouth opening, placement of oropharyngeal or nasopharyngeal cannula and two-handed V-E technique, in a patient with optimal positioning and strong NMB | 80 [28; 3; 4] |
| 61. | Declaration of failed FMV implies immediate transition to SADV | 85.7 [30; 2; 3] |
| Front-of-neck Access | ||
| 62. | Failure of all 3 non-invasive plans (primary and rescue), regardless of SpO2value,requires verbalisation of the need for and subsequent FONA | 90.6 [29; 0; 3] |
| 63. | Cricothyrotomy is the technique of choice in a CICO situation | 91.4 [32; 2; 1] |
| 64. | The scalpel-bougie-tube surgical technique is recommended for cricothyrotomy | 91.4 [32; 2; 1] |
| 65. | A FONA should be feasible wherever AW is managed | 100 [35; 0; 0] |
| 66. | Emergency cricothyrotomy should be converted to an ETT or tracheostomy because there is insufficient evidence for it as long-term management | 85.7 [30; 3; 2] |
| 67. | Failure of a cricothyrotomy to secure the AW makes it advisable for tracheostomy to be performed by a skilled operator | 94.3 [33; 1; 1] |
| 68. | Any AW management practitioner must acquire and maintain the necessary competency to perform a surgical or percutaneous cricothyrotomy with Seldinger’s technique | 100 [35; 0; 0] |
| Cuff pressure monitoring | ||
| 69. | Cuff pressure must be established at the minimum pressure necessary to ensure an effective and safe seal. Pressure should remain between 20−30 cm H2O for ETT and tracheostomy and cricothyrotomy tubes, and<60 cm H2O for SADs | 94.3 [33; 1; 1] |
| Extubation | ||
| 70. | Any reintubation can be considered potentially difficult as its management involves additional complexity | 97.1 [34; 1; 0] |
| 71. | Leak testing, preferably quantitative, ultrasound assessment and laryngeal visualization with VL or FOB can facilitate decision-making | 97.1 [34; 1; 0] |
| 72. | Awake extubation with use of advanced techniques is the most appropriate method for DAW | 94.3 [33; 2; 0] |
| 73. | Capnography should be available in recovery units and used in high-risk cases | 97.1 [34; 1; 0] |
| Documentation | ||
| 74. | A history of failure of previous procedures is the most accurate predictor of failure in subsequent management | 97.1 [34; 1; 0] |
| Management in the field of the airway | ||
| 75. | Location of an AW leader in each institution is recommended | 100 [35; 0; 0] |
| Teaching and training | ||
| 76. | Competency acquisition should be gradual, through a cognitive phase, simulation, and clinical training with problem-solving until the learning curve is complete, with assessment and feedback from the instructor at each phase | 100 [35; 0; 0] |
| 77. | Ongoing teaching and regular training are required for the development of new skills or techniques and the maintenance of competencies, preferably on an annual basis | 97.1 [34; 1; 0] |
- •
Manuel Á. Gómez-Ríos: drafting of the manuscript, preparation of all cognitive aids and graphic material, tables and annexes, literature review, critical reading, levels of evidence, final revision of the document.
- •
José Alfonso Sastre: Draft of RSI sections, SAD, and checklist, risk factor tables, information document models, literature review, critical reading, levels of evidence, final revision of the document.
- •
Xavier Onrubia-Fuertes: FONA contribution, unanticipated difficult tracheal intubation, literature review, final revision of the document.
- •
Teresa López: Draft of SAD and ECMO sections, literature review, critical reading, levels of evidence, final revision of the document.
- •
Alfredo Abad-Gurumeta: literature review, critical reading, levels of evidence, final revision of the document.
- •
Rubén Casans-Francés: literature review, critical reading, final revision of the document.
- •
David Gómez-Ríos: literature review, critical reading, final revision of the document.
- •
José Carlos Garzón: literature review, critical reading, final revision of the document.
- •
Vicente Martínez-Pons: Algorithm for unanticipated difficult tracheal intubation, literature review, final revision of the document.
- •
Marta Casalderrey-Rivas: literature review, critical reading, final revision of the document.
- •
Miguel Ángel Fernández-Vaquero: Algorithm for unanticipated difficult tracheal intubation, literature review, and critical reading aimed at AW predictors and assessment, final revision of the document.
- •
Eugenio Martínez-Hurtado: Algorithm for unanticipated difficult tracheal intubation, final revision of the document.
- •
Ricardo Martín-Larrauri: Algorithm for unanticipated difficult tracheal intubation, final revision of the document.
- •
Laura Reviriego-Agudo: Algorithm for unanticipated difficult tracheal intubation, literature review, final revision of the document.
- •
Uxía Gutierrez-Couto: literature search strategies.
- •
Javier García-Fernández: critical reading, final revision of the document.
- •
Alfredo Serrano Moraza: prehospital setting section, literature review, critical reading, final revision of the document.
- •
Luis Jesús Rodríguez Martín: prehospital setting section, final revision of the document.
- •
Carmen Camacho Leis: prehospital setting, final revision of the document.
- •
Salvador Espinosa Ramírez: prehospital setting, final revision of the document.
- •
José Manuel Fandiño Orgeira: critical reading, final revision of the document.
- •
Manuel José Vázquez Lima: critical reading, final revision of the document.
- •
Miguel Mayo-Yáñez: FONA contribution, final revision of the document.
- •
Pablo Parente-Arias: FONA contribution, final revision of the document.
- •
Jon Alexander Sistiaga-Suárez: critical reading, final revision of the document.
- •
Manuel Bernal-Sprekelsen: critical reading, final revision of the document.
- •
Pedro Charco-Mora: coordination, algorithm for unanticipated difficult tracheal intubation, draft ergonomic options, draft teaching and training, literature review, critical reading, final revision of the document
MAGR received lecture honoraria from Medtronic.
XOF received honoraria for lecture and practical workshop on neuromuscular blockade from Merck Sharp &Dohme.
RCF received honoraria for lectures from FreseniusKabi.
AAG received honoraria for lectures from Merck Sharp&Dohme and 3 M Edwards.
Delphi expert panelManuel Á. Gómez-Ríos, José Alfonso Sastre, Xavier Onrubia-Fuertes, Teresa López, Alfredo Abad-Gurumeta, José Carlos Garzón, Vicente Martínez-Pons, Marta Casalderrey-Rivas, Miguel Ángel Fernández-Vaquero, Eugenio Martínez-Hurtado, Ricardo Martín-Larrauri, Laura Reviriego-Agudo, Javier García-Fernández, Pedro Charco-Mora, Raquel García Álvarez, Alfredo Panadero Sánchez, Alejandra Prieto Gundín, María Luisa Santos Marqués, David Domínguez García, Irma María Barrio, Uxío García-Aldao, Aixa Espinosa, Carmen M. Holgado Pascual, Jesús Carazo Cordobés, Cristobal Añez Simón, Natividad Quesada Gimeno, Marina Gómez-Morán Quintana, Silvia Bermejo, Pilar Cabrerizo Torrente, Francisca Llobell, Roque J. Company Teuler, Teresa del Castillo Fernández de Betoño, Carlos González Perrino and Paola Hurtado.
External reviewersJaideep Pandit, Luis Gaitini, Tomasz Gaszyński and Pavel Michalek.
CollaboratorsYou can consult the list of collaborators in supplementary material.