Although tuberculosis (TB) is endemic in Colombia, with a prevalence of 26 cases per million, there are no recent and clear data regarding the prevalence of latent tuberculosis (LBT) in the population with rheumatoid arthritis (RA), candidates for biotechnological therapy.
MethodologyA cross-sectional study with an analytical component to determine the prevalence of LBT in patients with RA who are candidates for biotechnological therapy.
ResultsThe prevalence of LBT in RA patients who are candidates for biotechnological therapy is high, 18.3% (95% CI: 14.7–21.9). In the exploratory analysis, a relationship between LBT and male gender was found (P < .001), as well as abnormal findings on chest radiography (P = .039), and smoking (P = .028).
ConclusionThe prevalence of LBT in patients with RA who are candidates for biotechnological therapy is high. Prospective studies are needed to evaluate the incidence of TB in this group of patients and corroborate this association.
Colombia es un país endémico para tuberculosis (TB), con una prevalencia de 26 casos por millón. Sin embargo, no se cuenta con datos recientes y claros respecto a la prevalencia de tuberculosis latente (TBL) en la población con artritis reumatoide (AR) candidata a terapia biotecnológica.
MetodologíaEstudio de corte transversal con componente analítico para determinar la prevalencia de TBL en pacientes con AR candidatos a terapia biotecnológica.
ResultadosLa prevalencia de TBL en pacientes con AR candidatos a terapia biotecnológica es alta: 18,3% (IC 95% 14,7–21,9), y en los cruces exploratorios se encontró una relación de TBL y la variable género masculino (P = <,001), hallazgos anormales en la radiografía de tórax (P =,039) y el tabaquismo (P =,028).
ConclusiónLa prevalencia de TBL en paciente con AR candidatos a terapia biotecnológica es alta. Se requieren estudios prospectivos para evaluar la incidencia de TB en este grupo de pacientes y así corroborar su asociación.
Tuberculosis (TB) affects one third of the world population, it is a global health emergency declared by the World Health Organization (WHO) since 1993, and Colombia is an endemic country for this disease. The incidence of all forms of TB in Colombia is 26 cases per 100,000 inhabitants,1 81% corresponds to the pulmonary form and the remaining 19% to extrapulmonary forms.2 In the specific case of latent tuberculosis (LTB), which occurs in people without signs or symptoms, there are usually no reports and the available epidemiological data on prevalence are mainly determined by research studies in special populations.3
There are multiple population groups considered at high risk, in which the prevalence of LTB and its progression to active TB is higher, among which the following stand out: healthy individuals in close contact with TB cases, healthcare workers, infection with human immunodeficiency virus (HIV), dialysis therapy, intravenous drug users, prisoners, transplant recipients, diabetes mellitus (DM), alcoholics, smokers, obese subjects and patients on therapy with anti-tumor necrosis factor agents (anti-TNF).3 There are few studies about the prevalence of LTB in patients with rheumatoid arthritis (RA) who will be taken to biotechnological therapy; in Latin America, most of the studies have been conducted in Brazil, an endemic country for TB, and have reported prevalence rates ranging from 4% to 32.7%.4,5
In endemic countries such as Colombia, a purified protein derivative (PPD) test is performed in all patients with RA who will receive biotechnological therapy, regardless of the mechanism of action of the drug that will be started. However, although some epidemiological data that estimate the incidence, prevalence and mortality of LTB on a worldwide scale are available, in Colombia there is not recent and sufficient information that allows to quantify and calculate the magnitude of this condition. Likewise, the behavior of LTB in population groups at risk, such as individuals with rheumatologic diseases, is not known. For this reason, this work seeks to determine the prevalence of LTB in a specific population with RA (patients who are candidates for biotechnological therapy).
MethodologyA cross-sectional study was conducted to determine the prevalence of LTB in a population with RA, whose members were candidates for initiation of biotechnological therapy, in two tertiary care clinical centers between 2015 and 2018.
The study included patients over 18 years of age with a diagnosis of RA, according to the criteria of the American College of Rheumatology (ACR) 2010, with a record of PPD previous to the initiation of the biotechnological therapy, a reported chest X-ray, and a record in the clinical history of the complete report of PPD. The exclusion criteria included the presence of autoimmune diseases other than Sjögren's syndrome, patients who had received treatment for LTB or with active TB before the PPD test was done, and patients with a diagnosis of active TB in the clinical history.
The diagnosis of LTB was determined with a result of the first recorded PPD larger than 5 mm, in the context of immunosuppressed patients, according with the current recommendations.6,7 In the former groups it should be an absence of symptoms or signs of active TB (absence of respiratory symptoms, normal chest X-ray or with a benign lung nodule or findings not suggestive of acute interstitial or alveolar involvement). The variables included in the study were: diameter of the PPD, value of the interferon gamma release assay (IGRA), findings in the chest X-ray, treatment and activity of the RA measured by the Disease Activity Score (DAS) 28, rheumatoid factor (RF), anti-citrulline antibodies (anti-CCP) and comorbidities.
The sample size was calculated with the formula for a confidence interval (CI) of a proportion; considering a population size of 2562 patients with RA, for an expected proportion of 50%, a confidence level of 95% and an absolute accuracy of 5%, and a minimum of 335 subjects was required. Initially, it was performed a simple random sampling of the list of clinical records obtained in the search in the information system. The randomized medical records that did not meet the inclusion criteria were replaced.
The data were collected directly from the clinical records of the patients, by trained personnel with knowledge of the diagnosis and management of the study pathology. These data were anonymously transcribed into an electronic form, which finally transferred them into a Microsoft Excel spreadsheet.
Data analysisOnce the data collection was completed, they were analyzed with the SPSS20 program, licensed by the University of La Sabana, Bogotá, Colombia. A descriptive analysis was conducted, in which the qualitative variables were summarized in frequencies and percentages and the quantitative variables in means and standard deviation, if their distribution was normal, or in median and interquartile range if the distribution was non-normal.
A PPD larger than 5 mm was considered a positive case and the number of positive cases was divided by the total of subjects evaluated for the calculation of the prevalence. Likewise, the prevalence of LTB by comorbidity (DM) and cigarette smoking was described, for which additional exploratory crosses between these variables and the gender, radiological findings and treatment with or without the presence of LTB were conducted. For these purposes, a statistically significant P-value less than .05 was considered and the confidence interval for the overall prevalence was calculated.
The authorization for the development of this study and the review of the medical records were evaluated and approved by the Research Committee of the University of La Sabana and the ethics committees of each institution.
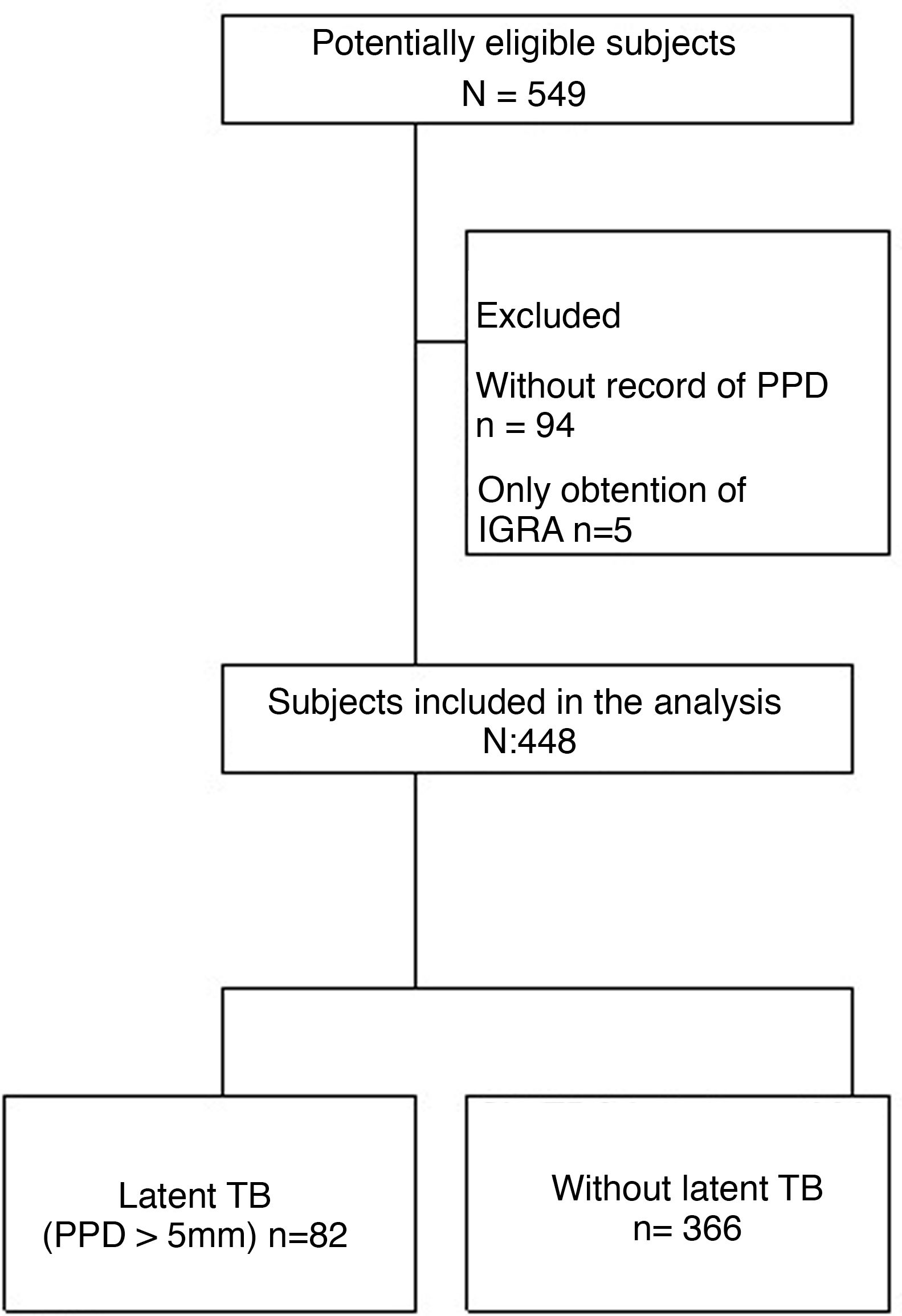
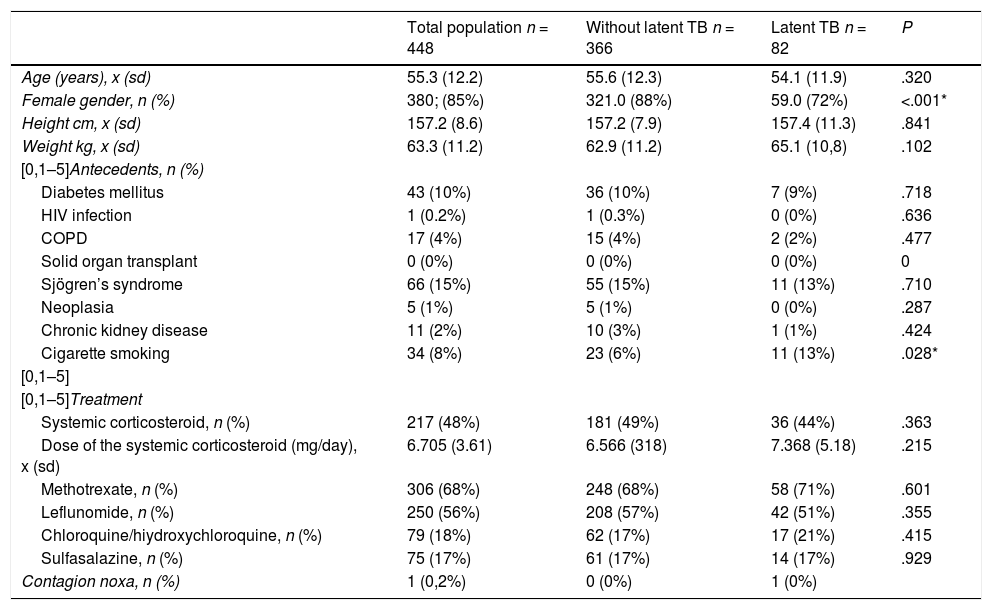
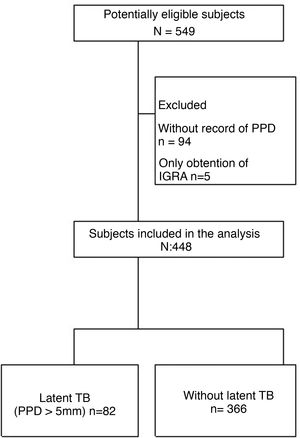
Results549 patients were collected from the 2 study centers, of them, 101 were excluded, since 94 lacked a PPD record and 5 only had a record of IGRA. Therefore, only 448 patients entered into the analysis (Fig. 1). The average age was 55 years and 80% of the patients were women; the presence of comorbidities in the studied subjects was less than 15% (Table 1).
General characteristics of the total population, subjects with latent tuberculosis and without latent tuberculosis.
| Total population n = 448 | Without latent TB n = 366 | Latent TB n = 82 | P | |
|---|---|---|---|---|
| Age (years), x (sd) | 55.3 (12.2) | 55.6 (12.3) | 54.1 (11.9) | .320 |
| Female gender, n (%) | 380; (85%) | 321.0 (88%) | 59.0 (72%) | <.001* |
| Height cm, x (sd) | 157.2 (8.6) | 157.2 (7.9) | 157.4 (11.3) | .841 |
| Weight kg, x (sd) | 63.3 (11.2) | 62.9 (11.2) | 65.1 (10,8) | .102 |
| [0,1–5]Antecedents, n (%) | ||||
| Diabetes mellitus | 43 (10%) | 36 (10%) | 7 (9%) | .718 |
| HIV infection | 1 (0.2%) | 1 (0.3%) | 0 (0%) | .636 |
| COPD | 17 (4%) | 15 (4%) | 2 (2%) | .477 |
| Solid organ transplant | 0 (0%) | 0 (0%) | 0 (0%) | 0 |
| Sjögren’s syndrome | 66 (15%) | 55 (15%) | 11 (13%) | .710 |
| Neoplasia | 5 (1%) | 5 (1%) | 0 (0%) | .287 |
| Chronic kidney disease | 11 (2%) | 10 (3%) | 1 (1%) | .424 |
| Cigarette smoking | 34 (8%) | 23 (6%) | 11 (13%) | .028* |
| [0,1–5] | ||||
| [0,1–5]Treatment | ||||
| Systemic corticosteroid, n (%) | 217 (48%) | 181 (49%) | 36 (44%) | .363 |
| Dose of the systemic corticosteroid (mg/day), x (sd) | 6.705 (3.61) | 6.566 (318) | 7.368 (5.18) | .215 |
| Methotrexate, n (%) | 306 (68%) | 248 (68%) | 58 (71%) | .601 |
| Leflunomide, n (%) | 250 (56%) | 208 (57%) | 42 (51%) | .355 |
| Chloroquine/hiydroxychloroquine, n (%) | 79 (18%) | 62 (17%) | 17 (21%) | .415 |
| Sulfasalazine, n (%) | 75 (17%) | 61 (17%) | 14 (17%) | .929 |
| Contagion noxa, n (%) | 1 (0,2%) | 0 (0%) | 1 (0%) | |
%: percentage; sd: standard deviation; n: number; x: average.
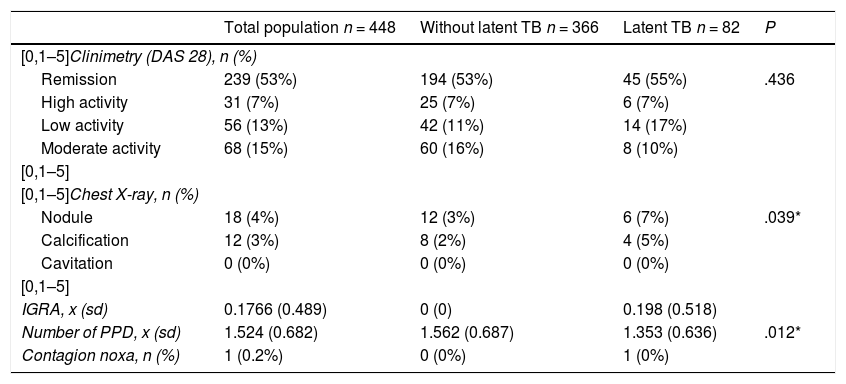
Anti-citrulline antibody titers (anti-CCP) were on average 279 U, with a percentage of weak titers of 3.5%, moderate 4.38% and strong 72.8%. The rheumatoid factor (RF) was positive (>20 IU/mL) in 86.8% of patients. 53.3% of the total sample was in remission by DAS 28. Only 17 patients had an IGRA, whose average value was 0.17 (negative) (Table 2).
Characteristics of clinimetry and paraclinical tests in general population, subjects with latent tuberculosis and without latent tuberculosis.
| Total population n = 448 | Without latent TB n = 366 | Latent TB n = 82 | P | |
|---|---|---|---|---|
| [0,1–5]Clinimetry (DAS 28), n (%) | ||||
| Remission | 239 (53%) | 194 (53%) | 45 (55%) | .436 |
| High activity | 31 (7%) | 25 (7%) | 6 (7%) | |
| Low activity | 56 (13%) | 42 (11%) | 14 (17%) | |
| Moderate activity | 68 (15%) | 60 (16%) | 8 (10%) | |
| [0,1–5] | ||||
| [0,1–5]Chest X-ray, n (%) | ||||
| Nodule | 18 (4%) | 12 (3%) | 6 (7%) | .039* |
| Calcification | 12 (3%) | 8 (2%) | 4 (5%) | |
| Cavitation | 0 (0%) | 0 (0%) | 0 (0%) | |
| [0,1–5] | ||||
| IGRA, x (sd) | 0.1766 (0.489) | 0 (0) | 0.198 (0.518) | |
| Number of PPD, x (sd) | 1.524 (0.682) | 1.562 (0.687) | 1.353 (0.636) | .012* |
| Contagion noxa, n (%) | 1 (0.2%) | 0 (0%) | 1 (0%) | |
%: percentage; sd: standard deviation; n: number; x: average.
Regarding the pharmacological management, 48% were managed with systemic corticosteroids, with an average dose of 6.7 mg/day; 68% with methotrexate; 55% with leflunomide; 17% with hydroxychloroquine/chloroquine and 16% with sulfasalazine.
The average number of tuberculin tests per patient was 1.5 and only one case presented a contagion noxa. The prevalence of LTB in patients with RA who were candidates for biotechnological therapy was 18.3% (95% CI 14.7–21.9) (Tables 1 and 2). The prevalence of LTB in subjects with DM, compared to those without DM, was 16.3% vs. 18.5%, while the prevalence of LTB in smokers vs. non-smokers was 32.4% vs. 17.1%. In the exploratory crosses it was found a prevalence ratio higher than 1 in the relationship between LTB and male gender (P ≤ .001), abnormal findings in the chest X-ray (P = .039) and cigarette smoking (P = .028). No association was found with treatment variables, other comorbidities or disease activity included in the study.
DiscussionThe calculated prevalence of LTB in this study was 18.3% (95% CI 14.7–21.9). Bonfiglioli et al., in a cross-sectional study in subjects with RA treated with anti-TNF, reported a prevalence of LTB of 32.7%.4 Conversely, Callado et al. described a prevalence of LTB of 13% in a rheumatologic population which included patients with RA, ankylosing spondylitis (AS) and psoriatic arthritis who were using infliximab.5 The characteristics of the population in these studies are similar to those found in the present study, with an age around 50 years, a higher proportion of women and therapy with anti-inflammatory agents and disease modifying drugs. The difference in the prevalence rates found by some authors could be explained by the dose of steroid used: doses higher than 15 mg per day for more than 3 months could increase the rate of false negatives of PPD and thus underestimate the prevalence of LTB in these patients. However, this has not been fully demonstrated.8
The city in which the study of Bonfiglioli et al. was conducted was Sao Paulo, Brazil, where an incidence of TB of 49.5/100,000 inhabitants is reported.9 On the other hand, Callado et al. conducted their study in the city of Fortaleza, Brazil, where the incidence of TB is 91.87/100,000 inhabitants.5 In comparison, the incidence in Bogota is 8.2/100,000 inhabitants,10 which can affect the variability of the reported prevalence of LTB. However, in order to be able to state it with greater certainty, it would be expected that the higher the prevalence of TB, the higher the prevalence of LTB, which is not reflected in the data obtained. Additional factors, unknown so far, that could explain these differences can then be considered. In the study of a population with rheumatologic disorders carried out by Callado et al., the prevalence of LTB (PPD > 5 mm) differs according to the type of rheumatologic disease, reaching 26% in AS and 4% in RA.5
The difference in prevalence found can also be explained by unique methodological aspects of each type of study; and individual conditions that may affect it cannot be completely ruled out.11 In this study it was found that the male gender and the antecedent of cigarette smoking would increase the prevalence of LTB in this population. Although it is true that the type of cross-sectional study prevents making associations due to lack of temporality, similar results between the male gender had been reported in South African studies, the explanation of the authors has been the greatest noxa of contagion in men.12 On the other hand, the association between LTB and cigarette smoking can be explained pathophysiologically by the alteration in the function of TNF alpha due to the action of nicotine, important in LTB and in the reactivation of active TB.13 It has also been described that the exposure to cigarette alters the activation of CD4 T lymphocytes and reduces the number of activated CD4 and CD8 T cells, which affects the neutralization of viral and bacterial microorganisms.14
We did not find differences in the frequency of LTB in our population according to comorbidities. The frequency of comorbidities in our study was low (<15%), and this situation affected their analysis, contrary to what was found in an observational study conducted in 12 centers in India, with a sample of 195 patients with rheumatic diseases who were managed with biotechnological therapy, in which a relationship between the development of TB and the presence of comorbidities such as DM, hypothyroidism and cardiovascular disease, with a 1.5-fold higher risk, and the use of corticosteroids with a 4.6-fold higher risk of developing the disease was reported.15 Other associated factors include contagion noxa and some disease modifying drugs.4,16
Associations have been found with other drugs different than those of the anti-TNF group, used in patients with RA and LTB, as is the case with leflunomide, which is related to opportunistic infections, mainly intracellular germs.17 In a study that assessed the risk for mycobacterial infection associated with anti-rheumatic medication, regarding the use of leflunomide it was found an odds ratio of 4.02 (1.08–15.0) (P = .04), the second highest, only surpassed by the use of anti-TNF with an odds ratio of 5.04 (1.27–20.0) (P = .02).16 In contrast, in the present study, pharmacological management was not related to the appearance of LTB.
In the analysis of variables such as disease activity, levels of rheumatoid factor, anti-CCP and findings on the chest X-ray, a relationship was found with the latter. The findings of pulmonary nodule or calcification could be related to the presence of LTB, since they could correspond to Ghon complexes in subjects, previous development of rheumatologic disease, or initiation of biotechnological therapy. Perhaps this finding is more related with the diagnosis than with the possible association with the disease, which limits its interpretation.6
Among the strengths of this study are the sample size to determine the prevalence of LTB and the rigor in the obtention of data from the medical records, as well as the verification in the diagnosis of RA and LTB and an adequate confidence interval. However, it is necessary to recognize that being retrospective and with a sample obtained in high-complexity hospitals, it is not exempt from possible selection bias when choosing patients with a more severe spectrum of the disease that may overestimate the calculation of the prevalence. Likewise, there is a probability of incurring information bias because it depends on the information recorded in clinical histories.
On the other hand, the diagnosis of LTB by PPD has limitations. Firstly, it can have a high rate of false positives when trying to measure a delayed cell-mediated hypersensitivity reaction using the purified protein derivative, which contains a mixture of antigens for Mycobacteria that is not very specific, being more relevant in people vaccinated with the Bacillus Calmette-Guérin. Second, its sensitivity is lower in immunocompromised patients, given a possible alteration in the function of T lymphocytes and cellular immunity. In addition, interobserver variability and factors related to difficulties in the displacement of the patient for the reading of the test can modify the final reading report.18 The specificity for the diagnosis of LTB in a high-risk population, such as health workers or population endemic for TB or HIV, is 93% (90%–96%) (Positive predictive value 90%), while the sensitivity is 57% (43%–71%) (Negative predictive value 65%),19 which could also be reflected in different prevalence values.
ConclusionThe prevalence of LTB in patients with RA who will receive biotechnological therapy is high: 18.3% and it is possible that there is a relationship between cigarette smoking and with male gender. It is required to develop cohort or case-control studies to verify these associations.
Conflict of interestThe authors declare that they do not have any conflict of interest.
Please cite this article as: Mora C, Bastidas Goyes AR, Patiño J, Vera JD, Beltrán A, Mutis C, et al. Prevalencia de tuberculosis latente determinada mediante la prueba de derivado proteico purificado (PPD) en una población de pacientes adultos con artritis reumatoide llevados a terapia biotecnológica. Rev Colomb Reumatol. 2021;28:178–183.