Flexible bronchoscopy is a diagnostic or therapeutic procedure with a low incidence of complications (0.08–6.8%). Bleeding after transbronchial biopsy is a rare complication (0–2.8% of the cases), usually resulting in minor bleeding that resolves with local measures. There is no clear definition of massive hemoptysis and due to the low incidence of this condition, there are no practical guidelines for the treatment of this complication that may be catastrophic. This case discusses the occurrence of massive hemoptysis during a transbronchial biopsy under flexible bronchoscopy, including a literature review on perioperative management.
La broncoscopia flexible es un procedimiento diagnóstico o terapéutico con baja incidencia de complicaciones (0.08% – 6.8%). El sangrado por biopsia transbronquial es una complicación rara (0-2,8% de los casos), suele ser leve y resuelve con medidas locales. No existe una definición clara de hemoptisis masiva y por su baja incidencia no hay guías de práctica clínica para el tratamiento de esta complicación que puede ser catastrófica. Presentamos un caso de hemoptisis masiva durante la realización de una broncoscopia flexible más biopsia transbronquial y revisamos la literatura acerca del manejo intraoperatorio.
Flexible bronchoscopy is one of the most frequent procedures used in the diagnosis and/or treatment of the airway and parenchymal lung disease. The incidence of complications is 0.08–6.8%.1–7
Mild bleeding is a complication that presents one or two days after the procedure and resolves spontaneously. It is more frequent in patients receiving antiplatelet or anticoagulant therapy, or with coagulopathy. Bleeding during transbronchial biopsy occurs in up to 2.8% of the cases and usually resolves with local measures (frozen saline solution).1
Intravenous and/or local anesthetic agents inhibit the cough reflex and increase the risk of respiratory depression. The volume of bleeding in the airway alters the ventilation/perfusion ratio and increases the risk of hypoxemic respiratory failure.8
There is no precise definition of massive hemoptysis with volumes between 100ml and 1000ml in 24h,9–12 and it has been suggested to use the level of functionality affected as a result of bleeding.11 Some authors include in the definition the need for blood products, hypoxemic respiratory failure, and/or hemodynamic instability, inter alia.12–16
As a result of the low incidence of massive hemoptysis, there are no practical clinical guidelines on the management of the condition during bronchoscopy.17 We present a case of massive hemoptysis following flexible bronchoscopy transbronchial biopsy.
Case report34-Year old patient with a history of HIV and non-compliant to medical therapy is being treated for meningeal cryptococcosis. The patient presents with respiratory symptoms with signs of alveolar occupation in the chest images and suspect opportunistic microorganism infection. The patient is scheduled for flexible bronchoscopy (FBC), bronchoalveolar lavage, and transbronchial biopsy. Functional class II/IV (NYHA scale). The physical examination rendered no positive findings and the clotting times and CBC were normal.
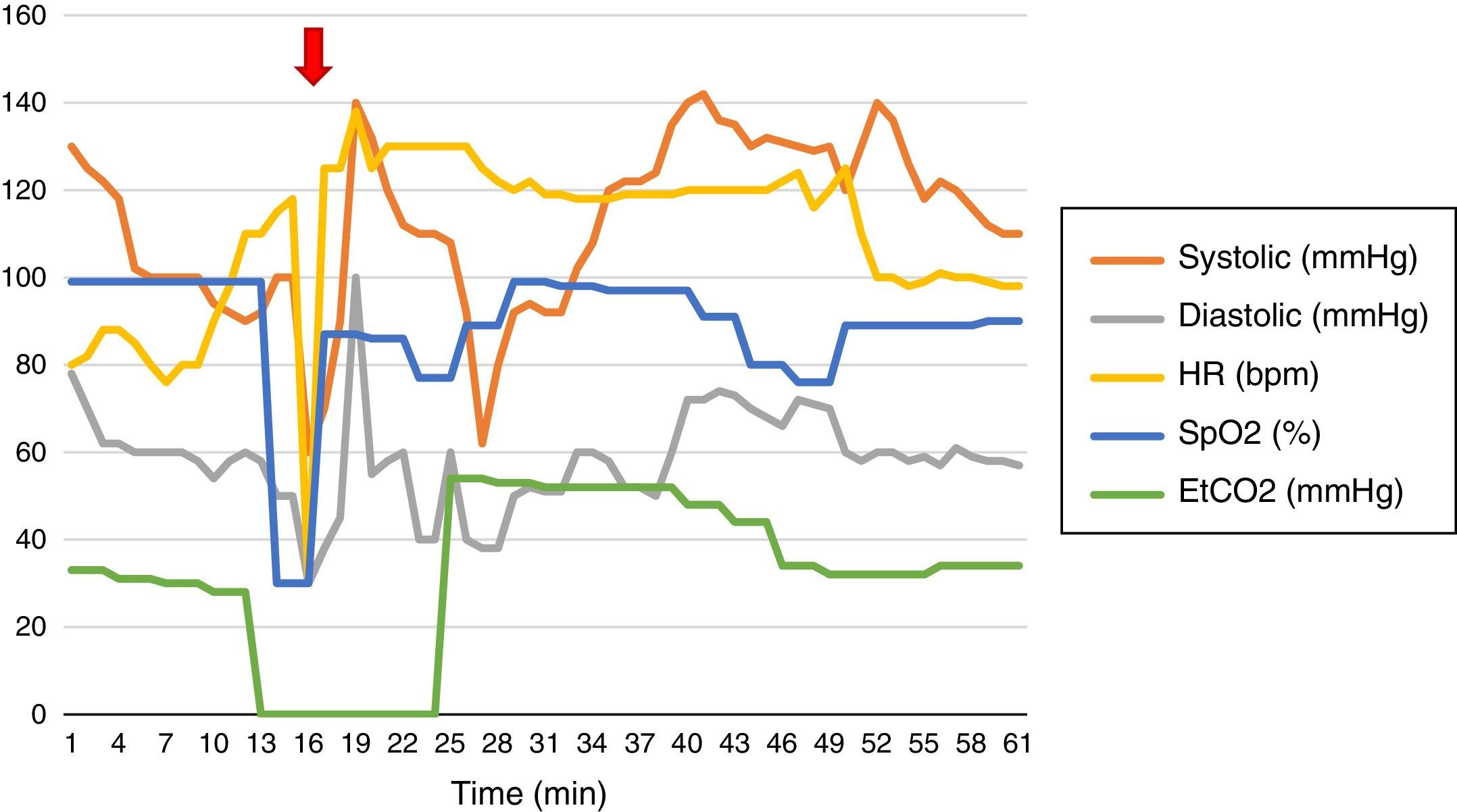
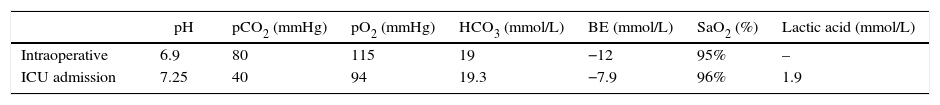
Basic monitoring and hemodynamic behavior are shown in Fig. 1. The induction of anesthesia was done with fentanyl (150μg [IV]), propofol (150mg IV), and succinylcholine (50mg IV), with placement of laryngeal mask # 5. Maintenance with continuous infusion of fentanyl and propofol.
When undergoing a transbronchial biopsy (Fig. 1) the patient presents with bleeding and subsequent desaturation, bradycardia, and hypotension. Atropine 1mg IV and Etilefrine 3mg IV were administered. The catheter was placed in the radial artery. A left dual lumen tube was placed blindly and checked with fluoroscopy. 2cc were administered to the pneumoplugger (hemoptysis prevents visualization using the fibrobronchoscope); protective one-lung ventilation was initiated (based on patient's body weight) with subsequent SpO2 77% and FiO2 100%. Alveolar recruitment maneuvers were initiated, H2O airway pressure 57cm, two-lung ventilation was then started with tidal volume 475ml, respiratory rate 20, inspiration:expiration ratio 1:2, positive end expiratory pressure 10cm H2O, FiO2 100%, maximal and plateau inspiratory pressure 66 and 23cm H2O, respectively, despite neuromuscular relaxation.
The fibrobronchoscopy failed to identify the bleeding site. The patient presented persistent hypotension and Norepinephrine vasopressor support was initiated resulting in hemodynamic stability. The arterial blood gases (Table 1) evidenced respiratory acidosis, hemoglobin of 4.4g/dl, and hematocrit 13%. The approximate estimate of intraoperative hemorrhage was 1300cc, so the decision was made to transfuse 2 units or red blood cells. The post-transfusion thromboelastography reported R: 6.1min, K: 3.6min, angle 46.5°, MA: 57.1mm, IC: −2.6 interpreted as normal. Based on the patient's comorbidities, the decision was made not to transfuse other blood products.
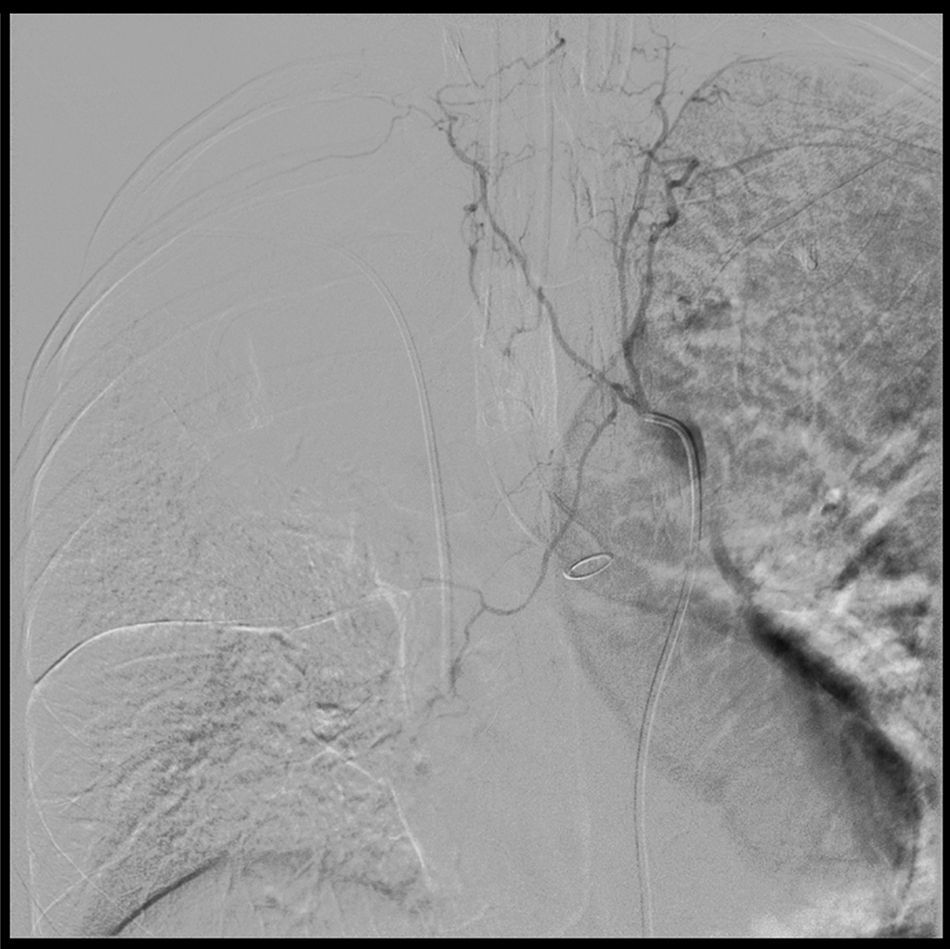
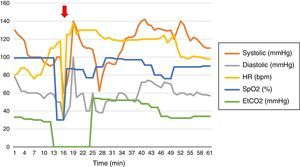
Arteriography under Interventional Radiology evidenced severe vasospasm at the bleeding site and thus the embolization was deferred (Fig. 2).
Aortogram and bronchial arteriography. The arteriography enables the selective catheterization of all the bilateral intercostal trunks above and below the left tracheobronchial angle, with failure to catheterize the bronchial artery. This is suggestive of vasospasm and is consistent with the arrest of the active bleeding. The hemorrhage is not visible in the aortogram. Dual lumen tube in the left source bronchus.
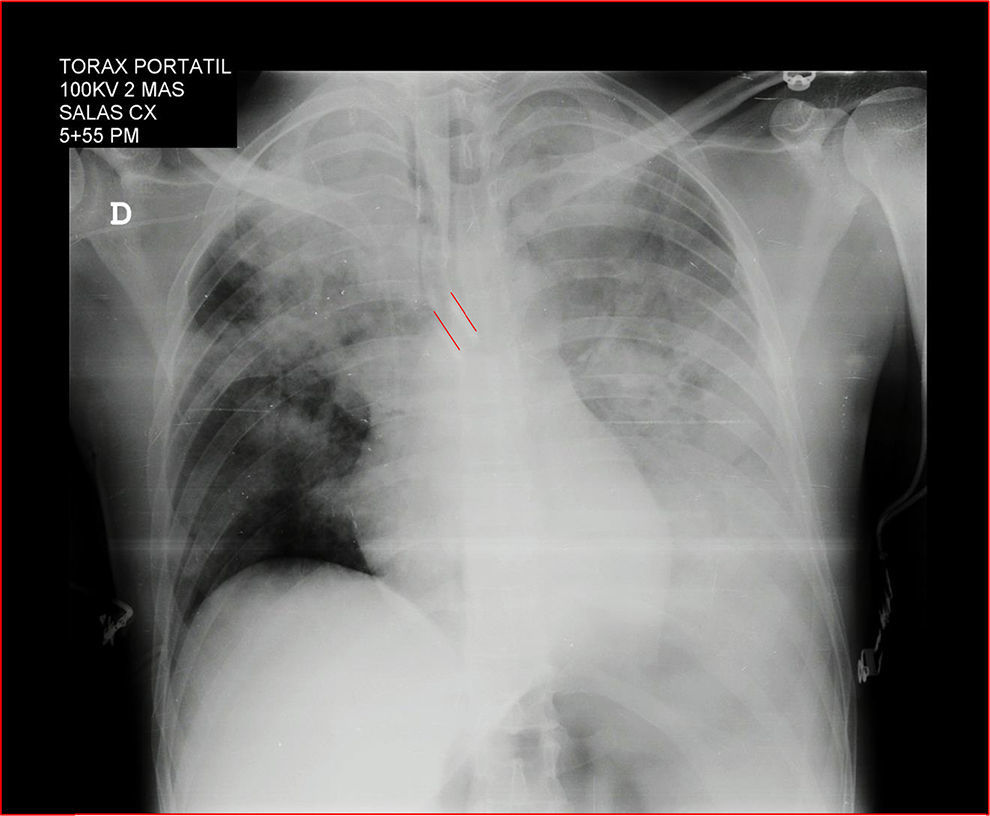
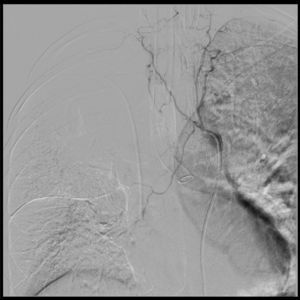
A portable chest X-ray confirms the position of the dual lumen tube and the bleeding in the left lung (Fig. 3). The patient was transferred to the ICU with stable vital signs, arterial blood gasses monitoring, improved respiratory acidosis, normal lactic acid, normal clotting times, hemoglobin 10.8g/dl, hematocrit 34%, manual platelet count 69,000, suggestive of potential intraoperative hemodilution, with normal MA. The decision was made to defer platelet transfusion.
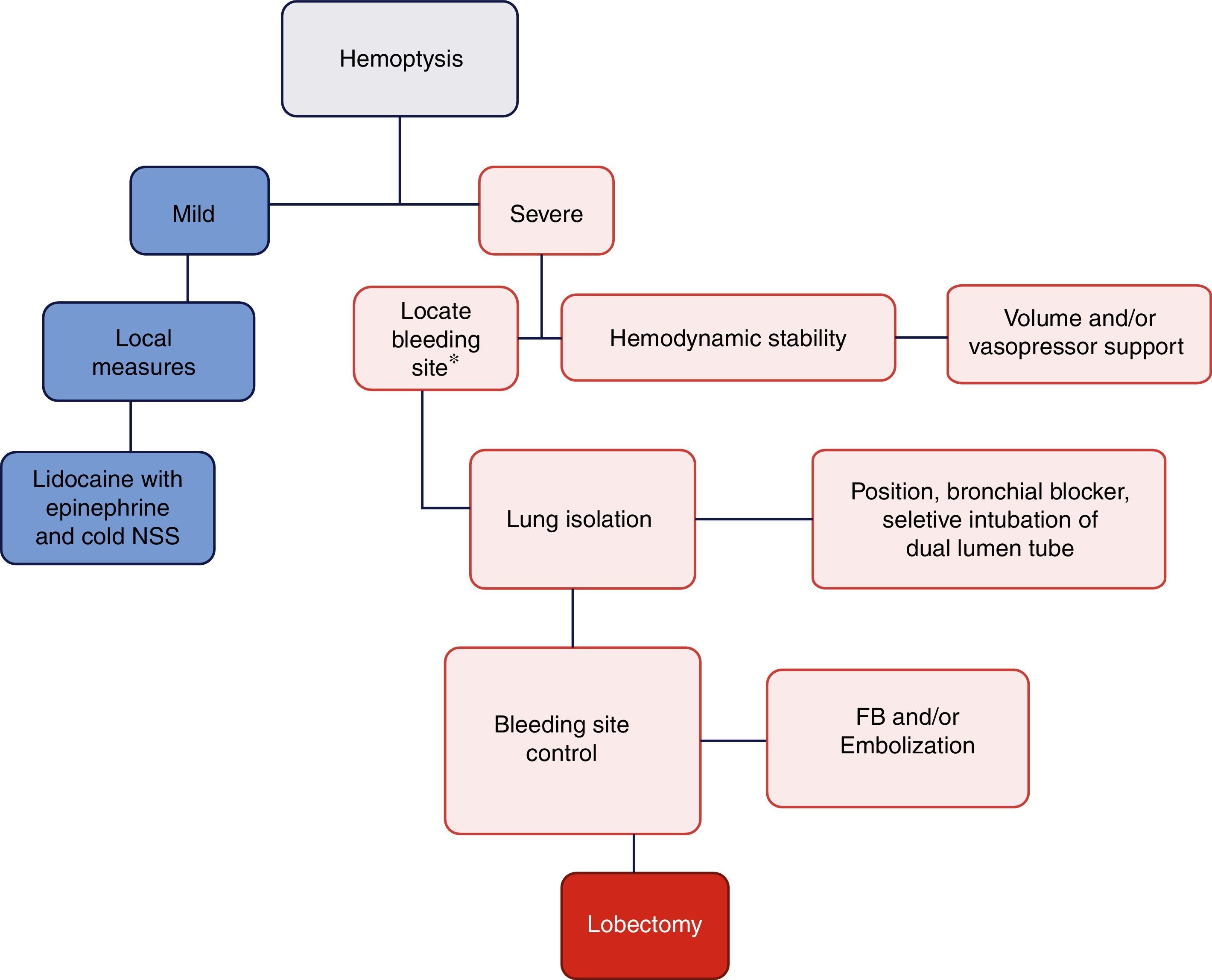
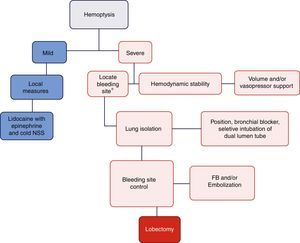
DiscussionThe objective of managing a massive hemoptysis during fibrobronchoscopy is threefold: hemodynamic stability, lung isolation, and bleeding control.17Fig. 4 illustrates the algorithm suggested for massive hemoptysis management.
Algorithm proposed for the management of massive hemoptysis during fibrobronchoscopy. *With rigid fibrobronchoscope (FB) if proximal bleeding is suspected or flexible FB if the bleeding is distal. NSS: normal saline solution. Mild hemoptysis: no hemodynamic involvement and self-limiting bleeding. Severe hemoptysis: causes hemodynamic instability, requires transfusion, or results in hypoxemic respiratory failure.
Any large volume of blood loss must be quickly replaced in order to avoid tissue ischemia, irreversible shock, and multiple organ failure. The use of crystalloids is the first line of therapy; colloids have failed to show improved outcomes in mortality, multiple organ failure, length of ICU or hospital stay, number of days on mechanical ventilation, or renal replacement therapy required.18 In our case we used Ringer Lactate because the NSS is associated with hyperchloremia and metabolic acidosis.19,20
Immediate massive transfusion shall be considered in case of uncontrolled hemorrhage and hemodynamic instability, defined as the use of more than 10 units over the first 24h, more than 4 units in 1h or volume replacement of over 50% of volemia over 3h.21–23
The massive transfusion protocols with fixed transfusional strategies of RBC/fresh plasma/platelets (1:1:1 or 2:1:1) have failed to show any benefit.23 In this case, only 2 units of RBC were needed. Based on the thromboelastography results, no additional blood products were used, neither was the massive transfusion protocol activated.
The use of vasopressors such as norepinephrine fails to treat the underlying cause of the hypovolemic shock and reduce the peripheral tissue perfusion. The decision to use vasopressors shall be based on the individual patient clinic.
In order to locate the bleeding site, the clinical signs (wheezing, pulmonary aggregates, dullness to percussion) may be unspecific. The use of the bronchoscope which has a larger diameter offers the advantage of a higher suction power and maintenance of the patent airway and hence is the preferred approach in case of proximal airway bleeding, but not in case of peripheral bleeding; hence, the flexible fibrobronchoscope is ideal in this case, but the narrower gauge limits any potential therapeutic intervention and prevents proper suction in massive hemoptysis.16
Positioning the patient on the side of the bleeding lung is intended to prevent the hemorrhage from reaching the healthy lung, which increases the pulmonary shunt and further impairs the ventilation/perfusion ratio. According to the literature reviewed, there are no studies measuring the benefits of such maneuver and the priority shall be to secure the airway.16
Intubation with dual lumen tube is ideal to secure the airway, isolating the bleeding lung and allowing for ventilation of the other lung. Proper positioning shall be done and checked under direct vision but this requires experience and is difficult to accomplish under profuse bleeding in the airway. Furthermore, repeated failed attempts may increase the patient's morbidity and mortality.16,24,25 In this particular case, we were able to intubate with a dual lumen tube on the first attempt. Other options include selective intubation with a standard orotracheal tube or the use of the bronchial blocker.
Timeliness in controlling the bleeding site is of the essence to improve the perioperative outcomes. The fibrobronchoscope enables the visualization of bleeding and if the bleeding is mild it may be controlled with the continuous administration of 50ml of cold water (4°C).26 Another option is the possibility to place a bronchial blocker, although the literature regarding the efficacy thereof is limited and in case of massive bleeding proper positioning of the blocker is quite difficult.16,26–29 Endobronchial techniques using electrical devices (laser, cryotherapy and argon plasma) could be an option for definitive therapy, but the implementation of these techniques could be difficult in massive hemoptysis.30
In our case, it was not possible to perform an arteriogram-based embolization because of the presence of severe vasospasm at the bleeding site (Fig. 2) which is a contraindication. This technique is a diagnostic and therapeutic option of the bleeding site.31,32 Around 90% of massive hemoptysis derive from the bronchial arteries33; selective embolization of these arteries controls bleeding in up to 90% of the cases.34,35
Emergency surgery (lobectomy or segmentectomy) is the last option to control severe hemoptysis. Mortality may be as high as 25%, though it may decrease with smaller resections.36
Finally, we did not use antifibrinolytic therapy (tranexamic acid) to control hemoptysis. The literature is scant, non-comparable, and has a low level of evidence.37 There is not enough evidence to know whether the antifibrinolytic therapy shall be used for the treatment of hemoptysis from any cause, although some trials suggest a shorter duration of bleeding.38
In conclusion, the management of massive hemoptysis shall be multidisciplinary (Anesthesiologist, Interventional Pulmonologist, Thorax Surgeon, and Interventional Radiologist). The three pillars of treatment are based on achieving hemodynamic stability, ensuring adequate ventilation of the healthy lung, and controlling the bleeding site (Fig. 4). Whilst the incidence of this complication is low, it may have fatal consequences and further studies are required for adequate treatment thereof.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingNone.
Conflict of interestThe authors have no conflicts of interest to disclose.
Please cite this article as: Segura-Salguero JC, Díaz-Bohada L, Lutz-Peña JR, Posada AM, Ronderos V. Manejo perioperatorio de hemoptisis masiva durante la realización de fibrobroncoscopia: Rev Colomb Anestesiol. 2017;45:256–261.