Patients with rheumatic diseases have an increased cardiovascular risk due to systemic inflammation and endothelial dysfunction, which promotes accelerated atherosclerosis. One way to measure cardiovascular risk, is through the carotid intima-media thickness which is an inexpensive and non-invasive predictor of cardiovascular events.
ObjectiveTo examine the evidence to determine the usefulness of ultrasound in the diagnosis of sub-clinical atherosclerosis in rheumatic diseases assessed by carotid intima-media thickness (IMT).
Material and methodsA systematic literature search was performed, using electronic databases of PubMed, for articles published from January 2005 to May 2015, with no language restriction. Observational cohort studies that assessed the prevalence of sub-clinical atherosclerosis using the IMT were included.
ResultsA total of 56 studies were identified for analysis, with almost all (95.7%) reporting an increased IMT in relation to the control group.
ConclusionsPatients with rheumatic diseases have an increased cardiovascular risk assessed using IMT. This measurement, assessed by carotid ultrasound, may help detect the risk of sub-clinical cardiovascular disease in these populations, allowing to establish a therapeutic strategy to reduce the risk of morbidity and mortality in these patients.
Los pacientes con enfermedades reumáticas tienen un aumento del riesgo cardiovascular debido a inflamación sistémica y disfunción endotelial, lo que promueve una acelerada aterosclerosis. Una de las formas de medir el riesgo cardiovascular es a través del grosor de la íntima-media, evaluada por ultrasonido carotídeo, el cual es un predictor de acontecimientos cardiovasculares de bajo costo y naturaleza no invasiva.
ObjetivoRevisar la evidencia que describe la utilidad y valor del diagnóstico ecográfico de aterosclerosis subclínica en enfermedades reumáticas, evaluadas mediante el grosor de la íntima-media carotídea (GIMc).
Materiales y métodosSe realizó una revisión de la literatura de la base de datos electrónica PubMed. Se incluyeron artículos desde enero de 2005 a mayo de 2015, sin restricción de idioma. Se incluyeron estudios observacionales de cohorte que evaluaron la prevalencia de aterosclerosis subclínica mediante la medición del GIMc y estudios de metaanálisis. Se verificó la calidad metodológica de los artículos y se extrajo la información relevante de cada uno.
ResultadosSe identificaron 56 artículos que cumplieron los requisitos. El 95,7% coincidió con el aumento del GIMc en relación con el grupo control, como marcador predictivo de aterosclerosis subclínica.
ConclusionesLos pacientes con enfermedades reumáticas tienen un aumento del riesgo cardiovascular medido a través del GIMc, como lo muestran varios estudios. Esta medición realizada por ultrasonido carotídeo podría ayudar a detectar el riesgo de enfermedad cardiovascular subclínica en estas poblaciones, lo que permitiría al clínico implementar medidas terapéuticas para reducir el riesgo de morbimortalidad en estos pacientes.
Atherosclerosis is considered a pathological alteration of the arteries characterized by the abnormal deposit of lipids and fibrous tissue in the arterial walls, which disrupts the architecture and function of the vessels and reduces, variably, the blood flow to the myocardium.1,2 It is a progressive process that begins in adolescence, becoming clinical manifest at a later age or, on the contrary, it may occur in a subclinical manner.3
Systemic autoimmune diseases are related to a chronic inflammatory state, an increased cardiovascular risk and, therefore, to an increase in the rate of morbidity and mortality, both because of the active disease, and because of the prolonged use of steroids.4 Many of the cardiovascular risk factors, such as: smoking, arterial hypertension, dyslipidemia and altered vascular homeostasis, contribute to endothelial activation during the early stages of the development of atherosclerosis, resulting in an increase in the expression of adhesion molecules such as P-selectin, E-selectin, intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 on the endothelial cell surface, which leads to the union and, finally, promotes the adhesion of monocytes.5,6 After the transmigration in the vessel wall, the monocytes are converted into macrophages and subsequently into foam cells. The process continues with the accumulation of lipids, release of cytokines and proliferation of smooth muscle cells, generating the formation of atheromatous plaque.7,8
Endothelial activation, common in rheumatic diseases such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), Sjögren's syndrome (SS), systemic sclerosis (SSc), gout, osteoarthritis (OA) and vasculitis, among others, is probably induced by autoantibodies. For the specific case of vasculitis, anti-neutrophil cytoplasmic antibodies (ANCA) and, in particular, those directed against proteinase 3 (PR3-ANCA), can activate the endothelial cells as demonstrated in vitro.9 It also occurs in vivo, due to the increased serum levels of vascular cell adhesion molecule-1 and E-selectin, which are involved in the process of systemic inflammation, contributing to the progression of cardiovascular damage.10 In the case of SLE, it is suggested that an alteration in lipid oxidation plays an important role in atherogenesis, contributing to the activation of the immune system, triggering inflammatory markers involved in the formation of the atheromatous plaque.11 In RA have been documented a variable number of factors for the development of atherogenesis, such as homocysteine, altered serum lipoprotein levels and prolonged pharmacological therapy. Recent findings indicate that the systemic inflammation may contribute to the development of atherosclerosis and confer an additional risk for cardiovascular death in patients with RA. The C-reactive protein has been considered as an independent prognostic marker for cardiovascular disease in these patients.12 For that purpose, the studies have demonstrated a close association between inflammation markers such as C-reactive protein, erythrocyte sedimentation rate and the markers for atheromatous cardiovascular disease (endothelial dysfunction and increased intima-media thickness of the carotid artery) in patients with these systemic autoimmune diseases, which significantly increases the prevalence of cardiovascular disease, as it has been reported in RA by 50%, similar to SLE with a risk of coronary heart disease, in women aged between 35 and 44 years, 50 times higher than in control subjects of the same age, and for ANCA-associated vasculitis with a higher than expected risk of 2.1 (CI 1.4–3.0), for cardiovascular events in the first 5 years of diagnosis.13,14
This review aims to examine the evidence that describes the usefulness and value of the echographic diagnosis of subclinical atherosclerosis in rheumatic diseases assessed by carotid IMT.
Measurement of the intima-media thicknessOne of the ways to measure cardiovascular risk is through the intima-media thickness by ultrasonography, which is a predictor of cardiovascular events.15 Ultrasound B-mode is a non-invasive technique that allows the visualization of the walls of superficial arteries such as the carotid branches.15 IMT has been widely used in the evaluation of atherosclerotic progression; it has been demonstrated that the increased thickness is associated with a higher prevalence and severity of coronary and cerebrovascular diseases.16,17
Epidemiological studies and clinical-therapeutic works of regression with lipid-lowering drugs have established that the carotid IMT is a marker sensitive to changes, valid for assessing the progression and regression of atherosclerotic disease, which would be the most appropriate method to evaluate the efficacy of pharmacological and non-pharmacological interventions, since it provides objective data on the changes in the arterial wall, data of risk and health of a specific population, to favor the implementation of preventive measures to decrease morbidity and mortality and improve the prognosis and, consequently, the patients’ quality of life.18,19
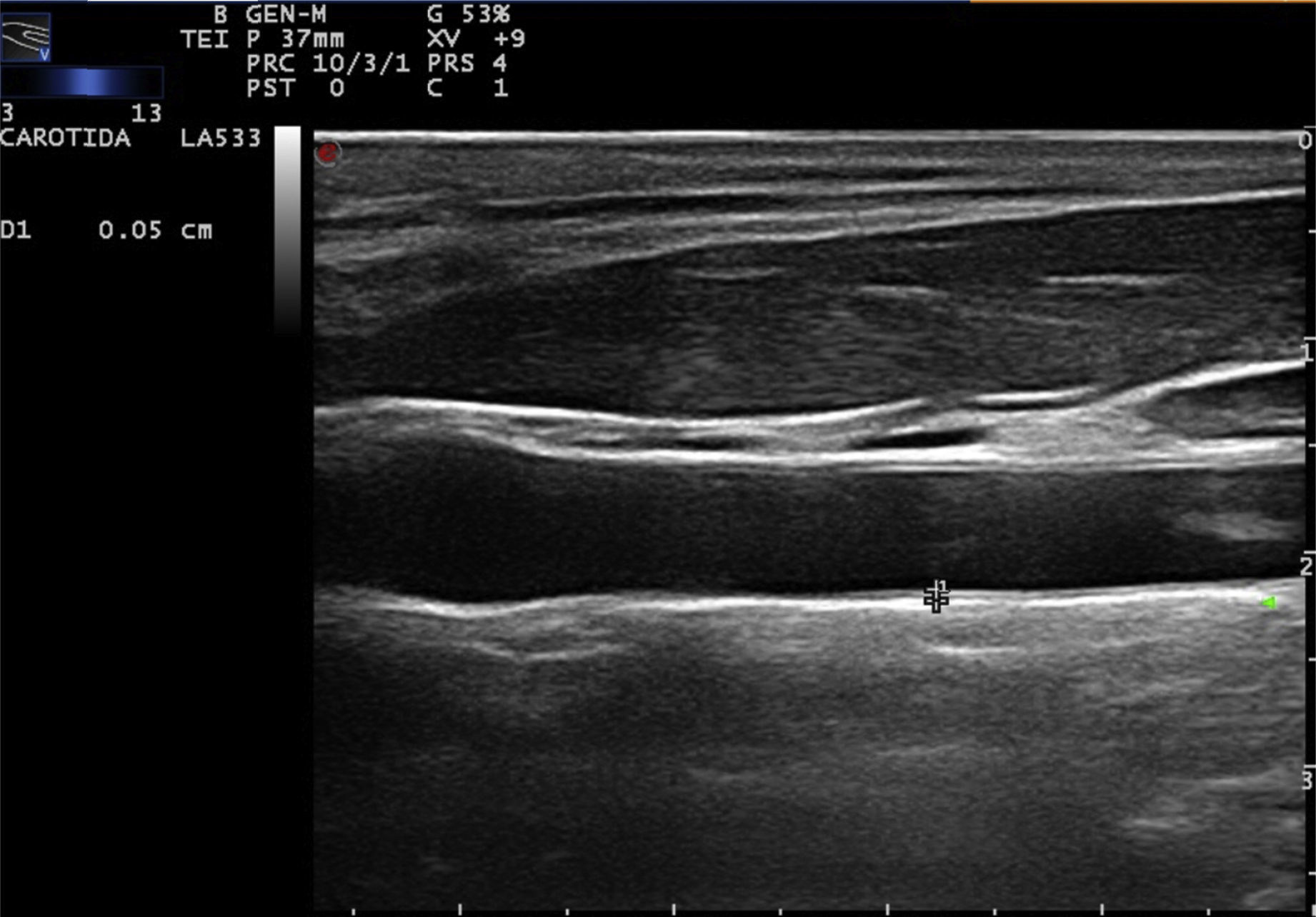
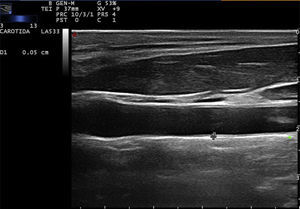
Carotid ultrasound based on radiofrequencyConventionally, the measurement of the intima-media thickness is carried out by the manual tracing of the interfaces between the layers of arterial tissue (Fig. 1). It should be noted that this method requires high competence, appropriate training and a level of specialization in the technique. As a general rule, mainly cardiologists or radiologists perform this type of examination. Despite the conventional approach to evaluate the carotid IMT, there are still some issues that must be overcome, such as the wide intra and inter-observer variability, which depends on the experts judgment and on the time it takes to perform the measurements of the carotid IMT.20
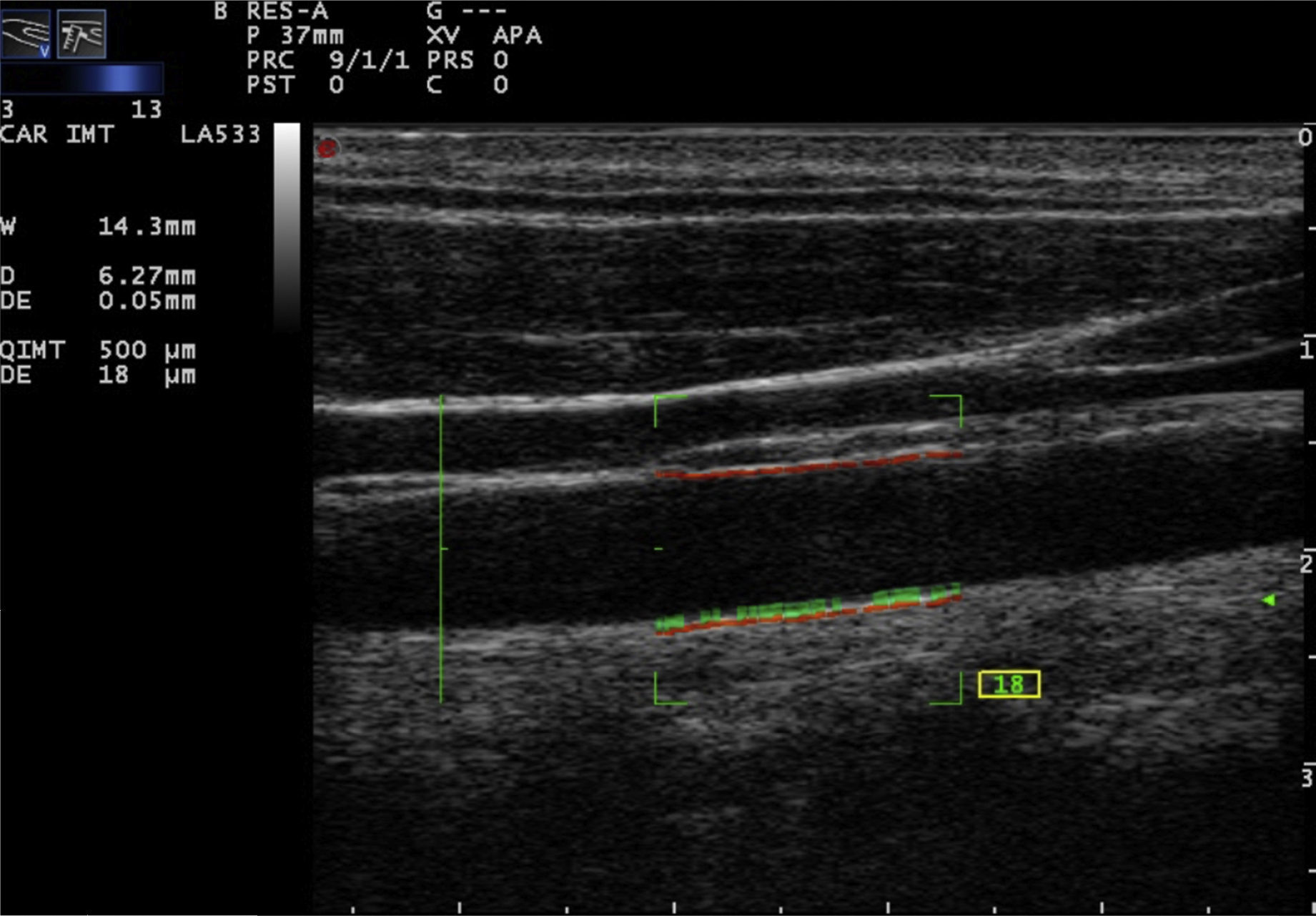
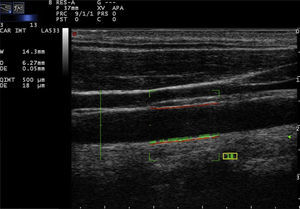
Recently, the technique of radiofrequency-based carotid ultrasound has been applied (Fig. 2). This method appears to be less dependent on the experience of the vascular ultrasound operator, since it provides an automatic measurement of the thickness of the interfaces of the arterial wall. Naredo et al. conducted a study whose objective was to assess the reliability of the automated radiofrequency-based ultrasound, comparing it with the B-mode of the conventional method, in patients with RA, demonstrating that the technique is valid, reproducible and reliable (intra-observer 0.61 and inter-observer 0.85) for the assessment of cardiovascular risk by the rheumatologist.21
Meanwhile, Di Geso et al. also compared both methods in 32 patients with chronic inflammatory rheumatic diseases. An expert cardiologist used the conventional manual method and a rheumatologist used the automated method for measuring the intima-media thickness. A good concordance between the two methods (0.69 and 0.77, for the right and left common carotid artery, respectively) was observed. Substantially good concordances were identified between the evaluators who adopted the conventional manual technique and the automated software.22
More recently, Naredo et al. conducted a prospective, longitudinal, multicenter study to compare the carotid IMT, evaluated by the automated method in 94 patients with RA treated with synthetic disease-modifying antirheumatic drugs (DMARDs) vs. biological DMARDs, compared with 94 controls. The carotid IMT was significantly greater in the patients with RA treated only with synthetic DMARDs than in the controls (591.4 vs. 562.1; p=0.035) and in patients treated with synthetic plus biological DMARDs vs. controls (591.4 vs. 558.8; p=0.040). The results suggest that carotid ultrasound can show significant differences between patients with RA, depending on the treatment they receive.23 The advantage of the automated method is that is faster than the conventional. The inter-observer variation for the detection of plaques is variable, and depends on the level of preparation of the sonographer and the quality of the ultrasound image.24
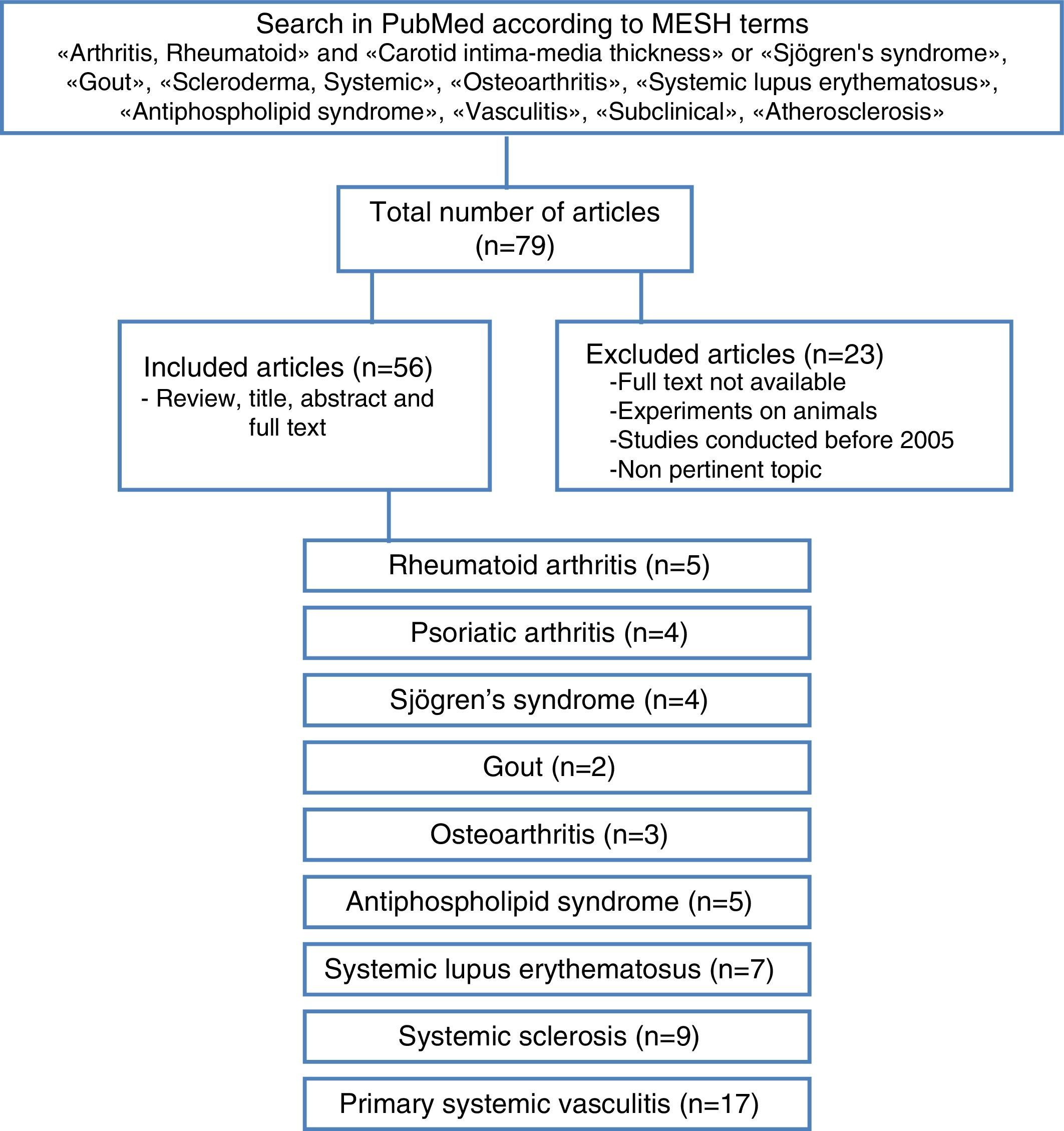
Materials and methodsA literature review of observational cohort studies and meta-analyses published between January 2005 and May 2015, with no language restrictions, was conducted in the database PubMed, including finally those in Spanish, English and Portuguese. The methodological quality of the articles was verified and the relevant information of each one was extracted. The articles in which the studies were not conducted in humans, the topic was not pertinent, the full text was not available or that have been published before 2005 were excluded. For the search were used MESH terms that included “arthritis, rheumatoid” and “carotid intima-media thickness” or “Sjogren's syndrome”, “gout”, “scleroderma, systemic”, “osteoarthritis”, “systemic lupus erythematosus”, “antiphospholipid syndrome”, “vasculitis”, “subclinical”, “atherosclerosis”.
The articles were selected by title and abstract, it was verified whether they were relevant and it was carried out the review of the full article. Using a collection format, from the selected articles were obtained relevant data regarding the carotid IMT and the formation of atheromatous plaques in each of the different rheumatic pathologies, compared with the control group (Fig. 3). The bibliographic citations of each article were reviewed looking for literature that was not recovered in the initial search.
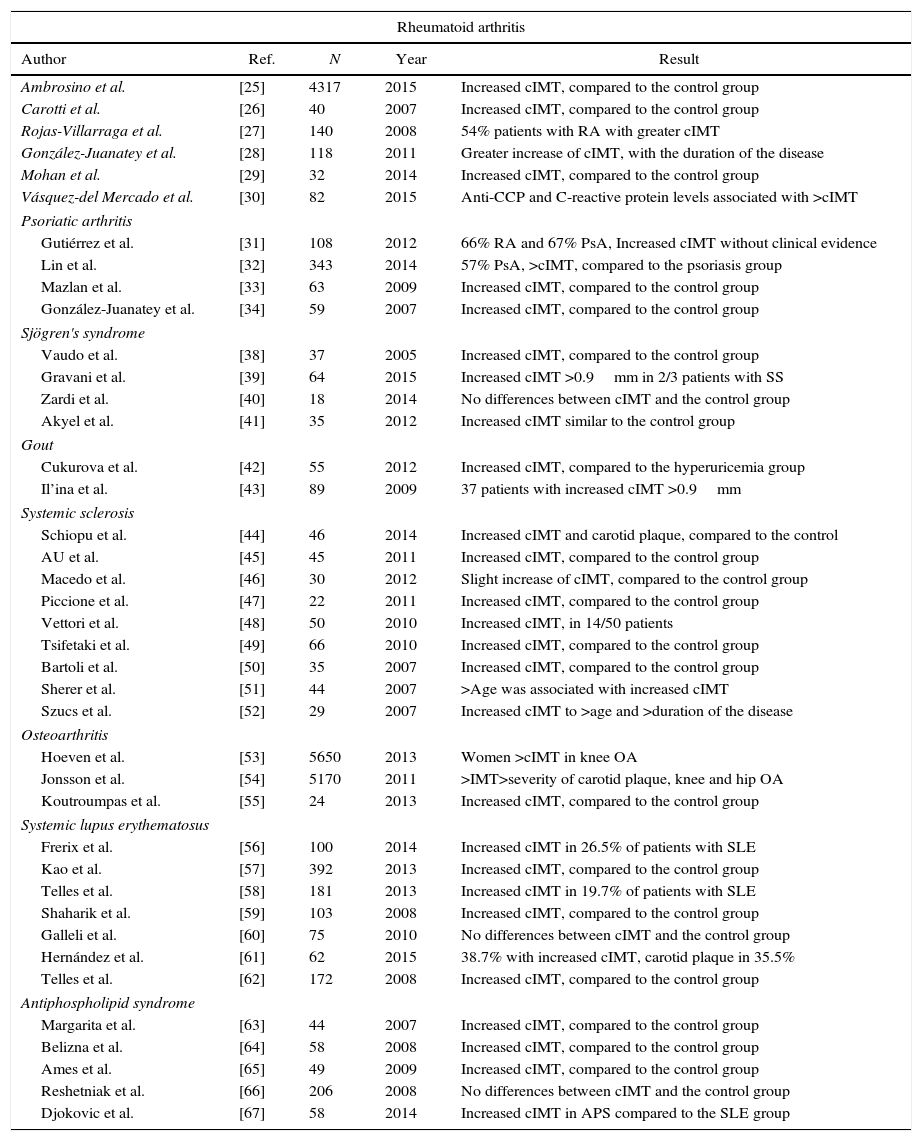
Carotid intima-media thickness in rheumatic diseasesThere are few studies in the literature regarding the measurement of carotid IMT in patients with rheumatic diseases, published in the last 10 years (Table 1).
Review of the medical literature of the last 10 years on the measurement of the carotid intima-media thickness (cIMT) as a predictive value for subclinical atherosclerosis in patients with rheumatic diseases.
| Rheumatoid arthritis | ||||
|---|---|---|---|---|
| Author | Ref. | N | Year | Result |
| Ambrosino et al. | [25] | 4317 | 2015 | Increased cIMT, compared to the control group |
| Carotti et al. | [26] | 40 | 2007 | Increased cIMT, compared to the control group |
| Rojas-Villarraga et al. | [27] | 140 | 2008 | 54% patients with RA with greater cIMT |
| González-Juanatey et al. | [28] | 118 | 2011 | Greater increase of cIMT, with the duration of the disease |
| Mohan et al. | [29] | 32 | 2014 | Increased cIMT, compared to the control group |
| Vásquez-del Mercado et al. | [30] | 82 | 2015 | Anti-CCP and C-reactive protein levels associated with >cIMT |
| Psoriatic arthritis | ||||
| Gutiérrez et al. | [31] | 108 | 2012 | 66% RA and 67% PsA, Increased cIMT without clinical evidence |
| Lin et al. | [32] | 343 | 2014 | 57% PsA, >cIMT, compared to the psoriasis group |
| Mazlan et al. | [33] | 63 | 2009 | Increased cIMT, compared to the control group |
| González-Juanatey et al. | [34] | 59 | 2007 | Increased cIMT, compared to the control group |
| Sjögren's syndrome | ||||
| Vaudo et al. | [38] | 37 | 2005 | Increased cIMT, compared to the control group |
| Gravani et al. | [39] | 64 | 2015 | Increased cIMT >0.9mm in 2/3 patients with SS |
| Zardi et al. | [40] | 18 | 2014 | No differences between cIMT and the control group |
| Akyel et al. | [41] | 35 | 2012 | Increased cIMT similar to the control group |
| Gout | ||||
| Cukurova et al. | [42] | 55 | 2012 | Increased cIMT, compared to the hyperuricemia group |
| Il’ina et al. | [43] | 89 | 2009 | 37 patients with increased cIMT >0.9mm |
| Systemic sclerosis | ||||
| Schiopu et al. | [44] | 46 | 2014 | Increased cIMT and carotid plaque, compared to the control |
| AU et al. | [45] | 45 | 2011 | Increased cIMT, compared to the control group |
| Macedo et al. | [46] | 30 | 2012 | Slight increase of cIMT, compared to the control group |
| Piccione et al. | [47] | 22 | 2011 | Increased cIMT, compared to the control group |
| Vettori et al. | [48] | 50 | 2010 | Increased cIMT, in 14/50 patients |
| Tsifetaki et al. | [49] | 66 | 2010 | Increased cIMT, compared to the control group |
| Bartoli et al. | [50] | 35 | 2007 | Increased cIMT, compared to the control group |
| Sherer et al. | [51] | 44 | 2007 | >Age was associated with increased cIMT |
| Szucs et al. | [52] | 29 | 2007 | Increased cIMT to >age and >duration of the disease |
| Osteoarthritis | ||||
| Hoeven et al. | [53] | 5650 | 2013 | Women >cIMT in knee OA |
| Jonsson et al. | [54] | 5170 | 2011 | >IMT>severity of carotid plaque, knee and hip OA |
| Koutroumpas et al. | [55] | 24 | 2013 | Increased cIMT, compared to the control group |
| Systemic lupus erythematosus | ||||
| Frerix et al. | [56] | 100 | 2014 | Increased cIMT in 26.5% of patients with SLE |
| Kao et al. | [57] | 392 | 2013 | Increased cIMT, compared to the control group |
| Telles et al. | [58] | 181 | 2013 | Increased cIMT in 19.7% of patients with SLE |
| Shaharik et al. | [59] | 103 | 2008 | Increased cIMT, compared to the control group |
| Galleli et al. | [60] | 75 | 2010 | No differences between cIMT and the control group |
| Hernández et al. | [61] | 62 | 2015 | 38.7% with increased cIMT, carotid plaque in 35.5% |
| Telles et al. | [62] | 172 | 2008 | Increased cIMT, compared to the control group |
| Antiphospholipid syndrome | ||||
| Margarita et al. | [63] | 44 | 2007 | Increased cIMT, compared to the control group |
| Belizna et al. | [64] | 58 | 2008 | Increased cIMT, compared to the control group |
| Ames et al. | [65] | 49 | 2009 | Increased cIMT, compared to the control group |
| Reshetniak et al. | [66] | 206 | 2008 | No differences between cIMT and the control group |
| Djokovic et al. | [67] | 58 | 2014 | Increased cIMT in APS compared to the SLE group |
| Primary systemic vasculitis | ||||
|---|---|---|---|---|
| Behçet's disease | ||||
| Author | Ref. | N | Year | Result |
| Ozturk et al. | [68] | 65 | 2015 | Increased cIMT, compared to the control group |
| Messedi et al. | [69] | 50 | 2011 | Increased cIMT, compared to the control group |
| Hong et al. | [70] | 40 | 2008 | Increased cIMT, compared to the control group |
| Ozturk et al. | [71] | 21 | 2008 | Increased cIMT, compared to the control group |
| Rhee et al. | [72] | 11 | 2007 | Increased cIMT with >age |
| Ozturk et al. | [73] | 34 | 2006 | Increased cIMT, compared to the control group |
| Keser et al. | [74] | 114 | 2005 | Increased cIMT, compared to the control group |
| Kawasaki disease | ||||
| Wu et al. | [75] | 35 | 2014 | Increased cIMT, compared to the control group |
| Meena et al. | [76] | 27 | 2014 | Increased cIMT, compared to the control group |
| Noto et al. | [77] | 18 | 2012 | Increased cIMT, compared to the control group |
| Cheung et al. | [78] | 72 | 2007 | Increased cIMT, associated with systemic arterial stiffness |
| Takayasu's arteritis | ||||
| Alibaz-Oner et al. | [79] | 32 | 2014 | Increased cIMT, compared to the control group |
| Schinkel et al. | [80] | 14 | 2014 | 50% had increased cIMT |
| Seth et al. | [81] | 56 | 2006 | Increased cIMT, compared to the control group |
| Granulomatosis with polyangiitis (GPA-Wegener) | ||||
| Nienhuis et al. | [82] | 28 | 2007 | Increased cIMT, compared to the control group |
| De Leeuw et al. | [83] | 29 | 2005 | Increased cIMT, compared to the control group |
| Giant cell arteritis | ||||
| Hafner et al. | [84] | 41 | 2014 | Increased cIMT in patients with rheumatic polymyalgia |
PsA: psoriatic arthritis; cIMT: carotid intima-media thickness; SLE: systemic lupus erythematosus; OA: osteoarthritis; SS: Sjögren's syndrome.
Patients with RA have a reduced life expectancy and a high cardiovascular morbidity and mortality compared with the general population. A meta-analysis analyzed the RA in relation with the markers of cardiovascular risk and the presence of carotid plaques, finding 51 studies with data of the carotid IMT (3600 patients with RA and 3020 controls) and 35 studies that reported on the prevalence of atheromatous plaques (2859 patients with RA and 2303 controls). RA patients showed a significant increase of the carotid IMT compared to the controls. The men had a more serious inflammatory state.25 On the other hand, Carotti et al. found an accelerated atherosclerosis by increase of the carotid IMT in 40 patients with RA, greater than in the control group.26 A Colombian study showed that 54% of patients with RA had a carotid IMT greater than 0.91mm correlating it with severe subclinical atherosclerosis, in the same way as the studies conducted by González-Juanatey, Mohan and Vázquez-Del Mercado, in which is also demonstrated an increase in the carotid IMT in this rheumatic pathology.27–30
Psoriatic arthritisGutiérrez et al. determined the prevalence of subclinical carotid atherosclerosis in 216 patients with RA or psoriatic arthritis, using the automated method; they observed that 66% with RA and 67% with psoriatic arthritis had a marked increase in the carotid IMT without clinical evidence.31 In another study was evaluated the relationship of the metabolic syndrome and the carotid IMT between 343 patients with psoriasis or psoriatic arthritis. 42.2% of patients with psoriasis and 57% with psoriatic arthritis had a higher prevalence of metabolic syndrome and measurements of the carotid IMT greater than patients with psoriasis, which contributes to an increased risk of cardiovascular disease.32
Mazlan et al. studied the carotid IMT and the cardiovascular risk factors in a population of 63 patients with psoriatic arthritis, using the conventional method, demonstrating a significant association between the cardiovascular risk and the increase in the carotid IMT, however, there was no association with the state of disease activity.33 González-Juanatey et al. demonstrated a higher prevalence of subclinical atherosclerosis in 59 patients with psoriatic arthritis, with respect to the controls.34
Sjögren's syndromeThere are some data on the risk of cardiovascular events in SS, in relation to the presence of valvular heart diseases, pericardial effusion, pulmonary hypertension, but few with measurement of the carotid IMT.35 Bartoloni et al. retrospectively analyzed a cohort of 1343 patients which was compared with a subgroup of 788 female patients, aged between 35 and 74 years and 4774 healthy women of the same age, finding a higher prevalence of cardiovascular risk factors such as systemic arterial hypertension and hypercholesterolemia.36 Likewise it is demonstrated in the study conducted by Kang et al., where hyperlipidemia and cardiac arrhythmias are the leading causes of comorbidity in patients with SS.37
In the last 10 years, 4 studies have been reported concerning the measurement of the carotid IMT. The first 2 were conducted by Vaudo et al. and Gravani et al. who demonstrated an increase in the carotid IMT compared to the control group.38,39 In contraposition with 2 studies carried out by Zardi et al. and Akyel et al. in which there was no significant difference in terms of the carotid IMT with respect to the control group.40,41
GoutWith regard to gouty arthritis, there are only 2 reports in the literature related with the evaluation of the carotid IMT and subclinical atherosclerosis. Cukurova et al. studied 55 patients with gout, finding an increase in the carotid IMT compared to the group of patients with asymptomatic hyperuricemia. Il’ina et al., in a population of 89 patients with gout, demonstrated that 37 had atheromatous plaques.42,43
Systemic sclerosisPatients with SSc have a higher prevalence of formation of atheromatous plaques compared to the control group, as demonstrated by Schiopu et al. In contrast, in another study was demonstrated a slight increase in the carotid IMT with respect to the control group, but without statistical significance.44
In a systematic review were included 14 studies that evaluated the carotid IMT, reporting a high prevalence of subclinical atherosclerosis in these patients.45–47 In the last 10 years have been reported in the literature several studies that analyze the increase in the carotid IMT in patients with SSc, as a predictive marker of subclinical atherosclerosis. A study conducted by Vettori et al. reported subclinical atherosclerosis in 14/50 patients, correlating the carotid IMT with the old age and the prolonged use of corticosteroids.48 Tsifetaki et al. also described in a population of 60 SSc patients a higher prevalence of carotid IMT compared with the control group.49 Bartoli et al. demonstrated that the carotid IMT is an indicator of subclinical cardiovascular disease in their population of 35 patients with SSc, where the carotid IMT is greater when compared with the control group, like in the study conducted by Sherer et al.50,51
Szucs et al. demonstrated that the age and the duration of the disease are directly proportional to the increase of the carotid IMT.52
OsteoarthritisThere were found 3 studies, 2 of them with a representative sample. In the ROTTERDAM study that included 2372 men and 3278 women, it was observed an increase of the carotid IMT particularly in women with knee OA, and also increased formation of carotid plaques in patients with OA of the metacarpophalangeal and distal interphalangeal joints.53 The AGES-REYKJAVIK study analyzed a population of 2195 men and 2975 women with hip and knee OA, finding that patients with total joint replacements showed increased severity in terms of formation of carotid plaques.54 Finally, a small study conducted by Koutroumpas et al., showed in 24 patients an increase of the carotid IMT in erosive OA.55
Systemic lupus erythematosusThere are several studies that assess the increase of the carotid IMT in this disease, most of them have demonstrated its increase compared to the controls. The study conducted by Frerix et al., with a population of 100 patients with SLE and 90 with SSc, reveals the existence of subclinical atherosclerosis measured by the carotid IMT in 26.5% of patients with SLE, compared with 28.9% in the SSc group.56 Kao et al. demonstrate that patients who have had cardiovascular incidents have increased carotid IMT and formation of carotid plaques compared with the lupus population without previous cardiovascular events.57
Telles et al. evaluated the progression of the atherosclerosis in a cohort of 181 lupus patients, of whom 157 were reevaluated after 39 months, demonstrating in 19.7% an increase of the carotid IMT.58 The increase in the IMT was also detected in 15.4% of patients with lupus nephritis.59 Only one study did not show significant differences regarding the carotid IMT vs. the control group.60 As for the formation of atheromatous plaques, 2 studies reported its increase, being the most recent of them conducted by Hernández et al., which showed that 35.5% of the SLE population had carotid plaques.61,62
Antiphospholipid syndromeWith respect to this syndrome, only 5 publications were found, 3 of them reported a marked increase in the carotid IMT compared to the control group, in contraposition with the study conducted by Reshetniak et al., which did not show significant differences.63–66. In a comparative study between antiphospholipid syndrome and SLE, was evidenced that the antiphospholipid syndrome exhibits a greater increase of the carotid IMT (48.3%) than SLE, however, no significant relationship was established between the type of antiphospholipid antibodies and the carotid IMT changes.67
Primary systemic vasculitisIn relation to primary systemic vasculitis, there are few studies. The most studied pathologies in the last 10 years, in order of frequency are: Behçet's disease,68–74 Kawasaki disease,75–78 Takayasu's arteritis,79–81 GPA-Wegener and, finally, giant cell arteritis.82,83 For Takayasu's arteritis, a study conducted by Seth et al., suggests that the carotid IMT might be a possible marker of disease activity. In this study, 74 common carotid arteries were evaluated in 37 patients with Takayasu's arteritis and 28 healthy controls, correlating their data with the presence of activity according to clinical criteria. The results found were a greater carotid IMT in 59% of the studied population, being prevalent in patients who exhibited disease activity, having the measurement of the carotid IMT a sensitivity of 82% and a specificity of 60%, as an activity marker.77
Studies with ANCA-associated small vessel vasculitis of the eosinophilic granulomatosis with polyangiitis type (Churg-Strauss) and microscopic polyangiitis are not found. In all studies analyzed there is a marked increase in the carotid IMT compared to the control group.84
ConclusionPatients with rheumatic diseases have an increased cardiovascular risk assessed by the carotid IMT, as demonstrated by several studies. This measurement could help to detect the risk of subclinical cardiovascular disease in these populations, which would allow the rheumatologist, in the clinical practice, to implement opportune therapeutic measures to reduce the morbidity and mortality, which already are increased. The automated radiofrequency-based ultrasound technique is still little used in rheumatology, however, it has been demonstrated that it is useful, valid, accessible, reproducible and reliable, so it is expected that in the future the evaluation of cardiovascular risk in rheumatic patients will be part of the quotidian assessment.
FundingNone.
Conflict of interestThe authors declare that they have no conflict of interest.
Please cite this article as: Saldarriaga Rivera LM, Ventura Ríos L, Hernández Díaz C, Pineda Villaseñor C. Medición del grosor de la íntima-media carotídea: utilidad y diagnóstico ecográfico de aterosclerosis subclínica en enfermedades reumáticas. Revisión de la literatura. Rev Colomb Reumatol. 2016;23:92–101.