The habit of consuming energy drinks is a life-threatening risk, because it can produce a syndrome characterised by a muscle necrosis. This promotes the release of enzymes and myoglobin from inside the myocyte into the circulation, creating lipid peroxidation and leading to acute kidney injury, and hyperkalaemia, together producing rhabdomyolysis. Rhabdomyolysis can be expected to be found within 24–48 h after strenuous activities, even more so with a modified BORG index greater than or equal to 5 points. However, energy drinks, due to their high content of caffeine and other components, can generate this little known adverse event.
Clinical caseA 37-year-old patient admitted to the emergency department due to clinical symptoms of myalgia, and dark urine that appeared 24 h after four days of low-intensity muscular exercises, and was associated with daily consumption of an energizing drink for 2 years. The diagnosis of rhabdomyolysis was confirmed by increased creatine kinase and transaminases. It was not possible to measure myoglobin levels. The patient was treated with aggressive fluid therapy. He never presented with any renal or electrolyte complications.
ConclusionThis case highlights the appearance of acute rhabdomyolysis in patients taking low intensity exercises, and not ruling out long-term consumption of energy drinks as the main cause. There are few cases currently reported in the literature. Owing to the timely treatment, progression to acute kidney injury was avoided.
un riesgo potencialmente mortal es el hábito de consumo de bebidas energizantes, porque puede producir un síndrome caracterizado por la necrosis muscular que promueve la liberación de enzimas y mioglobina proveniente del interior del miocito hacia la circulación, creando una peroxidación lipídica llegando a generar lesión renal aguda e hiperkalemia; conocido como rabdomiólisis. La rabdomiólisis la esperaríamos encontrar entre 24 a 48 horas después de actividades extenuantes, aún más con índice de BORG modificado mayor o igual a 5 puntos; sin embargo, las bebidas energizantes por su alto contenido de cafeína y otros componentes pueden generar este evento adverso poco conocido.
Caso clínicopaciente de 37 años, obeso, que ingresó al servicio de urgencias por cuadro clínico de mialgia y orina oscura que aparece 24 horas después de cuatro días de ejercicio muscular de baja intensidad, asociado a consumo diario de bebida energizante por 2 años. El diagnóstico de rabdomiólisis se confirma por hiperCKemia e hipertrasaminemia; no fue posible medir los niveles de mioglobina. El paciente fue tratado con fluidoterapia agresiva. Nunca presentó complicaciones renales ni hidroelectrolíticas.
Conclusiónnuestro caso destaca la aparición de rabdomiólisis aguda en pacientes sometidos a ejercicios de baja intensidad no descartando como causa principal el consumo crónico de bebidas energizantes. Son pocos casos actualmente reportados en la literatura. Gracias al tratamiento oportuno se evitó la progresión a lesión renal aguda.
Due to the high content of caffeine and other ingredients, energy drinks may lead to a potential life-threatening event known as rhabdomyolysis. The condition is defined as a syndrome exhibiting muscular necrosis and the release of intracellular muscular components into the circulation,1–7 characterized by elevated creatine-kinase (CK) levels, myalgia and myoglobinuria.8 The severity of the disease ranges from asymptomatic to lethal, because of electrolyte imbalance and acute renal injury.9 Muscle pain is present in over 50% of the patients, as well as weakness and edema of the affected muscles; dark color urine (myoglobinuria) presents in over 90% of patients.10–12
The most affected muscle groups are the gastrocnemius and the lower back muscles.13–15
The mechanism whereby the symptoms develop is the depletion of adenosine-triphosphate (ATP) and the breakdown of the sarcolemma as a result of oxidative stress, which increases intracellular calcium and consequently promotes the release of intracellular toxic substances into the extracellular fluid.16–20 There are several risk factors that may contribute to the development of the syndrome. 21,22 The data from clinical trials on energy drinks-associated rhabdomyolysis have not been thoroughly summarized to guide the optimal therapy for this population. There are few studies and it is believed that the underlying cause is the high caffeine content in those energy drinks.23–25 In case of clinical suspicion of rhabdomyolysis, laboratory tests should be conducted including CK serum level, myoglobin, lactate dehydrogenase (LDH), uric acid (UA), renal function tests, urine test strip to identify myoglobinuria and urinary sediment analysis. Urine drug screening test should be considered to identify any drugs that may cause rhabdomyolysis, and electrocardiography (EKG) if hyperkalemia is suspected.26–30 A muscle biopsy may be indicated in acute rhabdomyolysis in suspicious cases of genetic or idiopathic inflammatory myopathy.31–33 Currently, the initial treatment focuses on treating the trigger; there is no medical consensus for treatment and only aggressive fluid therapy with isotonic saline is suggested to maintain a urinary output between 1−3 cm3/kg/h and dialytic therapy as needed.34–37
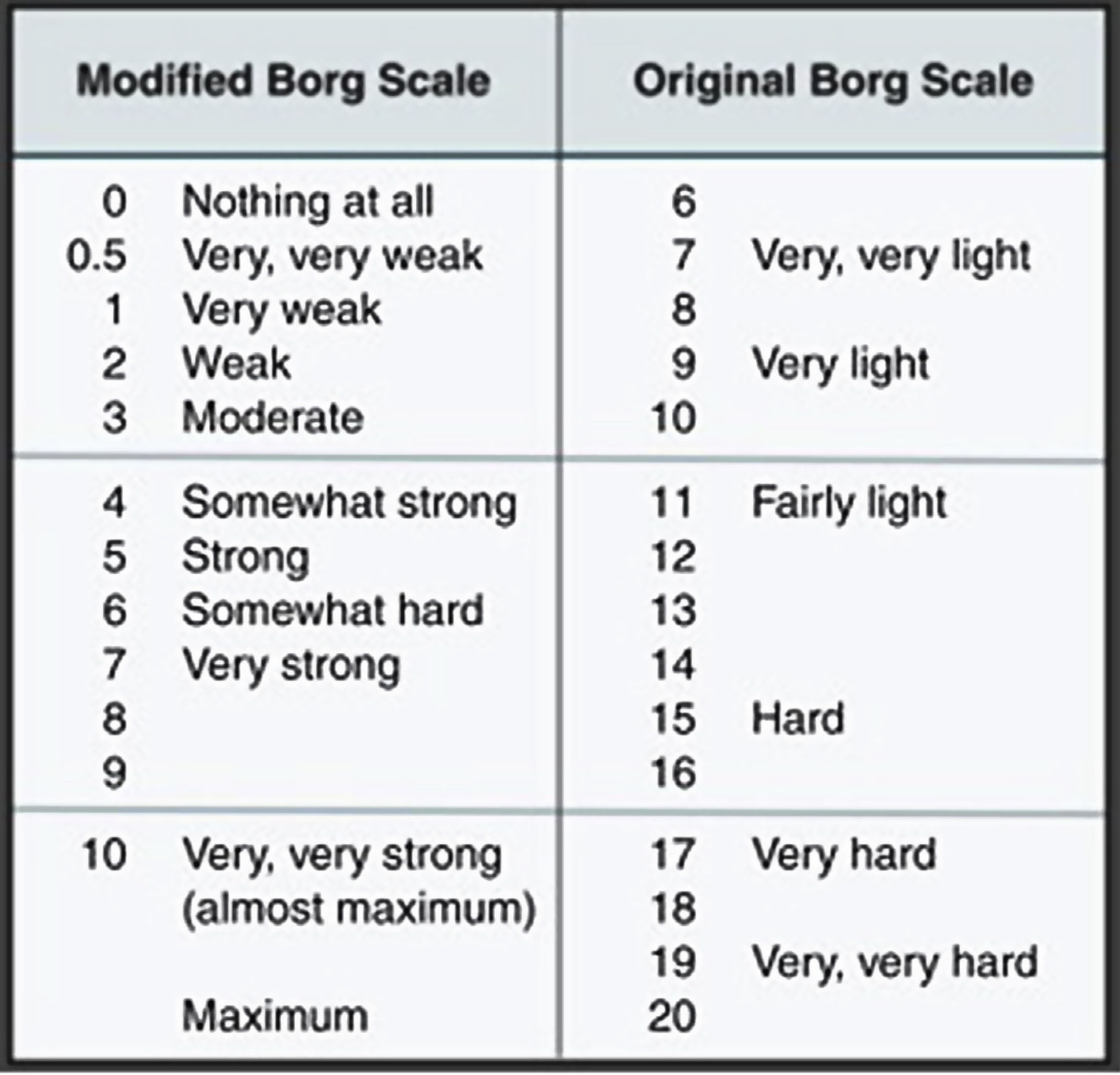
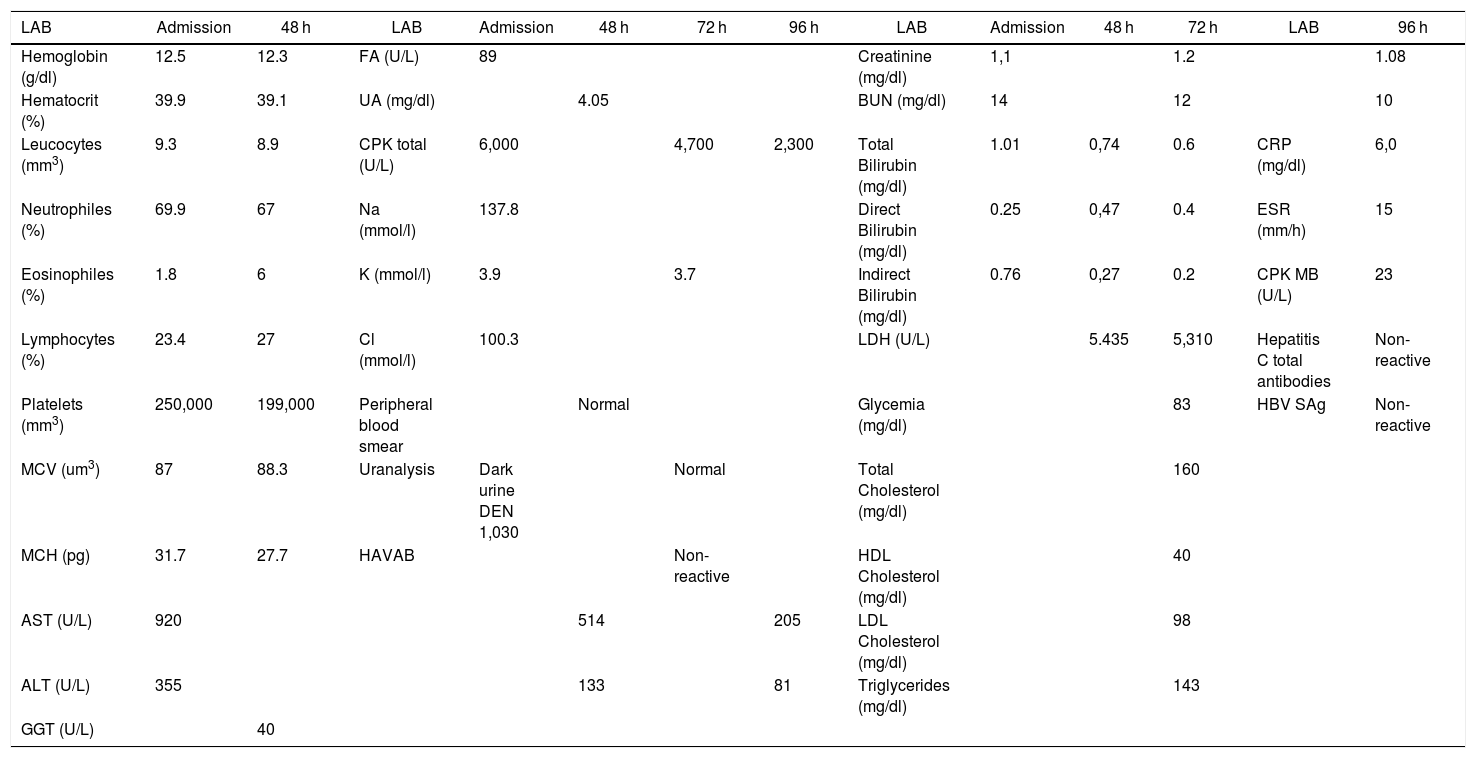
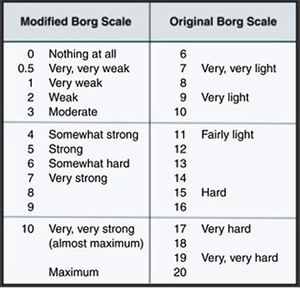
Clinical caseThis is a 37-year-old black male patient, business administrator from Cartagena city, Colombia. The patient has a history of allergic rhinitis since childhood but currently is not being treated; has obesity grade II based on the WHO classification, and is a chronic user of energy drinks – 2, 400 ml bottles per day for the last two years (he said he just likes drinking them) – for an average intake of 240 mg/L of caffeine per day. He does not go to the gym and hasn’t practice any routine exercise for the last 2 years. He said he does not drink any alcohol or smoke. The patient came to the myalgia clinic after 4 days of physical exercise, and was classified based on the subjective perceived exertion scale (modified Borg) with 2 points (Fig. 1).38 Additionally, his urine was dark. The physical examination revealed bilateral pain on touching the gastrocnemius, biceps and brachial triceps with no clinical signs of compartment syndrome. There were no hematomas and the vital signs were within the normal ranges, with no fever. The paraclinical tests revealed elevated transaminases AST 920 U/L and ALT 355 U/L, with otherwise normal blood test, renal function and bilirubin; the uranalysis reported dark color, turbid, elevated density of 1030, pH 5, and negative nitrites, ketones, bilirubin, leucocytes and RBC. Based on these results, additional tests were ordered because of the presence of signs and symptoms compatible with rhabdomyolysis secondary to mild intensity exertion and chronic consumption of energy drinks. Paraclinical tests to investigate other causes for the elevated transaminases, the muscle pain and dark urine were prescribed, including blood tests for hepatotropic viruses and imaging studies: total abdomen ultrasound focusing on the hepatobiliary study to analyze the liver morphology, rule out steatosis or obstructive masses. The complementary paraclinical tests included lipid profile and fasting glucose to screen for metabolic syndrome; EKG, chest X-ray, renal function tests, uric acid and ionogram to rule out rhabdomyolysis complications. creatine-phosphokinase (CPK) 6.000 U/L, creatinine 1,1 mg/dl, BUN 14 mg/dl, uric acid 4.05 mg/dl, Na 137.8 mmol/l, K 3.9 mmol/l, Cl 100.3 mmol/l, Ca ion 1.07 mmol/l, LDH 5435 U/L, normal arterial blood gases. Control tests were order after 24 and 96 h depending on half-life (Table 1) (taken from the medical record). The total abdominal ultrasound and EKG were normal.
Paraclinical tests ordered during hospitalization.
| LAB | Admission | 48 h | LAB | Admission | 48 h | 72 h | 96 h | LAB | Admission | 48 h | 72 h | LAB | 96 h |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hemoglobin (g/dl) | 12.5 | 12.3 | FA (U/L) | 89 | Creatinine (mg/dl) | 1,1 | 1.2 | 1.08 | |||||
| Hematocrit (%) | 39.9 | 39.1 | UA (mg/dl) | 4.05 | BUN (mg/dl) | 14 | 12 | 10 | |||||
| Leucocytes (mm3) | 9.3 | 8.9 | CPK total (U/L) | 6,000 | 4,700 | 2,300 | Total Bilirubin (mg/dl) | 1.01 | 0,74 | 0.6 | CRP (mg/dl) | 6,0 | |
| Neutrophiles (%) | 69.9 | 67 | Na (mmol/l) | 137.8 | Direct Bilirubin (mg/dl) | 0.25 | 0,47 | 0.4 | ESR (mm/h) | 15 | |||
| Eosinophiles (%) | 1.8 | 6 | K (mmol/l) | 3.9 | 3.7 | Indirect Bilirubin (mg/dl) | 0.76 | 0,27 | 0.2 | CPK MB (U/L) | 23 | ||
| Lymphocytes (%) | 23.4 | 27 | Cl (mmol/l) | 100.3 | LDH (U/L) | 5.435 | 5,310 | Hepatitis C total antibodies | Non- reactive | ||||
| Platelets (mm3) | 250,000 | 199,000 | Peripheral blood smear | Normal | Glycemia (mg/dl) | 83 | HBV SAg | Non- reactive | |||||
| MCV (um3) | 87 | 88.3 | Uranalysis | Dark urine DEN 1,030 | Normal | Total Cholesterol (mg/dl) | 160 | ||||||
| MCH (pg) | 31.7 | 27.7 | HAVAB | Non- reactive | HDL Cholesterol (mg/dl) | 40 | |||||||
| AST (U/L) | 920 | 514 | 205 | LDL Cholesterol (mg/dl) | 98 | ||||||||
| ALT (U/L) | 355 | 133 | 81 | Triglycerides (mg/dl) | 143 | ||||||||
| GGT (U/L) | 40 |
Reference values: laboratory (LAB), hemoglobin (12–16 g/dl), hematocrit (36–50%), leucocytes (5,000–10,000 mm3), neutrophiles (50–70%), eosinophiles (0–3%), lymphocytes (25–40%), platelets (150,000–400,000 mm3), mean corpuscular value (MCV) (80–100 um3), mean corpuscular hemoglobin (MCH) (26–34 pg), aspartate aminotransferase (AST) (10–41 U/L), alanine aminotransferase (ALT) (5–37 U/L), gamma glutamyl transferase (GGT) (5–85 U/L), FA (U/L), uric acid (UA) (< 7,5 mg/dl), total creatine-phosphokinase (CPK) (70–110 U/L), sodium (Na) (135–145 mmol/l), potassium (K) (3.5–5.0 mmol/l), chlorine (Cl) (96–106 mmol/l), uranalysis urinary density (DEN) (1010–1020), hepatitis A virus antibodies (HAVAB), creatinine (0.7–1.2 mg/dl), urea nitrogen (BUN) (6–20 mg/dl), Total bilirubin (0.1–1.2 mg/dl), direct bilirubin (0.1–0.6 mg/dl), indirect bilirubin (0.1–1.2 mg/dl), lactate dehydrogenase (LDH) (115–225 U/L), glycemia (60–100 mg/dl), total cholesterol (< 200 mg/dl), High density lipoprotein (HDL) (> 40 mg/dl), low density lipoprotein LDL (< 100 mg/dl), triglycerides (< 150 mg/dl), C-reactive protein (CRP) (< 10 mg/dl), erythrocyte sedimentation rate (ESR) (0–15 mm/h), creatine-phosphokinase fraction MB (CPK MB) (0–25 U/L), total antibodies hepatitis C, Hepatitis B surface antigen (HBV SAg).
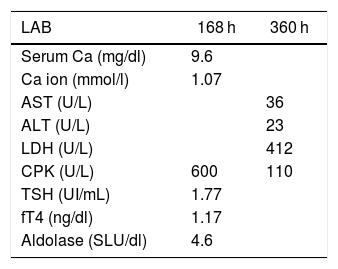
Aggressive fluid therapy was initiated with isotonic saline 1000 cm3/h for 2 h and then continued with 100 cm3/h for 72 h; the urinary output was maintained at 1−2 ml/kg/h; no urine alkalinization or electrolyte correction was conducted. The patient received analgesia for pain control with acetaminophen (1 g / 8 h). The paraclinical control tests never showed progression of the rhabdomyolysis to acute renal injury or hyperkalemic complications (Table 1) during hospitalization. Based on the patient’s good clinical and paraclinical evolution, the patient was discharged fifth days after hospital admission, emphasizing the importance of making life-style changes, diet and exercise. Control paraclinical tests were conducted 7 and 15 days after discharge, with no evidence of alterations (Table 2) (these were provided by the patient in an outpatient visit).
Paraclinical control tests after hospital discharge.
| LAB | 168 h | 360 h |
|---|---|---|
| Serum Ca (mg/dl) | 9.6 | |
| Ca ion (mmol/l) | 1.07 | |
| AST (U/L) | 36 | |
| ALT (U/L) | 23 | |
| LDH (U/L) | 412 | |
| CPK (U/L) | 600 | 110 |
| TSH (UI/mL) | 1.77 | |
| fT4 (ng/dl) | 1.17 | |
| Aldolase (SLU/dl) | 4.6 |
Reference values: Serum calcium (Ca) (9.1–10.2 mg/dl), calcium (Ca) ion (1.0–1.3 mmol/l), thyroid stimulating hormone (TSH) (0.4–4.7 UI/mL), free thyroxine (fT4) (0.72–1.46 ng/dl), aldolase (3–8.2 SLU/dl).
The definition of rhabdomyolysis is based on the physical examination and the medical record to identify any triggering risk factors. This particular patient presented symptoms of dark urine and myalgia with risk factors including obesity, long-term consumption of energy drinks, and physical inactivity. The consumption of energy drinks is very frequent among young adults between 11 and 35 years old, in order to enhance their tolerance to physical exercise. There is up to 57% addiction among this population with withdrawal symptoms.39 Our patient had been a chronic user for over two years of an energy drink containing 18 ingredients: taurine, caffeine, green tea, artificial and natural additives, and vitamins. The pharmacological action of caffeine is based on its ability to competitively antagonize the A1, A2A, A2B and A340,41 adenosine receptors, leading to the release of a large number of neurotransmitters.42,43 The intake of more than 200 mg of caffeine has been associated with adverse,44,45 and life-threatening effects at doses between 10 and 20g,46 inhibiting the phosphodiesterase enzyme activity, increasing the intracellular concentration of adenosine monophosphate, stimulating the skeletal muscles, boosting calcium release from the sarcoplasmic reticulum and generating tetanic contractions.47,48 Based on the above information, this patient consumed 240 mg of caffeine per day.
There are few reports on cases of rhabdomyolysis associated with the intake of caffeine. One of them is a case by Chiang et al., of a 44-year-old woman who presented with nausea, vomiting, palpitations and dark urine 6 h after drinking one liter of black coffee, resulting in elevated plasma CK level (7315 U/L).25 Our patient had similar CK values (6000 U/L), associated with myalgia and dark urine. Another ingredient is taurine, a cysteine-derived Sulphur compound that behaves as a neurotransmitter by reducing intracellular calcium induced by glutamate, inhibiting the release of cytochrome C and the apoptosis cascade; hence it is believed to improve endothelial vascular dysfunction, producing muscular necrosis and rhabdomyolysis.3,49,50
The expectation is that the rhabdomyolysis would be caused by strenuous exercise and ATP depletion leading to the release of intracellular contents of the damaged myocyte, which in turn may lead to renal failure and other systemic complications such as arrhythmia and disseminated intravascular coagulation.51–53 In contrast, our patient reported a modified Borg index of 2 points (low intensity exercise). There are no paraclinical tests able to confirm the causal relationship between the triad of long-term consumption of energy drinks, physical exercise and rhabdomyolysis. The risk factors that could account for this condition in our patient are: lack of physical training, exercising under extreme heat and humidity conditions (Cartagena city), obesity grade II and long-term consumption of energy drinks, as previously documented.52,54 Exercise-associated rhabdomyolysis may present in individuals with metabolic myopathy and should be suspected in patients with recurrent muscle symptoms during exercise.55
There are three major groups of metabolic myopathies: fatty acid β-oxidation disorders, muscular gluconeogenesis and mitochondrial diseases.56 Additionally, there are elevated transaminases, increased LDH and UA resulting from cellular lysis caused by the release of these enzymes, electrolytes, sarcoplasmic content and myoglobin.57 Besides, an LDH ≥ 2000 units and/or UA ≥ 6 mg/dl, regardless of the serum creatinine level, represents a high risk of developing acute renal injury as a result of rhabdomyolysis.58 Our patient had a high risk of acute renal injury due to his LDH levels.
Gagliano et al. reported the case of a 30-year-old male admitted to the hospital due to painful muscle swelling and dark urine, 24 h after low intensity exercise, with CPK of 70,962 UI/L. The patient was treated with IV sodium chloride and sodium bicarbonate and finally developed acute renal injury but survived.59 In our case, the patient had lower CPK values, did not develop acute renal injury and urine alkalinization was not required. It should be highlighted that the admission CPK level is a predictor of renal injury.60
The consumption of energy drinks is certainly a cause for silent morbidity and mortality. The high content of sugar increases the risk of metabolic syndrome, while the high content of caffeine involves a higher risk of cardiovascular dysfunction. However, probably the most severe negative effects are acute renal injury and electrolyte imbalance if a rhabdomyolysis syndrome develops. It should be kept in mind that this syndrome is more prevalent among individuals who exercise strenuously, but in our case there was a causal relationship. Energy drinks are frequently used by individuals who practice sports but it is not considered a risk factor and should alert us to suggest adequate hydration, warm-up exercises, and avoid using energy drinks.
ConclusionRhabdomyolysis is a clinical syndrome with a mortality of over 70% when associated with hyperkalemia, acidosis and acute renal injury. There are several risk factors and clinical triggers for the disease; the causes of hyperCKemia are heterogenous and the progression of the disease is predictable, based on the initial CPK values. The condition rarely presents after low intensity exercise, and the consumption of energy drinks has shown to be an associated risk factor. Timely aggressive fluid therapy improves the prognosis; urine alkalinization is not always necessary and in our case there were no complications due to hyperkalemia or acute renal injury.
FinancingNone.
Conflict of interestsNone.
Please cite this article as: Vergara Serpa OV, Reyes Jaraba CA, Cortina Gutiérrez A, Montoya Jaramillo ME, Echenique Torres OD. Rabdomiólisis inducida por consumo crónico de bebidas energizantes asociado a ejercicio físico de baja intensidad: reporte de caso. Rev Colomb Reumatol. 2021;28:145–151.