Multisystem inflammatory syndrome in adults (MIS-A) is a rare but severe complication in adults infected with SARS-CoV-2. However, the pathophysiology remains elusive, as the limited number of reports preclude a broader understanding of this syndrome. We conducted this systematic review to explore the clinical spectrum of MIS-A, in particular its rheumatological manifestations. Meta-analyses of case-series were also performed. We identified 28 patients from 14 case reports and two case series of MIS-A. This disease occurred equally in both genders, with a mean age of 33+10 years old, and predominantly in those of African descent (40%). Rheumatological manifestations consisted of Kawasaki Disease (KD)-like symptoms. Ninety percent of patients had positive COVID-19 serology tests, while 48% of patients were negative for COVID-19 RT-PCR test. Twelve patients were admitted to ICU and unfortunately two died. Although the signs and symptoms of MIS mimicked KD, the gastro-intestinal findings were more prominent in the former group. The demographic make-up was also different, with MIS-A occurring mostly in those of African descent. Importantly, unlike their paediatric counterparts, the adult group did not have coronary artery abnormalities. Long-term monitoring is needed as safety data is scarce. Of note, although the prognosis of MIS-A is excellent, the life-threatening nature of this syndrome demands intensive care unit level of care and mechanical support. During the COVID-19 pandemic, a constellation of KD symptoms in an adult patient should alert the clinician to the possibility of MIS-A.
El espectro clínico del síndrome inflamatorio multisistémico en adultos (MIS-A) es una complicación rara, pero grave en adultos infectados por el coronavirus del síndrome respiratorio agudo grave de tipo 2. Realizamos una búsqueda bibliográfica en varias bases de datos, y también se hizo en metanálisis. Identificamos 28 pacientes de 14 informes de casos y 2 series de casos de MIS-A. Esta enfermedad se manifestó por igual en ambos sexos, con una edad media de 33+10 años, y se presentó predominantemente en afrodescendientes (40%). Las manifestaciones reumatológicas consistieron en síntomas similares a la enfermedad de Kawasaki (EK). El 90% de los pacientes tuvo pruebas positivas de serología de la enfermedad por el coronavirus de 2019 (COVID-19), mientras que el 48% dio negativo para la prueba de reacción en cadena de la polimerasa con transcriptasa inversa de la COVID-19. Doce pacientes ingresaron en la unidad de cuidados intensivos y, lamentablemente, 2 fallecieron. Encontramos que, si bien los signos y los síntomas de MIS imitaban a EK, los hallazgos gastrointestinales eran más prominentes en el primer grupo. Además, la composición demográfica fue asimismo diferente, con MIS-A que se presentó principalmente en afrodescendientes. Es importante destacar que, a diferencia de sus homólogos pediátricos, el grupo de los adultos no experimentó anomalías en las arterias coronarias. Se necesita un seguimiento a largo plazo, ya que los datos de seguridad son escasos. Es de destacar que, aun cuando el pronóstico de MIS-A es excelente, la naturaleza potencialmente mortal de este síndrome exige el nivel de atención y el soporte mecánico de la unidad de cuidados intensivos. Durante la pandemia por la COVID-19, una constelación de síntomas de EK en un paciente adulto debe alertar al médico sobre la posibilidad de MIS-A.
Since the emergence of coronavirus disease of 2019 (COVID-19) from Wuhan, China on December 31, 2019, this disease has become the deadliest pandemic in modern human history, with mortality worldwide has soared to more than two million people and affecting more than 100 million people.1 At first, in the early pandemic, this disease was thought to be innocuous in the pediatric population.2 Then, on April 7, 2020, it was found that COVID-19 was associated with life-threatening post-infectious disease in pediatric populations identified as pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) or multisystem inflammatory syndrome in children (MIS-C).3 Later, in June 2020, it was also identified to occur in the adult population known as MIS-A.4 Nevertheless, the pathophysiology remains elusive, as a limited number of reports preclude a broader understanding of this syndrome. Therefore, we conducted this systematic review to explore the clinical spectrum of MIS-A, precisely its rheumatological manifestations. Subsequently, we hoped that this finding would fill in, in part, the knowledge gap, and help us with generating hypotheses. Moreover, this article was previously presented as a meeting abstract at the “ReumatologiKlinikBandung 2021” event on February 14, 2021.
MethodsSystematic reviewWe performed a systematic review of the literature consisting of case reports and case series following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.5
Information sources and search strategyWe performed a literature search through Pubmed, EuropePMC, and EMBASE. Keywords used were “multisystem inflammatory disease in adults” OR “multisystem inflammatory syndrome in adults” OR “Kawasaki-like in adults” AND “COVID-19” OR “SARS-CoV-2” AND “rheumatic” OR “vasculitis” OR “arthritis” OR “rheumatoid” OR “autoimmune” OR “rash”. We finalized the search on 29 January 2021.
Inclusion and exclusion criteriaThe inclusion criteria were case reports or case series that reported clinical manifestations or characteristics of patients meeting the criteria of MIS-A.4 We exclude non-English articles, articles without pertinent data, and articles without full-text availability.
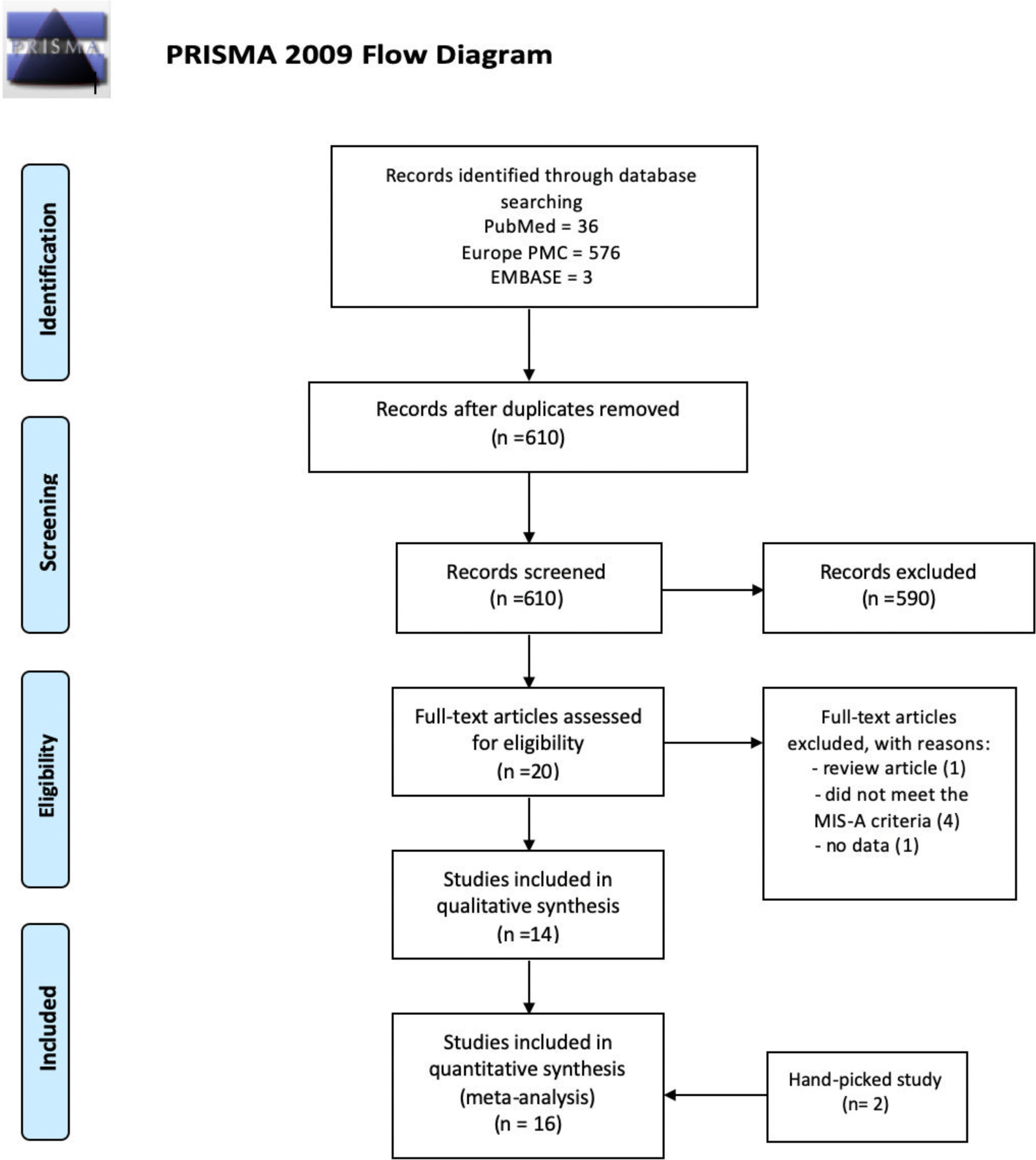
Study selectionTwo independent reviewers (SL and JH) screened the titles and abstracts for full-text eligibility and applied protocol inclusion and exclusion criteria to the full-text publication. Any discrepancies were discussed with third reviewers (AC). The study selection flowchart were shown in Fig. 1.
Data extractionWe collected the data regarding the first author name, country, study type, demographic characteristics (age, sex, ethnicity, comorbidities, body mass index [BMI]), SARS-CoV-2 RT-PCR and serological status, clinical manifestations, inflammatory markers (c-reactive protein [CRP], erythrocyte sedimentation rate [ESR], d-dimer, procalcitonin, fibrinogen, interleukin-6 [IL-6]), treatment characteristics, and outcomes. Outcomes consist of mortality, ICU admission, and length of hospital stay.
Risk of bias evaluationRisk of bias of included studies was evaluated by using Joanna Briggs Institute Critical Appraisal Checklist (Table S3). Each study was assessed for their risk of bias according to their type of studies. Each tool for case report and case series were consisted of eight and ten questions, respectively. Subsequently, overall evaluation of the bias assessment risk was interpreted with low risk of bias, high risk of bias, or unclear. Studies with suspected high risk of bias were excluded.
Meta-analysisMeta-analysis of proportions was performed to calculate the individual and pooled studies proportions of baseline characteristics, clinical findings, treatment, and outcome related to MIS-A. Random effect models was used to account heterogeneity between studies after applying the Freeman–Tukey Double Arscine Transformation of variances through R ver. 4.00 (R Foundation for Statistical Computing, Vienna, Austria) with the metaprop package. I2 was utilized to assess statistical heterogeneity. Finally, the meta-analyses was visualized with a forest plot generated through Microsoft Excel (Redmon, WA, USA).
ResultsIn total, we identified 28 patients from sixteen studies consisting of fourteen case reports and two case series that reported clinical manifestations of MIS-A.4,6–20 All of the extracted variables and risk of bias analysis are in the supplementary file.
Demographic characteristics of patients with MIS-AOf 28 patients with MIS-A, 14 (50%) were male. The mean age (SD) of patients with MIS-A was 33 (10) years. Of twelve studies that reported data on ethnicity (n=20), eleven (55%) were African, five (25%) were Latin or Hispanic, three (15%) were African, one (5%) was Pakistan, one (5%) was American, one (5%) was Caucasian, and one (5%) was Asian.4,6,20,21,8–10,12,13,17–19 The mean (SD) of BMI was 28.58 (5.6)kg/m2.6,9,12–14,17,19 Of 22 patients from ten studies, nine patients (40.9%) did not have any comorbidity, six (27.3%) were obese, four (18.2%) had hypertension, three (13.6%) had diabetes, two (9.1%) had blood disorder (sickle cell disease and thalassemia major), and one (4.5%) had asthma.4,6–9,12–14,18–20Table 1 depicts the demographic characteristic of patients with MIS-A in this study.
Demographic characteristic of MIS-A patients.
| Category | n/N | % | Mean (SD) |
|---|---|---|---|
| Age | 33 (10) | ||
| Sex | Male | 14/28 | 50 |
| Female | 14/28 | 50 | |
| Ethnicity | African | 11/20 | 55 |
| Latin/Hispanic | 5/20 | 25 | |
| Pakistan | 1/20 | 5 | |
| American | 1/20 | 5 | |
| Asian | 1/20 | 5 | |
| Caucasian | 1/20 | 5 | |
| BMI | 28.58 (5.6) | ||
| Comorbidity | DM | 3/22 | 13.6 |
| Hypertension | 4/22 | 18.2 | |
| Asthma | 1/22 | 4.5 | |
| Obesity | 6/22 | 27.3 | |
| Blood disorder (sickle cell & thalassemia minor) | 2/22 | 9.1 | |
| None | 9/22 | 40.9 | |
| SARS-CoV-2 | PCR (+) | 14/27 | 51.8 |
| Serology Ab IgG/IgM (+) | 18/20 | 90 |
Of 27 patients reported, fourteen (51.8%) had a positive test of SARS-CoV-2 reverse-transcriptase polymerase chain reaction (RT-PCR). Interestingly, ninety percent (n/N: 18/20) of the patients reported had a positive test of SARS-CoV-2 serology antibody.4,6–10,12–20
Clinical manifestations of MIS-AOf 28 patients reported, 21 patients (75%) came with fever. Besides, signs and symptoms of MIS-A from reported cases in this study could be divided into five major categories, including cardiovascular, respiratory, gastrointestinal, neuromusculoskeletal, and Kawasaki Disease-like symptoms.
The most common signs and symptoms from the first four categories were dyspnea without hypoxemia (N=9; 32.1%), diarrhea (N=13; 46.4%), fatigue or malaise (N=7; 25%), chest pain, and shock/hypotension (N=6; 21.4%, respectively). In addition, five typical signs and symptoms of KD occurred in patients with MIS-A, consisting of rash (N=13; 46.4%), conjunctival injection (N=11; 39.3%), lymphadenopathy (N=10; 35.7%), palmar erythema (N=4; 14.3%), and strawberry tongue (N=3; 10.7%). Nine patients showed complete KD's characteristic. All signs and symptoms reported in patients with MIS-A are available in Table 2.
Clinical manifestations of MIS-A patients.
| Sign and symptom | n (28) | % | |
|---|---|---|---|
| Fever | 21 | 75.0 | |
| Respiratory | Dry cough & sore throat | 8 | 28.6 |
| dyspnea | 9 | 32.1 | |
| Anosmia | 1 | 3.6 | |
| GIT | Abdominal pain | 6 | 21.4 |
| Diarrhea | 13 | 46.4 | |
| Nausea vomiting | 10 | 35.7 | |
| Neuromusculoskeletal | Myalgia | 4 | 14.3 |
| Fatigue/asthenia | 7 | 25.0 | |
| Headache | 6 | 21.4 | |
| Cardiovascular | Chest pain | 6 | 21.4 |
| Shock/hypotension | 6 | 21.4 | |
| KD like | Rash | 13 | 46.4 |
| Conjunctival injection | 11 | 39.3 | |
| Lymphadenopathy | 10 | 35.7 | |
| Palmar erythema | 4 | 14.3 | |
| Strawberry tongue | 3 | 10.7 | |
We pooled all of the inflammatory markers’ values in patients with MIS-A and presented the data in mean (SD). The amount of denominator in each inflammatory marker was not similar because not every case reported all of the inflammatory markers’ values. The overall pooled means of the inflammatory markers of MIS-A patients were increasing. Table 3 presented the pooled mean of the inflammatory markers in patients with MIS-A.
Inflammatory markers of MIS-A patients.
| Inflammatory marker (total patients) | Pooled mean | SD |
|---|---|---|
| ESR (mmHg) (4) | 64.8 | 41 |
| Ferritin (ng/mL) (25) | 3347.8 | 4455.3 |
| CRP (mg/dL) (25) | 13177.3 | 20744.6 |
| D-dimer (ng/mL) (22) | 2738.3 | 2182 |
| Procalcitonin (mg/L) (6) | 22221.5 | 25554.2 |
| Fibrinogen (ng/dL) (6) | 29656.5 | 22675.3 |
| LDH (u/L) (10) | 437.5 | 345.3 |
| IL-6 (pg/mL) (7) | 512 | 781.4 |
Treatment used among 25 patients reported were corticosteroid (n=12; 54.5%), intravenous immunoglobulin (n=9; 40.9%), anticoagulant (n=8; 36.4%), vasopressor and or inotropes (n=7; 31.8%), aspirin (n=5; 22.7%), antibiotics (n=4; 18.2%), and immunosuppressants consisting of tocilizumab (n=3; 13.6%), cyclosporine, and eculizumab (n=1; 4.5%, respectively). A full description of treatment characteristics is available in Table 4.
Treatment characteristic of MIS-A patients.
| Treatment | N (28) | % |
|---|---|---|
| Corticosteroid | 12 | 54.5 |
| IVIG | 9 | 40.9 |
| Anticoagulant (Heparin & LMWH) | 8 | 36.4 |
| Vasopressor & inotropes (dobutamine, norepinephrine, midodrine) | 7 | 31.8 |
| Aspirin | 5 | 22.7 |
| Antibiotic | 4 | 18.2 |
| Tocilizumab | 3 | 13.6 |
| Mechanical ventilation | 3 | 13.6 |
| Cyclosporine | 1 | 4.5 |
| Apixaban | 1 | 4.5 |
| Eculizumab | 1 | 4.5 |
| Convalescent plasma | 1 | 4.5 |
Of 20 patients, 12 (60%) were admitted to ICU. Unfortunately, of 22 patients hospitalized, two patients died (1.1%). The mean length of stay among 19 patients was 11 (6) days.
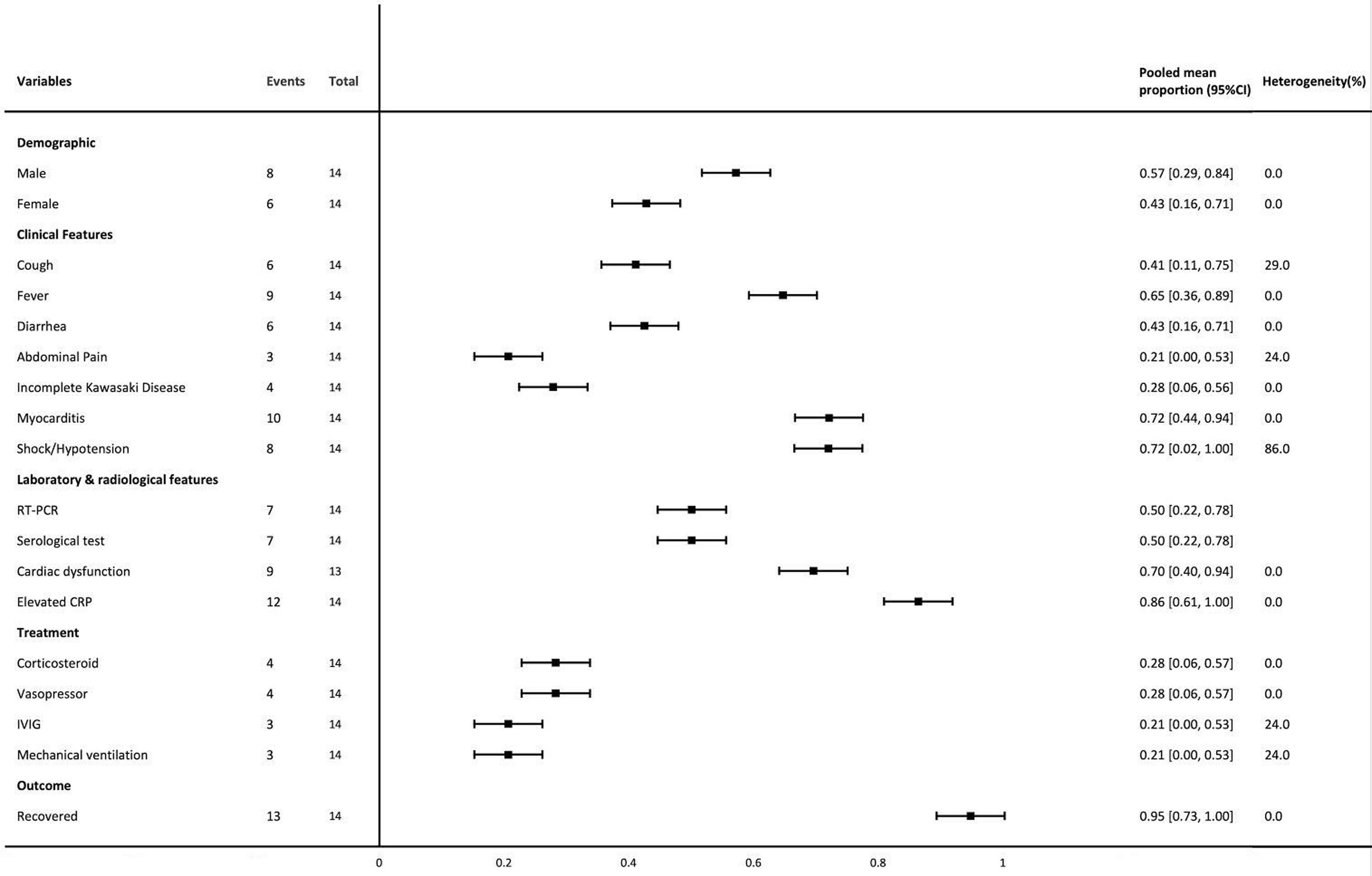
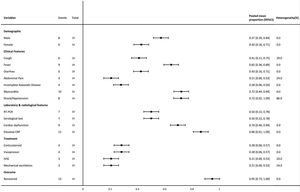
Meta-analysis resultsThe forest plot shows the baseline characteristics, clinical findings, treatment, and outcome related to MIS-A (Fig. 2).
Forest plot of meta-analyses of proportion. The meta-analyses of proportion included two case-series. Important variables that were not included e.g. ethnicity, are only available in one case-series, which preclude us from performing the meta-analysis. RT-PCR: reverse transcriptase – polymerase chain reaction, IVIG: intravenous immunoglobulin.
A hyperinflammation syndrome that manifests late in the course of SARS-CoV-2 infection was firstly reported in children and has become an identifiable syndrome. This condition was subsequently known as a multisystem inflammatory syndrome in children (MIS-C).22 Nevertheless, although initially it was only reported in children patients under 21 years of age, ever since June 2020, there were some case reports with similar conditions that occurred in adults, which was then called multisystem inflammatory syndrome in adults (MIS-A).4 However, interim guidance for MIS-A has not been elucidated yet since the clinical guidance released by the American College of Rheumatology was only limited to children.23
In this study, we identified sixteen studies consisting of fourteen case reports and two case series. In our study, the mean age (SD) was 33 (10) years old. Some studies reported cases of young adults who met the MIS-A criteria except for the age category were excluded from this study. Several ethnicities reported from this study were African-American (n/N=11/20; 55%), Latin or Hispanic (n/N=5/20; 25%), Caucasian (n/N=1/20; 5%), and Asian (n/N=1/20; 5%). This may show that although MIS-A was more commonly reported in African descent, it should have been a concern as this might occur in various ethnicities. However, ethnicity and genetic predisposition should further be explored. In contrast with acute COVID-19 infection which mostly occurred severely in patients with comorbidities, 40.9% (n/N=9/22) of MIS-A patients did not have any comorbidity. Nonetheless, almost 30% of the patients were obese, with the mean (SD) BMI of 28.58 (5.6) kg/m2, and the others had hypertension, diabetes, a blood disorder, and asthma.
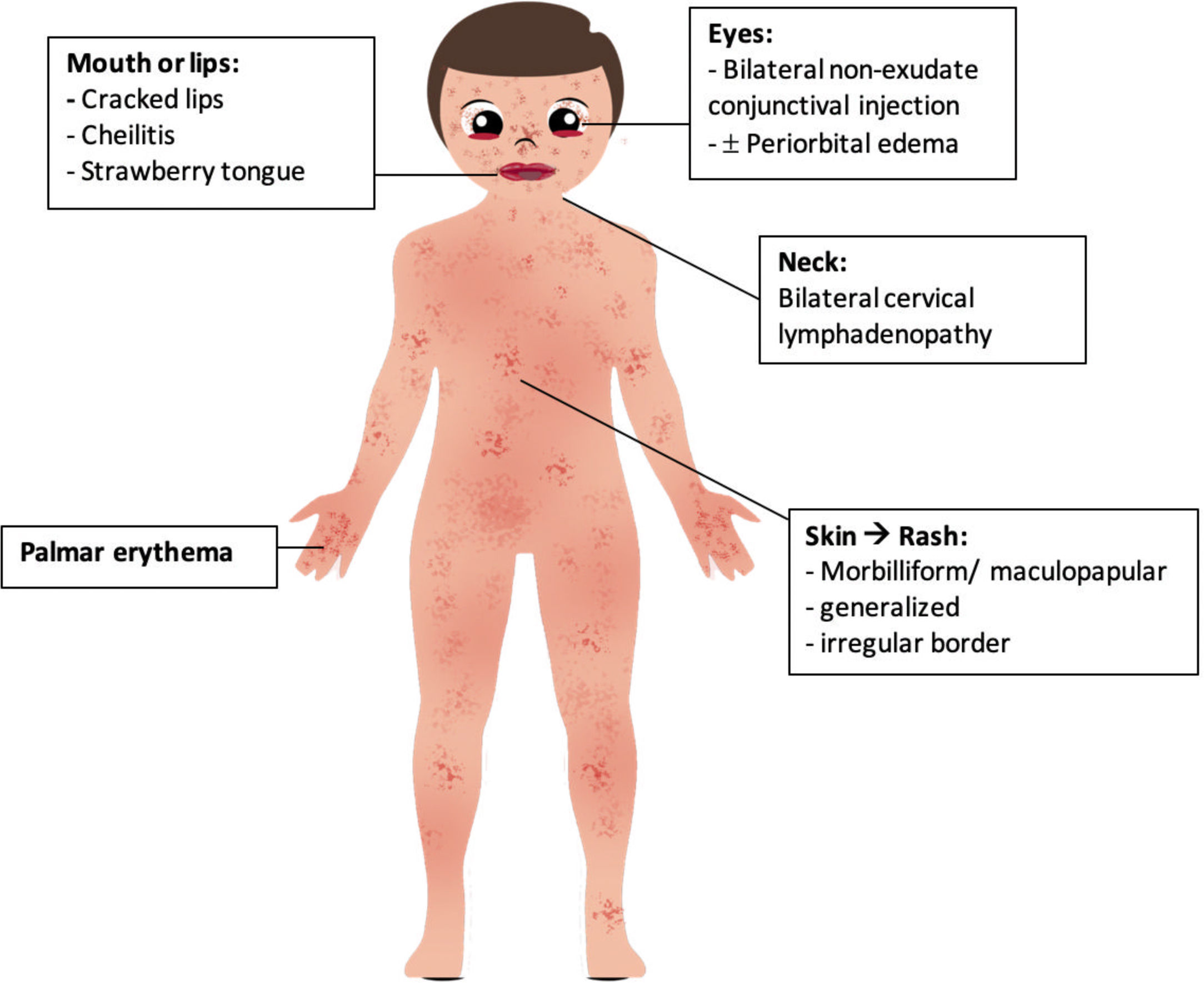
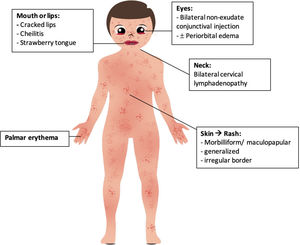
We collected all clinical manifestations of patients with MIS-A associated with COVID-19 and divided them into five main categories, including cardiovascular, respiratory, gastrointestinal, neuromusculoskeletal, and Kawasaki disease-like symptoms. Apart from nine patients who were reported to have classical KD's signs and symptoms, the remainder showed incomplete form of this disease. Five descriptions of KD-like symptoms in MIS-A including: (1) generalized morbilliform or maculopapular rash, evenly spread mostly in abdomen, upper extremities, back, pelvis, groin, scalp, or legs, with irregular borders, (2) bilateral conjunctivitis without exudation, sometimes associated with swollen eyelid or periorbital edema, (3) oral mucositis with cracked lips and/or strawberry tongue, (4) bilateral palmar erythema, and (5) cervical lymphadenopathy (Fig. 3). These manifestations appear in one17 to four10 days after the onset of the first symptom. Among all studies reported, fever was the most common complaint among all. We proposed these clinical spectrum subgroups to assist in refining the diagnostic criteria of MIS-A, considering that the preliminary case definition for this syndrome published by CDC is potentially nonspecific and may lead to misclassification.4
Kawasaki disease was initially thought to be generally rare in adults. Nevertheless, patients with MIS-A were reported for having KD-like symptoms. We found that although MIS's signs and symptoms mimicked KD, the gastrointestinal findings were more prominent in the former group. Importantly, unlike the pediatric counterparts, the adult group rarely experiences coronary artery abnormalities.14 Adults were more prone to experience myocarditis, which was proven by cardiac MRI and had moderate to severe impairment of left ventricular ejection fraction, which subsequently normalized but temporarily mandates intensive care support including vasopressors and inotropes.24 Notably, most of the patients did not develop any severe pulmonary manifestations or any primary viral pneumonia's features, suggesting that it was probably not in the continuum of a severe COVID-19 infection.14
The pathomechanism that underlies the occurrence of these symptoms has not yet been elucidated. Nonetheless, despite limited cases and literature reported related to MIS-A, we proposed three hypotheses to assist in defining the pathomechanism of this relatively new clinical syndrome. First, in line with MIS-C, this syndrome might be associated with a post-infectious state that occurs several weeks after getting primary SARS-CoV-2 infection.25 This study also corroborated this hypothesis as 90% of patients reported had a positive antibody serology test of SARS-CoV-2. No cases were reported before the commencement of the COVID-19 vaccine administration, so it can be assumed that a positive result in SARS-CoV-2 antibody serology was related to the previous primary infection. However, no quantitative titer was reported. Moreover, better improvement and favorable response to immunomodulation further suggest that MIS-A is driven by post-infectious immune dysregulation rather than by the direct acute infection of the virus itself. This post-infectious phenomenon are thought to be related with hyperinflammatory reaction due to antibody-mediated dependent enhancement.26,27 Prior SARS-CoV-2 infection results in the development of antibodies, which could probably bind to mast cells and trigger histamine release if they cross-reactivate with different SARS-CoV-2 strains.27 This might also be the culprit of such patients with positive test results of RT-PCR as well as serology. Such non-neutralizing antibodies trigger the Fcγ receptor of mast cells and eventually enhance disease severity and subsequently lead to cytokine storms. The release of histamine into the circulation will manifest symptoms or signs related to inflammation. It can also impede blood flow through capillaries by contracting the endothelial and pericyte cells, resulting in vasculitis, myocarditis, and edema of hands and feet, as described in MIS-A patients. However, this hypothesis is yet to be confirmed as if MIS is mediated by antibodies, there could be important implications for vaccine safety.28
Second, the coronavirus have an ability to block type I and type III interferon responses. Thus, patients with initially high viral load cannot overcome or control virus replication and could probably lead to excessive responses and delayed cytokine storm.26 Third, complement system activation might have a role in this multi-inflammatory syndrome. One histological study among a patient with MIS-A by Boudhabay et al found the presence of glomerular C3c and C5b9 deposits in addition with increasing levels of sC5b9. Moreover, improvement and symptoms resolution after administering Eculizumab, a monoclonal anti-C5 antibody which act by blocking the membrane attack complex (MAC) formation on the surface of endothelial cells, also suggest a probable role of complement system as one of MIS-A mechanism.9
As COVID-19 vaccine rollout is increasing throughout the world, clinicians should be cognizant of MIS-A's clinical spectrum because this syndrome is theoretically a potential side effect of COVID-19 vaccination that cannot be confidently ruled out, especially for vaccines which mechanism of action rely on stimulating B lymphocytes. Moreover, the post-vaccination antibody titer that will rise or increase after two doses of injection will obscure MIS-A patients’ identification. Nonetheless, regardless of this possibility, the benefit of COVID-19 vaccination will likely transcend its harm.
Managing MIS-A will need to involve a multidisciplinary care team, with a rheumatologist at the forefront and lead the patient care, considering other autoimmune diseases that resemble this novel syndrome, such as the macrophage activation syndrome (MAS) or secondary hemophagocytic lymphohistiocytosis (HLH)18 and Kawasaki disease, as well as their experience on immunomodulators and immunosuppressants.
There were several limitations of this study. First, we could not rule out the possibility of selection bias, i.e., whether all cases or only a few selected individuals are described. In addition, we could not rule out ascertainment bias as we could not ensure whether all case reports or case series are self-reported or through clinical records or administrative codes, in which the latter are more reliable than the earlier. Finally, reliable conclusions could not be drawn, and more prospective observational cohort studies are needed. Nevertheless, our systematic review and meta-analysis compiles 16 studies, which will help with the rapid dissemination of these important findings. Further reports or studies related to the hyperinflammatory reaction among MIS-A patients are still needed to better elucidate its immunopathology and define the best treatment approach.
ConclusionRheumatologic spectrum of MIS-A mimics all typical signs of Kawasaki disease, with gastrointestinal and cardiovascular perturbations more prominent in the former than the latter. Therefore, amidst the COVID-19 pandemic, a constellation of KD symptoms in an adult patient should alert the clinician of the possibility of MIS-A.
FundingNone.
Conflict of interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
None.