The coronavirus disease 2019 (COVID-19) produced by SARS-CoV-2 has taken on great importance in recent months, and is under constant investigation by different areas of medicine, including rheumatology, in search of the best scientific evidence. In the case of the pediatric population, it is particularly important as it was first thought that the impact of the pandemic in this population would be less due to the low presence of severe cases. Evidence is now being reported of clinical pictures in children with a diagnosis of COVID-19 who are characterized by an altered inflammatory state consisting of a storm of pro-inflammatory cytokines that produces manifestations similar to those presented in autoimmune diseases, such as Kawasaki disease. It has been called Multisystemic Inflammatory Syndrome in children, temporarily associated with SARS-CoV-2 which, in many cases requires hospitalization in pediatric intensive care units and multidisciplinary management by various specialties.
La enfermedad por coronavirus 2019 (COVID-19) producida por el SARS-CoV-2 ha tomado una gran importancia en los últimos meses y se encuentra bajo constante investigación por distintas áreas de la medicina, incluida la reumatología, en la búsqueda de la mejor evidencia científica. En el caso de la población pediátrica cobra especial importancia puesto que en un principio se pensaba que el impacto de la pandemia en esta población sería menor, debido a la baja presencia de casos severos, pero la evidencia actual reporta la existencia de cuadros clínicos en niños con diagnóstico de COVID-19 que se caracterizan por un estado inflamatorio alterado consistente en una tormenta de citocinas proinflamatorias que produce manifestaciones similares a las presentadas en enfermedades autoinmunes como la enfermedad de Kawasaki. Se le ha denominado síndrome inflamatorio multisistémico en niños asociado temporalmente con SARS-CoV-2, el cual en muchos casos precisa internación en unidades de cuidados intensivos pediátricos y el manejo multidisciplinario por diversas especialidades.
Coronaviruses are positive-sense single-stranded RNA viruses which are classified into 4 categories: alpha, beta, gamma and sigma. They have a rigid spherical shape and an irregular surface on which the protein S (Spike) is located.1 In the SARS-CoV-2 virus, the structure of the protein S largely resembles that of SARS.2,3 It is currently known that the coronaviruses that infect humans are divided into seven strains, including the SARS-CoV-2,4–6 which has caused more than 350,000 deaths worldwide in a period of 6 months. SARS-CoV-2 infection is asymptomatic in a large proportion of patients, in others occurs with mild symptoms and in a small proportion the symptoms are severe. The current panorama of the COVID-19 infection in the pediatric population is described below, giving full details on the multisystem inflammatory syndrome in children temporarily associated with SARS-CoV-2, which has a wide spectrum and can be serious and even fatal in some patients.
Materials and methodsA literature search was conducted in the PubMed, Science Direct and Scopus databases using the search terms «COVID-19», «coronavirus», «2019-nCoV», «SARS-CoV-2», «Severe Acute Respiratory Syndrome», «Kawasaki Disease», «Multisystem Inflammatory Syndrome in Children». There were no date restrictions during the search. The articles that fulfilled the search terms in the title or in the abstract were taken into account and those which were relevant for the review were selected, from which the most important information was extracted.
ResultsWith the search terms used, 185 articles were found, the majority of which were found in Medline. Articles that were not fully accessible and those that were duplicated were excluded. Finally, a total of 114 articles were selected for the topic review.
General aspects of COVID-19At the end of 2019, a series of cases of pneumonia of unknown origin was registered in Wuhan, China. Subsequently, the new coronavirus was isolated as the causative agent from samples obtained from the lower respiratory tract of the patients, which facilitated its differentiation from the coronaviruses described in previous years.7 According with the information published by the World Health Organization (WHO) at the beginning of January 2020, the source of the new coronavirus was linked to a seafood market place in Wuhan, and for this reason it was closed.8 Later, fecal, blood and respiratory samples were taken from 59 suspected cases from which the identification of the new coronavirus was conducted by means of the polymerase chain reaction (PCR).9
Based on the aforementioned samples, the diagnostic test for identification of the virus was obtained, which confirmed the diagnosis in 41 of these patients. By this time, thanks of the research conducted by Huang et al., the possible spectrum of the disease associated to the new coronavirus, called 2019-nCOV, was brought to light. The existence of human-to-human transmission, the radiographic patterns, as well as the role played by proinflammatory cytokines and their direct relationship with the severity of the disease could also be evidenced.9
In February 2020, the WHO assigned the name of new coronavirus disease 2019 (COVID-19) to this condition and in less than one month declared it an international emergency.10 Guan et al. published the clinical findings of 1099 hospitalized patients with a diagnosis of COVID-19 in China. The diagnostic confirmation was performed by means of the real-time reverse transcriptase polymerase chain reaction (RT-PCR), from samples obtained from nasopharyngeal swabs. This sample of patients accounted for 14.2% of the total of infected and hospitalized patients until January 29, 2020 in China. Fever an cough were reported as the cardinal symptoms; the first occurs in up to 43% of the patients at the beginning of the disease.11
The most frequently reported symptomatology at the onset of symptoms is fever, cough, myalgia, fatigue, diarrhea, nasal congestion and respiratory distress,12 which occurs in half of the cases one week after the onset of symptoms. With the passage of the days, the clinical picture becomes more florid and multisystemic involvement could appear in a proportion of patients.
In severe cases, acute respiratory distress syndrome, acute cardiac injury, secondary infections or overlapping of these,3 or neurological involvement such as cerebrovascular accident, seizures, ataxia, encephalopathy, variants of the Guillain-Barré syndrome, ophtalmoparesis, Miller-Fisher syndrome, meningitis and delirium may occur.13 Even the cerebrovascular accident behaves as a factor of poor prognosis in these patients.14 At the dermal level, a wide variety of lesions have been reported, including maculopapular eruptions (similar to pityriasis rosea, erythema multiforme, palmar erythema, and enanthema), vesicular eruptions, urticarial eruptions, necrotic lesions, and pseudo-chilblains.15
The hypothetical transmission mechanisms of this disease described to date are through respiratory droplets, direct contact, feces, urine and saliva.16 Maternal-fetal transmission is a matter of discussion. Lamouroux et al. synthetize arguments in favor and against vertical transmission based on 12 publications until April 4, 2020, in which 68 deliveries in mothers positive for COVID-19 were reported, with a total of 71 live newborns, 3 of them with a diagnosis of COVID-19 within the first 48 h.17–19 Other studies report in utero infection based on high titers of immunoglobulin M (IgM) for SARS-CoV-2 in blood obtained from the newborns.20–22 The position at this moment is uncertain and more evidence is required to confirm maternal-fetal transmission.23
By March 4, 2020, a total of 90,870 cases had been notified globally, with an extension to 72 countries.24,25 Five months later, a total of 24,257,989 cases were reported, a clear sample of the propagation capability of this virus.25 In the case of Colombia, the number of confirmed cases of COVID-19 is 607,938, with 136,702 active cases and 19,364 deaths as of August 31, 202026. The information in real time can be consulted in the website of the National Institute of Health (Instituto Nacional de Salud): https://www.ins.gov.co/Noticias/Paginas/Coronavirus.aspx.
Immune response in COVID-19The first defense response against the invasion of the virus is innate immunity, which can be evaded by the SARS-CoV and SARS-CoV-2 viruses.3,27 The detection of the virus by the immune system is carried out through endosomal sensors or cytoplasmic RNA sensors, which mobilize and activate transcription factors. This triggers a high expression of innate pro-inflammatory cytokines and type 1 interferons in search of containing the virus. In addition, the SARS-CoV-2 can alter ubiquitination and degrade the signaling protein, thereby evading the immune system.28–30
Adaptive immunity plays also an important role. Activated cytotoxic T cells destroy the infected cells. The B cells produce antibodies that are directed against the viral antigens. Lymphocyte counts are markedly low and the plasma concentrations of inflammatory cytokines are high in patients with severe pneumonia.3,9,31,32 It has been observed that T CD4 + lymphocytes, T CD8 + lymphocytes and natural killer cells are considerably reduced in seriously ill patients, compared to the slightly ill patients.31 Xu et al. suggest that the severe immune lung injury occurs in part due to the increase of cytotoxic T lymphocytes and proinflammatory subsets of T lymphocytes.33
High plasma concentrations of cytokines from Th1 and Th2 lymphocytes such as IL-2, IL-7 and IL-10, granulocyte-colony stimulating factor, interferon gamma-induced protein 10 (IP-10), macrophage chemoattractant protein-1, macrophage inflammatory protein-1α and the TNF were recorded in the patients with COVID-19 in the intensive care unit.9 Likewise, the plasma concentrations of interleukin-6 (IL-6) were increased in patients with severe symptoms, compared with mild cases and healthy individuals31,34. In addition, severe cases of COVID-19 have a cytokine profile similar to that seen in secondary hemophagocytic lymphohistiocytosis, which is characterized by an increase in cells with hemophagocytic activity and an uncontrolled cytokine storm due to aberrant immune activation that can cause serious complications and even death in the final stages of the disease.33,35,36 Alveolar macrophages expressing ACE2 receptors as the primary target cells in SARS-CoV-2 infections are also mentioned. Once active, they can play an important role in the cytokine storm.37
Role of the angiotensin-converting enzyme 2 receptorThe ACE2 receptor is a type 1 membrane protein,38 considered in different investigations as the entry of the SARS-CoV-2.39,40 These receptors are expressed in different cells of the body, including those of the lung and intestinal epitelium and some immune cells.41–43 Hwang et al. suggest that the SARS-CoV-2 is transmitted more easily between people because the binding affinity between the protein S and the ACE2 receptor is 10–20 times higher than in SARS.2 Taking into account that this receptor is not present in all cells of the immune system, it is possible that other receptors or other mechanisms, such as the process of phagocytosis of immune complexes that contain the virus are involved.28
COVID-19 in the pediatric populationThis entity has had a different behavior in the pediatric population. Various studies bring out how the severity in this case is usually lower. It has been observed that more than 90% of pediatric patients present with a mild or moderate clinical picture, or are asymptomatic at diagnosis.44,45 In the observational study conducted by Dong et al. with 2135 pediatric patients with COVID-19, it was observed that 34.1% corresponded to cases confirmed by RT-PCR and 65.9% to suspected cases. The foregoing is a sample of the obstacle that represents the obtention of truly confirmed cases, since the speed of contagion exceeds the speed at which the diagnostic tests are performed.45
Why are children less susceptible to COVID-19 infection?There are several hypotheses that seek to support the differences between the pediatric and the adult population in the number of cases of COVID-19, the severity of the symptoms and the severity of the complications.46–48 Among the theories raised are the lower risk of contact with the virus in the pediatric population as they are less present in crowded activities and less exposed to cigarette smoke and hospital environments.46,47 Other theories speak of a possible competition of SARS-CoV-2 with other viruses that are frequently present in the respiratory tract of the children such as influenza, respiratory syncytial virus and rhinovirus27,48; and other theories point to the difference in the immune response and in the number of ACE2 receptors in children and in adults.49–51
Bénéteau-Burnat et al. report that serum levels of ACE2 vary in the pediatric population compared with the adult population.52 Newborns have higher ACE2 levels, at 6 months the values decrease and at 4 years they become stable. Later, they increase in puberty and decline in the adolescence until reaching normal levels in adulthood.53
Clinical findings in COVID-19 in childrenThe incubation period ranges between 1 and 14 days. In general, 90% of the cases in the pediatric population are mild or asymptomatic.45 The infection is more frequent in men than in women, but some studies have reported that the difference with respect to sex is not statistically significant.45 Initially, children may present nonspecific symptoms such as fever, dry cough, fatigue or even gastrointestinal symptoms such as diarrhea and vomiting.54,55 Headache and nasal discharge are present in up to 50% of the mild or atypical forms, as well as anosmia and abdominal pain, with a frequency higher than 20%.56
The hospitalization rate is low (5.7%), in most cases associated with dyspnea.57 The time elapsed between admission and discharge from hospital ranges between 24.9 and 30.9 days in patients under 20 years of age,55 with a period of one to two weeks to be free of symptoms.58,59 Children under one year of age with comorbidities such as asthma or immunosuppression have a higher rate of complications, with a probability of 10.6% of having a severe or critical clinical picture.57 The prevalence of critical and severe cases decreases with age: 7.3% between one and five years and only 3% in those over 15 years of age.59
In a recent study that included 345 children with confirmed diagnosis of COVID-19 it was found that 23% (n = 80) had at least one underlying condition, among which asthma, heart disease and immunosuppression stood out.44 In the group of hospitalized patients (n = 37), 77% had at least one underlying condition, being higher when compared with the patients who did not required hospitalization (12%). DeBiasi et al. also reported that the presence of underlying conditions was more common in the group of hospitalized patients (63%) than in the non-hospitalized cohort (32%).60 It is concluded that the presence of underlying conditions, among which asthma, heart disease and immunosuppression stand out, is associated with a greater probability of severe forms of the disease in the pediatric population.
Laboratory findings in COVID-19 in childrenIn most cases, the pediatric population do not present marked alterations in the laboratory tests, being infrequent the presence of lymphopenia, leukopenia and elevation of the C-reactive protein, in contrast to what occurs in adults.61 The elevation of ALT, AST, LDH, procalcitonin and d-dimer is also more frequent in the adult population.9,11,32,62 It has been reported an increase in the MB fragment of creatine kinase (CK-MB) > 200 U/l.13
Radiological findings in COVID-19 in childrenAt the imaging level there are some differences between adults and children. The pediatric population exhibits peripheral lung lesions, while adults present lesions in the interlobar fissures and the pleura. The ground glass pattern and the presence of alveolar consolidations can be observed both in one population and in the other.61 In children, it is characteristic to find nodular lesions or small unilateral patches, while in adults the presence of larger, bilateral patches with a cobblestone pattern is more frequent.
The early findings on the chest computed axial tomography are radiopaque lesions in the outer third of the lung and with subpleural location.63 The lung disease progresses and generates radiopaque lesions of alveolar occupation that predominate in the lower pulmonary lobes, so that later in the critical phase, multilobar consolidations that generate a “white lung” can occur.63 It should be mentioned that the radiological manifestations are closely related to the inflammatory response of the host.61
Diagnostic tests in COVID-19The adequate collection of the samples is the most important step to establish a rapid diagnosis of COVID-19, in order to initiate early management and control the propagation of the virus; therefore, it is necessary that the health personnel be trained in the collection, packaging, transportation and storage of the samples, and they should have all the personal protection measures when taking them.64,65 In the cases of ambulatory patients, material of the upper airway as oropharyngeal or nasopharyngeal swab or lavage should be collected.65
In hospitalized patients with severe respiratory disease it may be necessary to take samples of the lower respiratory tract such as sputum or bronchoalveolar lavage, or endotracheal aspirate in those patients with invasive ventilatory support. High loads of viral SARS-CoV-2 RNA have been also detected in fecal samples.49 The nasopharyngeal sample is of choice because is better tolerated by the patient and much safer for the health personnel.50 In addition, oropharyngeal samples are associated with lower sensitivity.51
The molecular test of choice for the diagnosis of COVID-19 is the RT-PCR, which is based on the detection of viral RNA by nucleic acid amplification50 and whose positive results range between 30 and 60% of the cases, depending on whether the sample was taken in early or late stages of the infection.51,65 Other possible causes of false negative results in the tests could be that the sample is insufficient, inadequate packing and transportation or technical reasons inherent to the test{65 In the work conducted by Wang et al., the rate of positive RT-PCR results was compared in different samples taken from 205 patients. The rates of positive results were: for bronchoalveolar lavage (93%), sputum (72%), nasopharyngeal swab (63%), oropharyngeal swab (32%), stool (29%), blood (1%) and urine (0%), so the performance of the RT-PCR depends on the type of sample and its adequate collection.47
The serology can be complementary or it may support the diagnosis in cases in which the RT-PCR is negative and there is a strong epidemiological nexus with SARS-CoV-2 infection.65 IgG and IgM antibodies develop between 5 and 10 days after the infection with SARS-CoV-266 and reach peaks at 12 and 17 days for IgM and IgG, respectively. In the case of IgM, positive titers remain for up to 35 days, while for the IgG they remain for at least 49 days.67 Serological tests under development have a range of sensitivity (SE) between 87% and 93% and the specificity (SP) is of 95%–100%. In the case of the Cellex qSARS-CoV-2 IgG/IgM Rapid Test the SE is 93.8% and the SP is 95.6%.67
Viral sequencing does not play an important role in the initial diagnosis of COVID-19 and is useful in epidemiological studies of mutations in the viral genome. Virus culture is not recommended either for diagnosis and its current use is directed to the study of the properties of the virus and the development of a vaccine.65
Pediatric multisystem inflammatory syndrome (MIS-C)Although COVID-19 infection is less severe in the pediatric population and its expected impact is lower, in recent months new evidence has emerged suggesting the appearance of an unusual form of hyperinflammatory syndrome in the pediatric population affected by this disease. This condition, called the multisystem inflammatory syndrome in children temporarily associated with SARS-CoV-2 (MIS-C), shares common characteristics with other pediatric inflammatory conditions including Kawasaki disease (KD, in its complete or incomplete form), streptococcal and staphylococcal toxic shock syndromes, bacterial sepsis, and macrophage activation syndrome. This situation has been put in evidence by authors from the United States, Europe and India and it can occur days or even weeks after the acute COVID-19 disease.68
This picture is characterized by the appearance of persistent fever associated with hypotension and multisystemic commitment at the cardiac, renal, gastrointestinal, hematological, dermatological or neurological levels. Non-purulent conjunctivitis, lymphadenopathies, skin rash and swollen hands and feet can also occur, making it very similar to KD. The latter, which is the most frequent primary systemic medium vessel vasculitis in pediatric age, is self-limited and most often affects the coronary arteries. It predominates in infants and children under 5 years of age,69 but its presentation in adults has also been described in the literature.70 Currently, KD is the main cause of acquired cardiovascular disease in children from developed countries71,72. Its etiology is still unclear, but it is suggested that it may be caused by infectious agents that trigger an abnormal immune response in genetically susceptible individuals.
Among the infectious agents that have been associated as possible triggers are the adenovirus, the respiratory syncytial virus, the dengue virus, enteroviruses, measles and Epstein–Barr virus.73–78 Likewise, it has been reported the association between KD and other forms of coronavirus.79Table 1 presents the main clinical findings of KD published by the American Heart Association in 2017, where the similarity in the form of presentation with the MIS-C is evidenced.80 The treatment of choice in patients with KD consists in the use of intravenous immunoglobulin (IVIG), but it should be remembered that between 10%–20% of cases are resistant to it and require adjuvant anti-inflammatory treatment with corticosteroids or other immunosuppressants.80–82
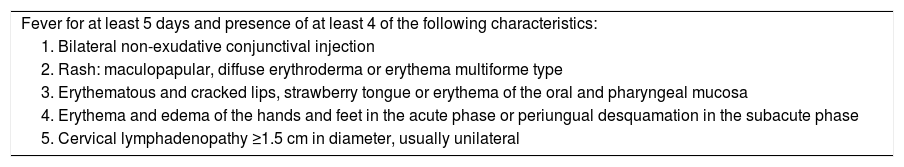
Diagnostic criteria of the American Heart Association 2017 for Kawasaki disease.
| Fever for at least 5 days and presence of at least 4 of the following characteristics: |
| 1. Bilateral non-exudative conjunctival injection |
| 2. Rash: maculopapular, diffuse erythroderma or erythema multiforme type |
| 3. Erythematous and cracked lips, strawberry tongue or erythema of the oral and pharyngeal mucosa |
| 4. Erythema and edema of the hands and feet in the acute phase or periungual desquamation in the subacute phase |
| 5. Cervical lymphadenopathy ≥1.5 cm in diameter, usually unilateral |
Other manifestations that may be present in the MIS-C are abdominal pain, diarrhea, emesis, headache, confusion, among others.83 Cardiac involvement in these patients is described with the appearance of myocarditis, valvulitis, pericardial effusion and, in more severe cases, acute heart failure with elevated levels of troponin and B-type natriuretic peptide (NT-ProBNP).84 At the ultrasound level there may be coronary findings similar to those present in KD.84–86 Associated with this, there is an elevation of inflammatory markers such as C-reactive protein, procalcitonin, ferritin, erythrocyte sedimentation rate (ESR), d-dimer, in addition to the presence of neutrophilia and lymphopenia.73 Respiratory symptoms are mild and they may even be absent in these patients.86
It is necessary to monitor closely signs that suggest clinical deterioration at the respiratory or cardiovascular level. Other signs such as increase in fever, worsening of gastrointestinal symptoms, prolonged skin rash, persistently elevated inflammatory markers, increase in ferritin, fibrinogen or d-dimer, as well as increased liver enzymes (AST, ALT) and LDH, low serum sodium or deteriorated kidney function may indicate a greater systemic inflammatory involvement.87
These patients often require admission to the pediatric intensive care unit due to the need for cardiac or respiratory support. An interdisciplinary management that includes specialists in intensive care, infectious diseases, cardiology and rheumatology is mandatory, considering the severity of the clinical picture and the possibility of severe outcomes such as multiple organ failure and shock.81,83,87–90
Verdoni et al. describe this phenomenon in 10 patients between 2 and 16 years of age. The RT-PCR for SARS-COV-2 of the nasopharyngeal swab was positive in two cases; subsequently, serology testing was performed, with positive IgG in 8 of the 10 patients and positive IgM in 3 of them. Five of the patients fulfilled complete criteria for KD, while the remaining 5 had the incomplete form of the disease. All patients received IVIG, methylprednisolone and aspirin, 2 of them at antiinflammatory doses with a favorable response.91 Due to the negative results in the nasopharyngeal swabs in 8 of the 10 patients, the possibility that this phenomenon represents a postinfectious inflammatory syndrome mediated by antibodies or immune complexes is not excluded. Likewise, it is not clear whether this condition is a new emerging disease, or if it is the same KD and SARS-CoV-2 acts as a triggering agent.
Riphagen et al.88 present a series of 8 patients between 4 and 14 years who exhibited characteristics of atypical KD or toxic shock syndrome. Four of the children had a family exposure to COVID-19, 6 of them were Afrodescendants and 7 were above the 75th weight percentile. Two children had non-significant comorbidities (alopecia areata and autism). All children had fever (38–40 °C), rash, conjunctivitis, peripheral edema, pain in the extremities and gastrointestinal symptoms (diarrhea, vomiting, abdominal pain). The totality of patients progressed to refractory shock and required the use of vasopressor agents. The majority of children did not have respiratory involvement, but 7 of them required mechanical ventilation to achieve cardiovascular stabilization.
The tests for SARS-CoV-2 were negative in the bronchoalveolar lavage and in the nasopharyngeal aspirate. There was elevation of C-reactive protein, procalcitonin, troponins and d-dimer. Adenovirus and enterovirus were isolated in one of the patients. All patients presented hyperrefringence in coronary vessels by echocardiogram and one of them developed a giant aneurysm after being discharged from the hospital. The treatment included IVIG (2 g/kg) in the first 24 h, and afterwards 6 of them received aspirin at doses of 50 mg/kg. One of the patients presented a giant cerebrovascular infarction associated with cardiac arrhythmia, refractory shock and death. The diagnosis of SARS-CoV-2 was confirmed post mortem in this patient.88 Other published works and reports reveal similar cases.81,92 The association of MIS-C with obesity93 and a higher frequency in individuals belonging to ethnic minorities is also described.94
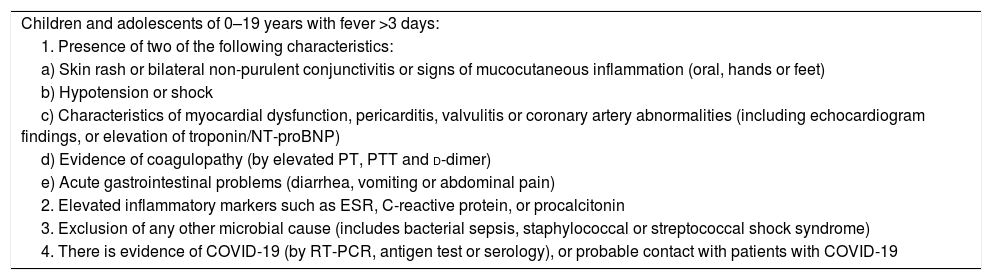
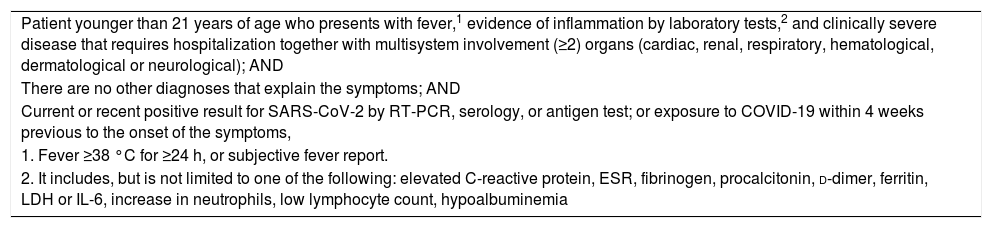
At the moment, and due to the lack of information, the WHO and the Center for Disease Control and Prevention have published a definition of suspected case or MIS-C.95,96 (Tables 2 and 3).
Definition of «preliminary case» of pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 of the WHO.
| Children and adolescents of 0–19 years with fever >3 days: |
| 1. Presence of two of the following characteristics: |
| a) Skin rash or bilateral non-purulent conjunctivitis or signs of mucocutaneous inflammation (oral, hands or feet) |
| b) Hypotension or shock |
| c) Characteristics of myocardial dysfunction, pericarditis, valvulitis or coronary artery abnormalities (including echocardiogram findings, or elevation of troponin/NT-proBNP) |
| d) Evidence of coagulopathy (by elevated PT, PTT and d-dimer) |
| e) Acute gastrointestinal problems (diarrhea, vomiting or abdominal pain) |
| 2. Elevated inflammatory markers such as ESR, C-reactive protein, or procalcitonin |
| 3. Exclusion of any other microbial cause (includes bacterial sepsis, staphylococcal or streptococcal shock syndrome) |
| 4. There is evidence of COVID-19 (by RT-PCR, antigen test or serology), or probable contact with patients with COVID-19 |
Case definition of the CDC for multisystem inflammatory syndrome in children.
| Patient younger than 21 years of age who presents with fever,1 evidence of inflammation by laboratory tests,2 and clinically severe disease that requires hospitalization together with multisystem involvement (≥2) organs (cardiac, renal, respiratory, hematological, dermatological or neurological); AND |
| There are no other diagnoses that explain the symptoms; AND |
| Current or recent positive result for SARS-CoV-2 by RT-PCR, serology, or antigen test; or exposure to COVID-19 within 4 weeks previous to the onset of the symptoms, |
| 1. Fever ≥38 °C for ≥24 h, or subjective fever report. |
| 2. It includes, but is not limited to one of the following: elevated C-reactive protein, ESR, fibrinogen, procalcitonin, d-dimer, ferritin, LDH or IL-6, increase in neutrophils, low lymphocyte count, hypoalbuminemia |
Currently there is no established treatment for COVID-19, but the scientific community is conducting different clinical trials in which treatment strategies and the development of a possible vaccine are being considered.97–99 Among the drugs that have been used in the treatment of COVID-19 are antimalarials,100,101 antivirals such as remdesivir102, mesenchymal stem cells103, transfusion of plasma from convalescent patients104, corticosteroids,105–107 anti IL-1108,109 and anti IL-6 such as tocilizumab,110–112 with promising results in some of them, but to date insufficient to promote their universal use.
In the case of MIS-C, the use of IVIG is recommended87, with good outcomes in most cases81,88,91,92,94. The recommendations suggest to use it at doses of 2 g/kg of body weight, which could stop the storm of inflammatory factors and improve the immune function of the patients.113 Regarding corticosteroids, their use at low or moderate doses could be considered for the treatment of MIS-C, and high doses in patients with life-threatening complications such as shock and in those who require high or multiple doses of inotropes or vasopressors. Low-dose aspirin (3–5 mg/kg/day) can also be used in patients who present MIS-C and characteristics similar to KD. The use of anakinra can be considered in those patients refractory to IVIG and corticosteroids.113 In the case of tocilizumab, favorable outcomes have been observed in some case reports,114 which leaves a door open for further research with this type of drugs in patients with MIS-C.
ConclusionCurrent COVID-19 pandemic has posed many challenges for the field of health, and the area of rheumatology is not the exception. Defining a transmission profile, a contagion rate, risk factors for suffering a more severe disease and basic measures of promotion and prevention are material for the study by different researchers and scientific groups. This review summarizes the most recent evidence available on the pathophysiological mechanisms of the immune response and the cascade of cytokines in pediatric patients with COVID-19 and its implications in rheumatology and in the development of new therapies.
MIS-C secondary to a cytokine storm can appear in a very similar way to KD and generate diagnostic difficulties and treatment challenges. It is important to suspect this entity in febrile pediatric patients with multisystem involvement. The progressive increase in the available evidence about COVID-19 will allow an adequate and timely diagnostic and therapeutic approach to the patients.
Conflict of interestThe authors declare that they have no conflict of interest.
Please cite this article as: Naranjo Arango YA, Farfán Cortés AYAA, García Henao JP, Arango Slingsby C, Saldarriaga Rivera LM. Síndrome inflamatorio multisistémico en niños con COVID-19: una visión desde la reumatología. Rev Colomb Reumatol. 2021;28:289–299.