Decreased blood flow disrupts the endothelium, changes the nitric oxide/endothelin-1 ratio, narrows the capillaries and results in microcirculatory dysfunction. Secondary anoxia leads to mitochondrial energy imbalance, depletion of adenosine-triphosphate and disruption of the intracellular hydrogen, sodium and calcium homeostasis. If the flow is restored, the reperfusion stimulates the endothelial expression of adhesion molecules attracting polymorphic nucleotides and platelets, with subendothelial infiltration of these cells and their entrapment in the microvasculature, as well as vasoconstriction, endothelial edema and reduced flexibility of the cellular membrane. Ischemia/reperfusion may result in inflammation and organ failure.
ObjectiveTo determine whether hypertonic saline solution reduces the ischemic/reperfusion injury in the liver, the kidney, and the ileum.
Materials and methodsExperimental trial in pigs. Aortic blood flow suppression (15min) and reperfusion (60min). The experimental group was pretreated with 7.5% hypertonic saline and the control group received normal 0.9% saline solution. Hemodynamic, gasometric, and biochemical measurements were taken, and the serum and tissue levels of ET-1, TNF-alpha, IL-10, and IL-2 were determined.
ResultsThere were no significant differences in the tissue expression of ET-1, TNF-alpha, IL-10, and IL-2 between the two groups. The hemodynamic behavior was similar in both groups. The group treated with hypertonic solution showed an increasing post-perfusion systolic rate up to the basal values, while the systolic rate in the control group dropped significantly (p=0.015).
ConclusionHypertonic solution prior to the ischemic insult improves the ventricular function after reperfusion.
La disminución del flujo sanguíneo altera el endotelio, cambia la relación oxido nítrico/endotelina-1, estrecha capilares y produce disfunción microcirculatoria. La anoxia secundaria lleva a desacople energético mitocondrial, depleción de adenosin-tri-fosfato y alteración de la homeostasis intracelular de hidrógeno, sodio y calcio. Si el flujo se reanuda, la reperfusión estimula la expresión endotelial de moléculas de adhesión que atraen polimorfonucleares y plaquetas, con infiltración subendotelial de estas células y su atrapamiento en la microvasculatura, así como vasoconstricción, edema endotelial y disminución de la flexibilidad de la membrana celular. La isquemia/reperfusión puede derivar en inflamación y fallo orgánico.
ObjetivoDeterminar si la solución salina hipertónica disminuye la lesión isquemia/reperfusión en hígado, riñón e íleon.
Materiales y métodosEstudio experimental en cerdos. Supresión del flujo sanguíneo aórtico (15 minutos) y reperfusión (60 minutos). El grupo experimental recibió pre-tratamiento con solución salina hipertónica al 7,5% y el grupo control solución salina normal al 0,9%. Se realizaron mediciones hemodinámicas, gasométricas, bioquímicas, y determinación sérica y tisular de ET-1, TNF-alfa, IL-10, IL-2.
ResultadosNo hubo diferencias significativas en la expresión tisular de ET-1, TNF-alfa, IL-10, IL-2 entre los grupos. Los grupos presentaron un comportamiento hemodinámico similar. El grupo tratado con hipertónica exhibió un índice sistólico post-reperfusión que aumentó hasta los valores basales, mientras que el índice sistólico del grupo control presentó una caída significativa (p=0.015).
ConslusiónLa solución hipertónica antes del insulto isquémico mejora la función ventricular después de la reperfusión.
Various models have been proposed to explain the mechanisms of tissue damage secondary to ischemia and reperfusion.1–7 With regards to ischemia, the variations in pulsatile flow on the arterial wall shear stress have been shown to disrupt the cellular microenvironment, generating an imbalance concentration of endothelin-1 (ET-1)/nitric oxide (NO)/prostacyclin, decelerating the blood flow down to non-reflux limits, and favoring the participation of erythrocytes to finally suppress the inflow of oxygen and glucose into the cell.1,8 The response of the cell to anoxia9,10 involves mitochondrial energy mismatch, depletion of adenosine-triphosphate (ATP) and disruption of the homeostasis of the hydrogen (H+), sodium (Na+), and calcium (Ca2+) ions. These events activate proteolytic enzymes and damage the cell volume regulatory capacity (cell edema), specifically affecting the endothelial cells and the macrophages. This phenomenon, together with a change in the NO/ET-1 production ratio, contributes to further narrowing of the lumen of the capillaries and to microcirculatory dysfunction. The narrowing of the capillaries results in the accumulation of neutrophils (PMNs) and the simultaneous activation of macrophages, stimulating the release of reactive oxygen species (ROS). These ROS are also released through the mitochondrial pathway or the xanthine/xanthine oxidase (X/XO) pathway of the endothelial cells, as well as by the action of inflammatory cytokines, including tumor necrosis factor alpha (TNF-alpha) and interleukins (IL).4 Cytokines induce the endothelial expression of adhesion molecules (intracellular adhesion molecule 1-ICAM- and vascular endothelial cell adhesion molecule-VCAM-), while chemokines lead to the activation and recruitment of PMNs. IL-1 and TNF-alpha recruit and activate CD4+ T-lymphocytes that release the granulocyte-macrophages colony stimulating factor (GM-CSF), interferon-gamma (IFN-γ), and TNF-beta.
However, the tissue injury further deteriorates after the blood flow is re-established and there is a significant oxygen input. Post-ischemic perfusion trials show that ET-1 is one of the key actors in organ microcirculatory changes; however, ROS also play a critical role, particularly when mediated via the X/XO pathway. The contribution of PMNs and macrophage-derived ROS is significant. Factors such as ischemia time and temperature also affect the ROS production pathway.11
The activation of the endothelium from mechanical stimuli triggers a chemical response initially mediated by NO and ET-1. The production of NO is not always associated with a beneficial vasodilatation process. In fact, unreleased amounts of NO under the action of inducible nitric oxide synthase (iNOS) may be harmful because the delayed release of NO, combined with superoxide anion, favors the production of peroxynitrite that acts as a powerful oxidant.6,12
The I/R injury on the endothelium stimulates the expression of adhesion molecules that interact with the PMNs and platelets, enabling the subendothelial infiltration of PMNs and their entrapment inside the microvasculature, further facilitated by vasoconstriction, endothelial edema, and a decreased cell membrane flexibility. The activation of the various subpopulations of T-lymphocytes is also favored through complement action.13 The mechanisms of PMN damage include the release of ROS following the respiratory explosion of the nicotinamide-adenine-dinucleotide-phosphate (NADPH) oxidase system, and the release of proteolytic enzymes. The stimulation of cytokine release from endothelial cells, recruiting larger numbers of PMNs and plugging the capillaries, results in worsening of non-reflux.14 Moreover, hypoxia alters the ability of the adaptive intracellular mechanisms to maintain the cell volume. Cell volume is maintained via intracellular signaling events such as changes in the transmembrane potential, changes in ion composition, in the second messenger cascades, the phosphorylation of various proteins, the expression of certain genes, and apoptosis.8,12,15,16 Such a process is essential for erythrocyte function, for epithelial transfer, the regulation of metabolism, hormone release and cell contraction, migration and proliferation; all of these mechanisms are involved in hypoxia tolerance and tissue repair.
7.5% hypertonic saline solution (HSS) has been used in the clinic in humans for hemorrhagic shock resuscitation and as a second line therapy in intracranial hypertension. Experiments have been designed in animal models with heart, kidney, lung, liver and gut ischemia. Their mechanism of action involves the rapid optimization of the intravascular volume and myocardial contractility, in addition to maintain the dilatation of the terminal arterioles and flow, preventing the “clogging” of erythrocytes and leukocytes.17–20 Hypertonic saline also maintains the cell volume by improving the cell and erythrocyte edema, reducing PMNs binding to the endothelium and inflammation.21,22
This paper discusses the hypothesis that HSS infused prior to ischemia reduces the systemic and tissue impact of the I/R injury. The objective of the trial is to determine the hemodynamic behavior and the serum and tissue expression of inflammation mediators using HSS.
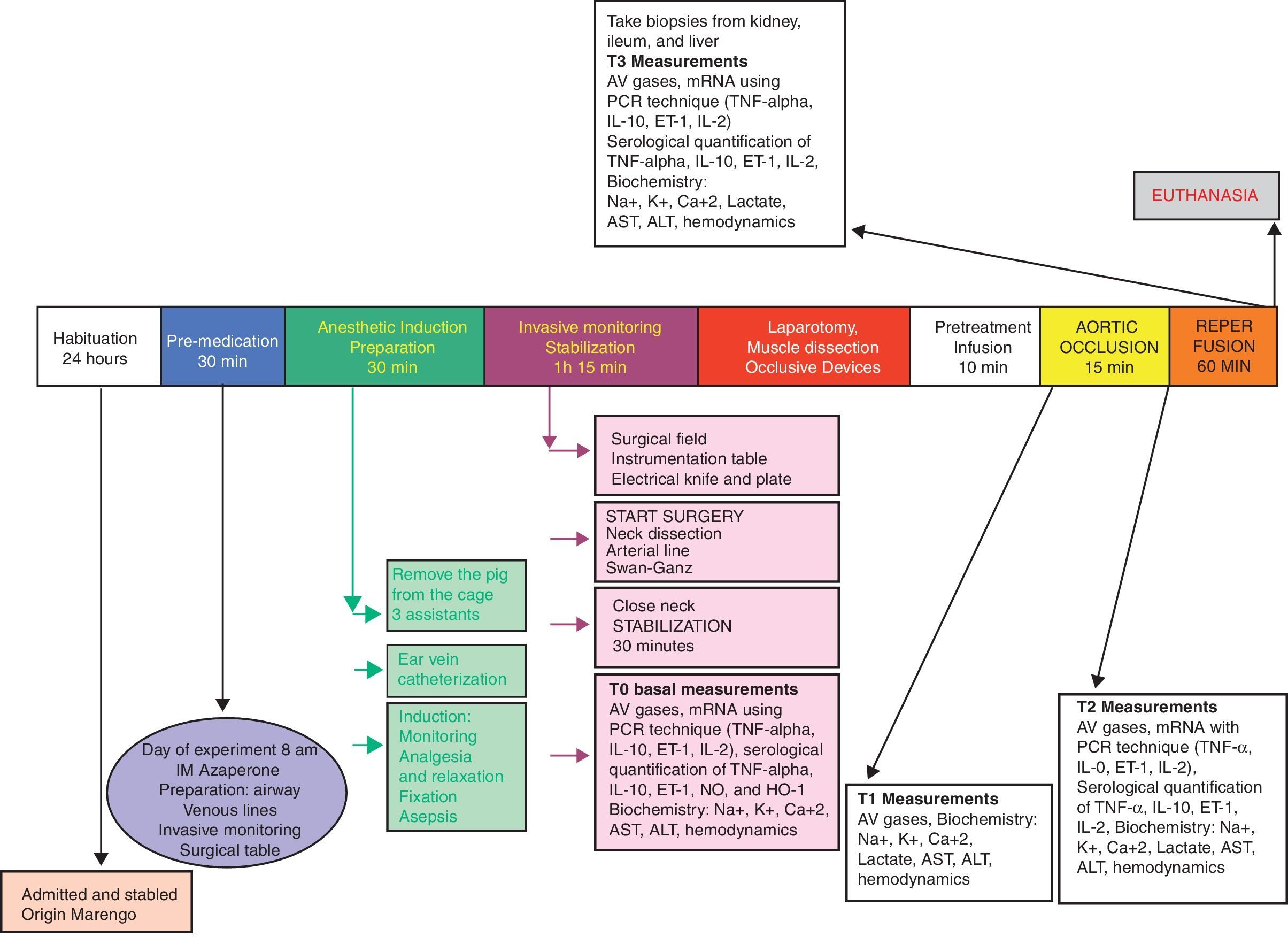
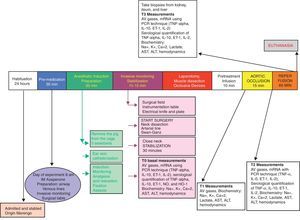
Experimental designFollowing the approval of the Ethics Committees of the School of Veterinary Medicine and Animal Health and the School of Medicine of the Universidad Nacional de Colombia, a randomized experimental trial was done in pigs. The animals underwent general anesthesia for the intervention, and the procedure was done according to a blood flow suppression model with extrinsic occlusion of the aorta above the celiac trunk for 15min, and then the flow was restored. The reperfusion injury was evaluated 60min after removal of the aortic occlusion (Fig. 1).
Animals14 terminal line crossbred pigs were used (Tecniagro X G&P X Landtrace), upon a request for litter programming and formalities for transport authorization. The Tecniagro line comprises animals from the Large White X Pietran line. The animals wore vaccination ear tags and had been dewormed. These procedures were implemented according to the guidelines and standards of the Instituto Colombiano Agropecuario. At the Universidad Nacional de Colombia, 20–25kg pigs were used supplied by the Centro Agropecuario Marengo, were used.
Group allocationThe animals were allocated to one of the two groups, using a simple randomization method, blinding the allocation using opaque envelopes. The researchers doing the measurements of the clinical and paraclinical outcomes were blinded to the distribution. The groups were as follows:
Hypertonic-GH solution group (n=7) – These animals received 7.5% HSS at a dose of 4ml/kg of weight, 10min prior to the aortic occlusion.
Control group-CG (n=7) – These animals received 0.9% NSS at a dose of 4ml/kg of weight, 10min prior to the aortic occlusion.
Anesthetic and surgical managementThe animals were admitted and stabled for 24–36h prior to the procedure at the facilities of the school of veterinary medicine, with water and feed ad libitum. During the 12h prior to surgery, the animals only had access to water but no feed. 30min prior to the induction of anesthesia, the animals were pre-medicated with IM azaperone (40mg/ml) at a dose of 2mg/kg. At admission to the OR, the animals were weighed and measured. The induction was done with inhaled isofluorane, inserting an 18G peripheral venous catheter into the pinna and 0.9% NSS was used for maintenance. IV sodium thiopental (10mg/kg) was administered for airway management with orotracheal intubation with a 6.5F tube. The tube was fixed in place and the animals were placed in supine decubitus. Mechanical ventilation was provided using the anesthesia machine (tidal volume: 10ml/kg and respiratory rate: 16/min); standard monitoring using pulse oximetry and continuous cardiac visioscope. Anesthesia was maintained with inhaled 1% isofluorane, fentanyl citrate (5mcg/kg/h IV), vecuronium bromide (0.2mg/kg IV every 2h), and intravenous fluids (0.9% NSS at 5ml/kg/h).
Under general anesthesia and restraining the four extremities, the animals were kept in supine decubitus to proceed with an anterolateral oblique cervical incision, sectioning of the platysma and lateral retraction of the sternocephalic muscle. The surgeon continued to dissect up to the internal jugular vein and the common carotid artery, and then sutured with 0 silk. A distal ligature of the internal jugular vein was completed, with venotomy and insertion of a Swan Ganz catheter, with pressure monitoring to check for proper placement of the tip of the catheter into the pulmonary artery. The common carotid artery was dissected, a distal ligature applied and the arterial pressure monitoring catheter was inserted. Then a medial laparotomy was performed, and then, the infra diaphragmatic aorta was dissected 1cm below the hiatus of the diaphragm, with cotton tape repair and preparation of Rummel tourniquet. Liver biopsies were taken at the end of the procedure (wedge 3cm×2cm dissection of the right medial lobe), as well as biopsies from the left kidney (2cm×1cm lower pole) and the small intestine – distal ileum (4cm long segment, prior ligature of the mesenteric vessels).
InterventionFollowing the above anesthetic and surgical procedure, the animal was stabilized for 30min. Then, unlabeled 20ml syringes prepared by the research assistant containing the solution for infusion were delivered to the researcher in charge of anesthesia, according to the sealed envelope instructions for each animal.
Hypertonic solution group (GH): 4ml/kg of 7.5% HSS were infused for 10min. At the end of the infusion, the aortic occlusion was maintained for 15min and then the occlusion was released, for a 60-min reperfusion.
Control group (CG): 4ml/kg of 0.9% NSS were infused for 10min. At the end of the infusion, the aortic occlusion was maintained for 15min, after which the occlusion was released, for a 60-min reperfusion.
Measurements- -
Hemodynamic variables measured: heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), right atrial pressure (RAP), pulmonary capillary pressure (PCP), cardiac output (CO).
- -
Hemodynamic variables calculated: cardiac index (CI), pulmonary vascular resistance index (PVRI) and systemic (SVRI), left ventricular stroke work index (LVSWI) and right (RVSWI).
- -
Gasometric variables: O2 arterial blood pressure (PaO2), CO2 arterial blood pressure (PaCO2), Arterial pH (pH art), arterial O2 saturation (SaO2), base excess arterial (BE art), arterial lactate concentration (Lactate art), O2 venous pressure (PvO2), CO2 venous pressure (PvCO2), venous pH (pH ven), venous O2 saturation (SatvO2), base excess venous (BE ven), PaO2/FiO2, rate of O2 extraction (ExtO2).
- -
Hemoglobin, hematocrit.
- -
Blood chemistry: Glycaemia, BUN, creatinine, transaminases (AST, ALT), sodium (Na+), potassium (K+), calcium (Ca2+).
- -
Molecular biology: Serum determination of TNF-alpha and IL-2 (inflammatory cytokines) levels, IL-10 (anti-inflammatory cytokines), ET-1 (markers of endothelial injury) in serum or plasma using the Enzyme-Linked-Immuno-Sorbent-Assay ELISA technique.
- -
Detection of protein expression with immunohistochemistry for TNF-alpha (inflammatory cytokines) and IL-10 (anti-inflammatory cytokine) in liver, kidney, and ileum tissue.
- -
Histological determination (using hematoxylin/eosin -HE- in the gut, the liver and kidney) of ischemia/reperfusion injury. The extent of congestion and hemorrhage, edema and the presence of intercellular dehiscence, necrosis, and apoptosis were analyzed.
- -
-T0: Baseline: Following the induction of anesthesia and after 30min of animal stability, the hemodynamic variables were recorded and blood samples were drawn for arterial and venous gases, blood chemistry, and molecular biology.
- -
-T1: At the end of the NSS or HSS infusion (10min): prior to the aortic occlusion, the hemodynamic variables were recorded and blood samples were drawn for arterial and venous gases, blood chemistry and molecular biology.
- -
-T2: After 5min of aortic occlusion: prior to removing the aortic occlusion devices, the hemodynamic variables were recorded and blood samples were drawn for arterial and venous gases, blood chemistry and molecular biology.
- -
-T3: 60min post-reperfusion: The hemodynamic variables were recorded and blood samples were drawn for arterial and venous gases, blood chemistry and molecular biology. Liver, kidney, and ileum biopsies were then taken.
Blood samples
- -
Arterial gases: from the single-lumen catheter inserted into the carotid artery.
- -
Venous gases: from the proximal line of the pulmonary artery catheter.
- -
Blood chemistry and molecular biology: plasma and serum samples were taken through the proximal line of the pulmonary artery catheter.
All samples were properly labeled and packaged as per the experimental protocol. The samples were sent to be processed at the Clinical laboratory of the School of Veterinary Medicine and the Laboratory of Physiology of the School of Medicine, Universidad Nacional.
Tissue samples
To avoid any bleeding that could hemodynamically compromise the animal during harvesting of tissue samples, the following steps were followed:
- -
Ileum wedge biopsy from the antimesenteric margin: 30cm away from the ileocecal junction, the proximal and distal intestine underwent painless clamping along a 10cm segment, avoiding clamping the mesentery. A knife wedge biopsy was taken from the antimesenteric margin, with a 3cm base and the apex at 5mm from the mesenteric margin. The gut was then sutured in one plane using 4-0 polypropylene.
- -
Free margin wedge biopsy of the liver: cold knife section, 5cm base and 1cm apex. The hemostasis was done with a 4-0 polypropylene suture.
- -
Wedge biopsy of the renal cortex: cold knife section, 5cm bases and 1cm apex, macroscopically checking for a complete inclusion of the cortex.
Every 0.3cm3 tissue fragments of the organs biopsied (ileum, liver, and kidney) were placed in non-sterile bottles with 10% formaldehyde to be forwarded to the Pathology laboratory of the School of Medicine.
EuthanasiaUpon completion of the protocol and with the animal under general anesthesia, a bolus of 40 milliequivalents of undiluted potassium chloride was administered via the distal pulmonary artery catheter. When the cardiac activity stopped, the anesthesia management was interrupted. Each animal was placed in a double bag for animal waste from experimental laboratories, and kept in the cold storage to be collected afterwards by the staff in charge of hospital waste disposal.
Statistical analysisThe data from the continuous variables were expressed as means, standard deviation and ranges. The data from the categorical variables were expressed as numbers and percentages. The quantitative variables were compared against Student-t and the qualitative variables against Chi square or Fisher's Test. For non-normal distribution variables, Mann–Whitney's U was used. A p≤0.05 value was estimated. The statistical analysis was based on STATA 10.1 (STATA Corp LP).
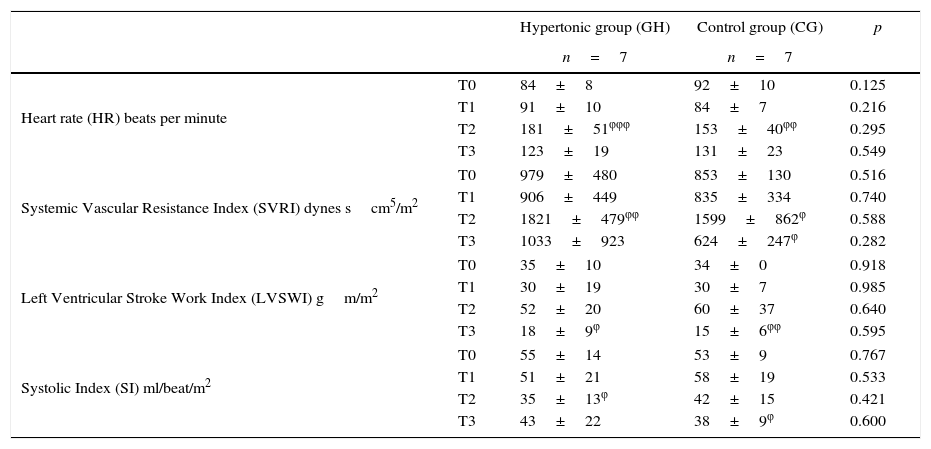
ResultsThe measurements of the hemodynamic changes throughout the experiment did not show any significant differences between the two groups (Table 1).
Hemodynamic behavior throughout the experiment.
| Hypertonic group (GH) | Control group (CG) | p | ||
|---|---|---|---|---|
| n=7 | n=7 | |||
| Heart rate (HR) beats per minute | T0 | 84±8 | 92±10 | 0.125 |
| T1 | 91±10 | 84±7 | 0.216 | |
| T2 | 181±51φφφ | 153±40φφ | 0.295 | |
| T3 | 123±19 | 131±23 | 0.549 | |
| Systemic Vascular Resistance Index (SVRI) dynes scm5/m2 | T0 | 979±480 | 853±130 | 0.516 |
| T1 | 906±449 | 835±334 | 0.740 | |
| T2 | 1821±479φφ | 1599±862φ | 0.588 | |
| T3 | 1033±923 | 624±247φ | 0.282 | |
| Left Ventricular Stroke Work Index (LVSWI) gm/m2 | T0 | 35±10 | 34±0 | 0.918 |
| T1 | 30±19 | 30±7 | 0.985 | |
| T2 | 52±20 | 60±37 | 0.640 | |
| T3 | 18±9φ | 15±6φφ | 0.595 | |
| Systolic Index (SI) ml/beat/m2 | T0 | 55±14 | 53±9 | 0.767 |
| T1 | 51±21 | 58±19 | 0.533 | |
| T2 | 35±13φ | 42±15 | 0.421 | |
| T3 | 43±22 | 38±9φ | 0.600 | |
Data reported as mean±standard deviation, 95% confidence interval.
During the aortic occlusion (T2), significant increases were found against the baseline values in each group, with regards to heart rate (p=0.0023 GH, p=0.0004 CG), mean systemic blood pressure (p=0.0005 GH, p=0.003 CG), and systemic vascular resistance (p=0.009 GH, p=0.04 CG).
Following reperfusion (T3), both groups showed decreased systemic vascular resistance. The group treated with HSS showed a drop in the SVRI values approaching the baseline. In the case of the control group, the drop in SVRI reached significantly lower values as compared to the baseline (p=0.049). During reperfusion, the left ventricular stroke work volume dropped significantly (p=0.019 GH, p=0.002 CG) as compared to the baseline values in both groups. During this time, the systolic index (SI) as a contractility variable, increased in the HSS-treated group. In the control group (CG) the SI decreased, reaching significantly lower levels (p=0.015) versus the baseline.
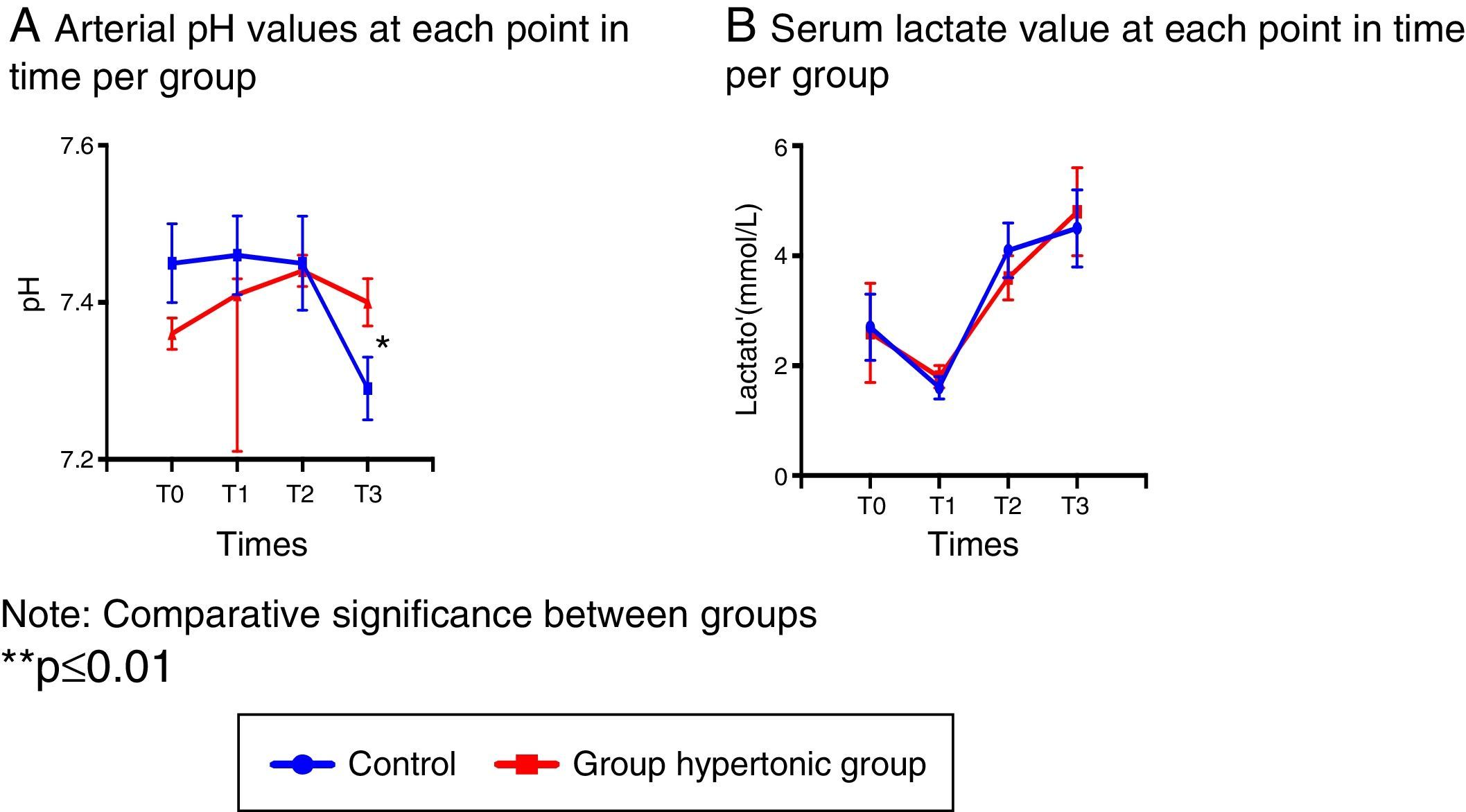
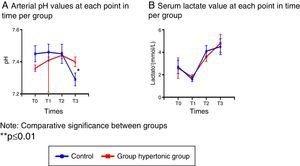
Blood gasometry analyses indicated arterial pH variations resulting in significant differences following reperfusion between the two groups (T3), and at that time the control group presented acidosis with a significant drop in the arterial pH (Fig. 2A).
With regards to the other blood gasometry parameters, both groups behaved similarly.
In particular, the serum lactate levels decreased with the intravenous fluid infusion (hypertonic solution – GH- or normal saline solution – CG-) in T1 in both groups, leading then to a sustained increase with significantly higher values as compared to T1. (T2: p=0.006 GH, p=0.001 CG, T3: p=0.002 GH, p=0.003 CG) (Fig. 2B).
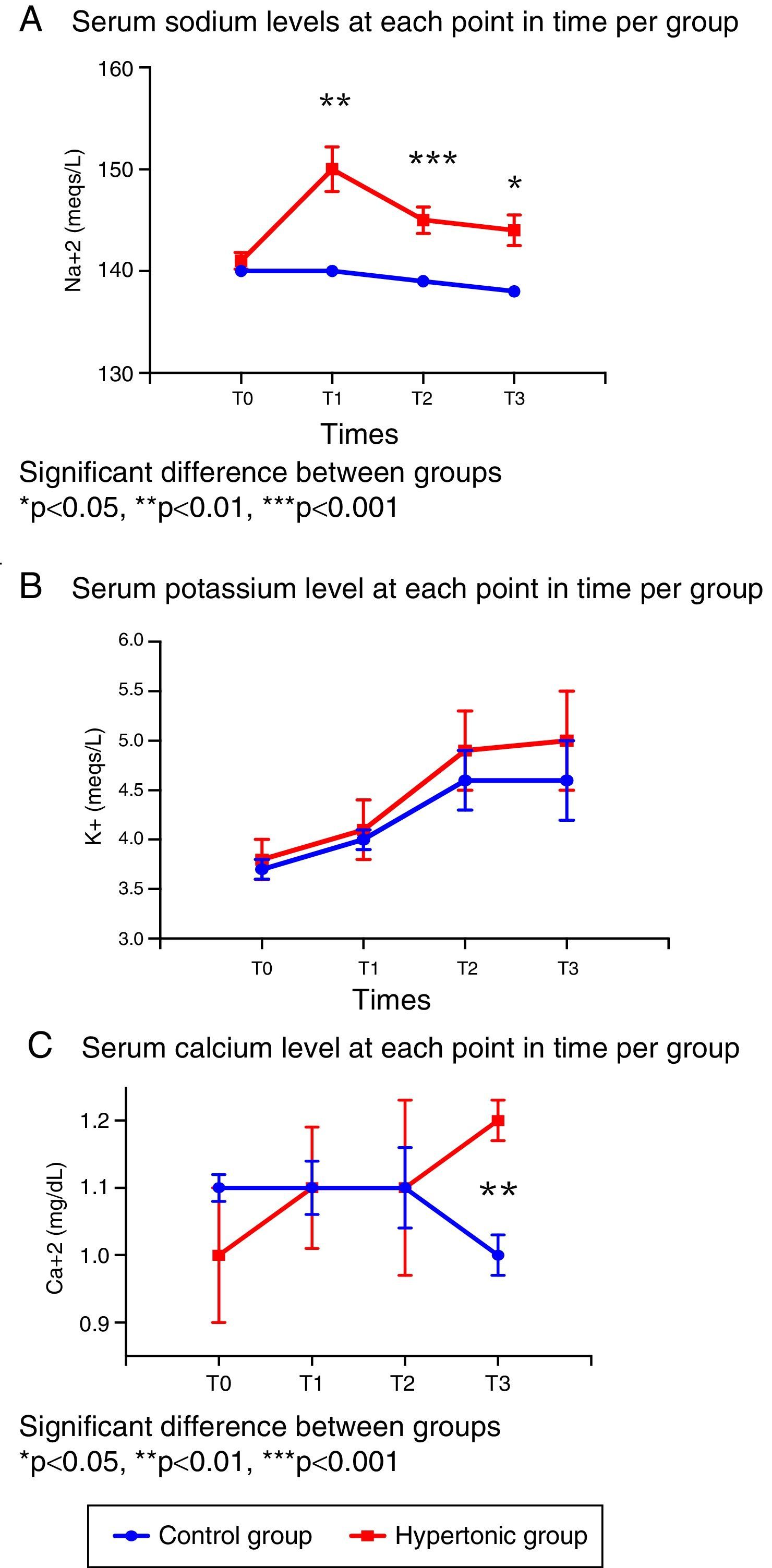
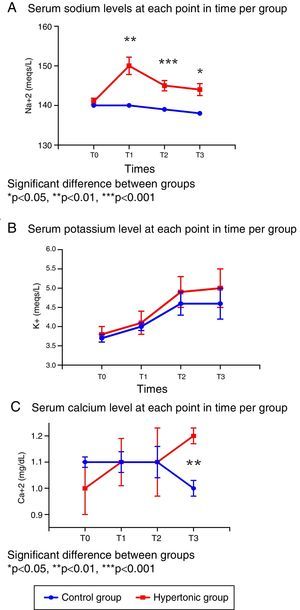
In terms of the serum electrolyte behavior, the baseline values (T0) were similar in both groups (Fig. 3).
There were significant differences in the sodium levels between the two groups. The group treated with HSS (GH) presented significantly higher sodium levels throughout the experiment, as compared with the control group (T1: p=0.001, T2: p=0.0006, T3: p=0.019). The peak value in the treated group was reached following the infusion (T1). The sodium levels for both groups were maintained within the normal ranges during the experiment. (Fig. 3A).
With regards to serum potassium, there were no differences between the two groups in the levels throughout the experiment (T0 to T3); the potassium levels increased progressively within the normal reference range at each time point (Fig. 3B).
The serum calcium levels were similar in both groups from T0 to T2; following reperfusion (T3), the levels are significantly different (p=0.006) as a result of the drop in calcium levels in the control group (Fig. 3C).
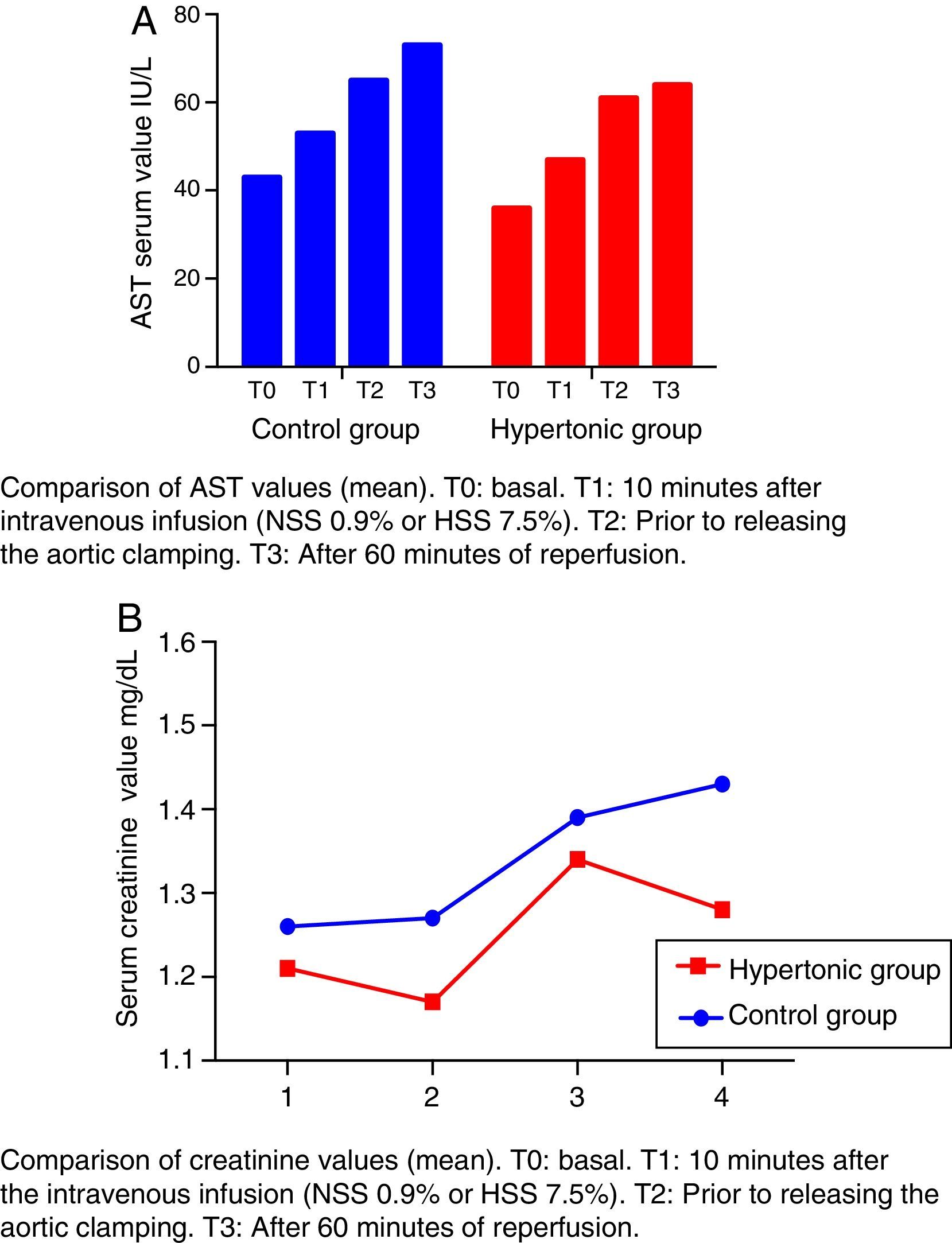
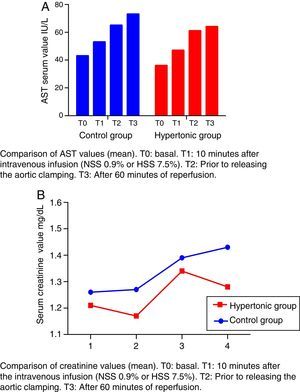
The creatinine and transaminases figures show a rising trend curve throughout the experiment (T0 to T3) in the two groups, with no significant differences between them.
The levels of transaminases aspartate-amino-transferase (AST) increase after T1 beyond the normal range (Fig. 4A). This elevation is significant in the control group (CG) versus the baseline value (T2: p=0.018, T3: p=0.034).
The creatinine levels are maintained within the range of normal values (Fig. 4B).
None of the referred values (creatinine and transaminases) decrease after reperfusion in either group (T3) (Fig. 4).
Serum TNF-alpha and IL-2 levels showed no significant differences throughout the experiment (T0 a T3). There was no significant elevation of cytokines during aortic occlusion (T2) or during reperfusion (T3). The observed values of both TNF-alpha and IL-2 were maintained within the normal range.
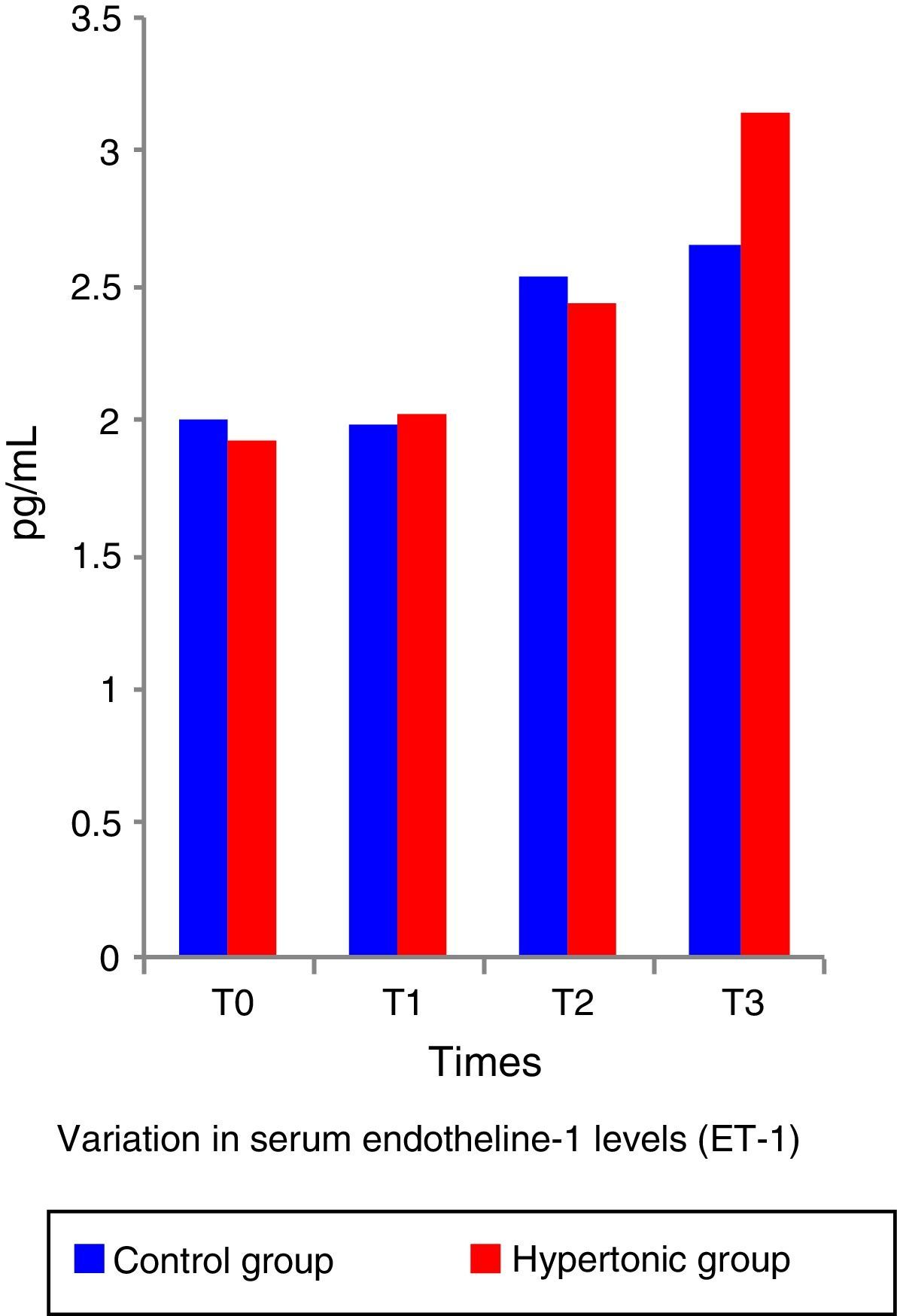
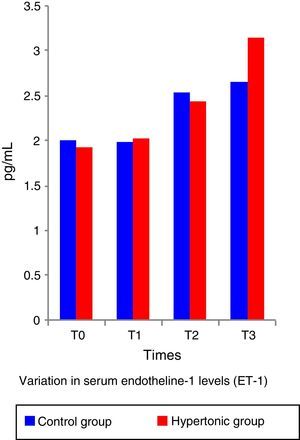
The ET-1 (Fig. 5) and IL-10 levels did not show significant differences between the two groups. Both, ET-1 and IL-10 showed normal figures above the reference levels throughout the experiment (T1 to T3). There was no correlation between the IL-10 and ET-1 values with the systolic index (SI – contractility parameter) at any of the time points during the experiment (pairwise comparison test with Bonferroni correction) in any of the two groups.
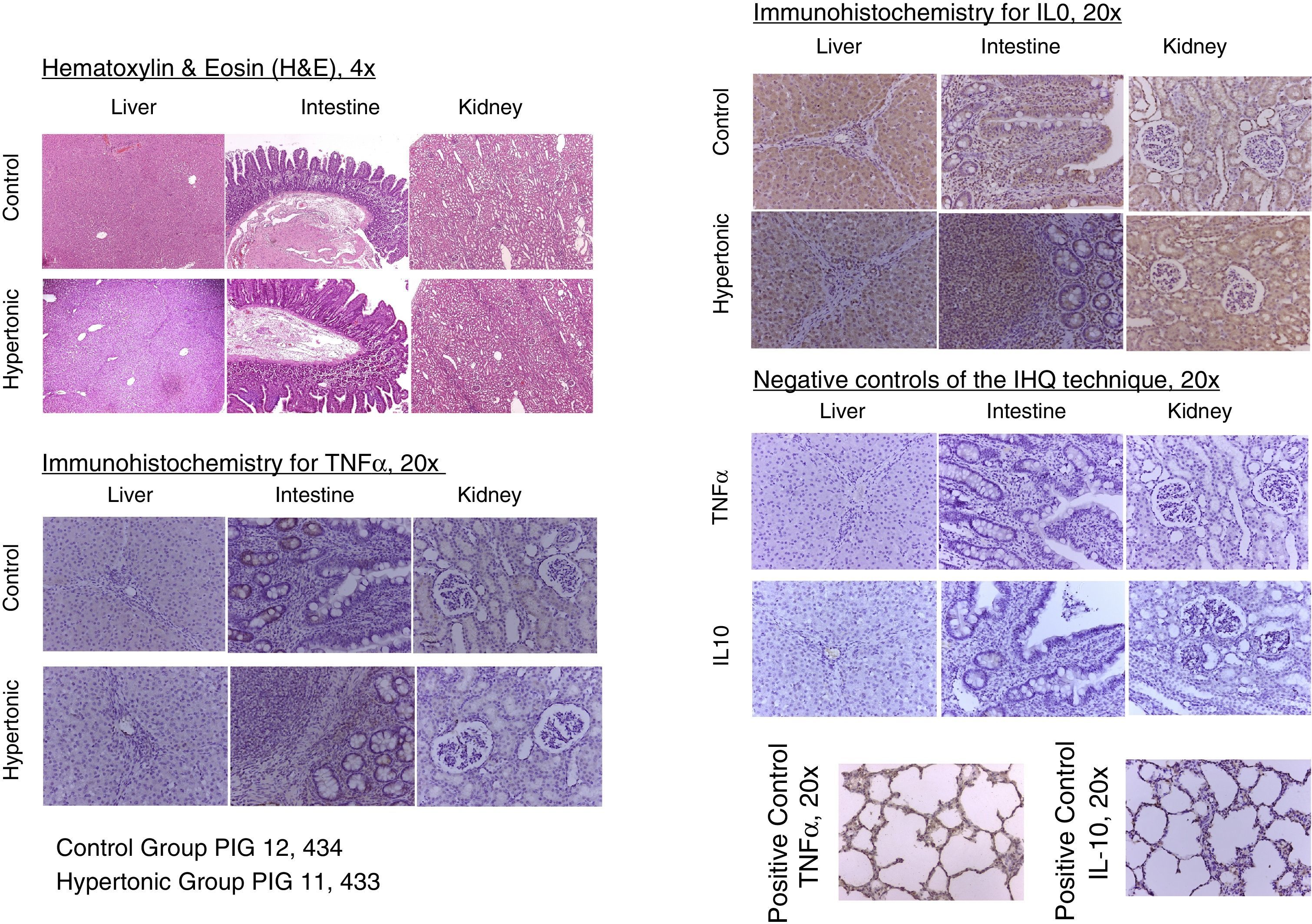
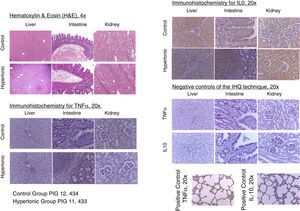
With regards to the histopathology changes, five parameters were used for their interpretation (edema, infiltrates, necrosis, apoptosis, and deposit substances), interpreted as present (P=1) or absent (A=0), to determine a score for each organ (liver, kidney, intestine). A general characterization was also evaluated as normal (N=0) or abnormal (A=1). The results of the pathological examination with hematoxylin/eosin showed no differences between the two groups. There was no evidence of necrosis or apoptosis in any of the samples. The general characterization of the histopathology slides was normal (N=0). Those with an abnormal pattern presented edema with no other alterations (Fig. 6).
The tissue immunohistochemistry analysis for TNF-alpha and IL-10, did not report any differences between the two groups. In order to standardize the analysis and its interpretation, three parameters were scored: mild immune-reactivity (1), moderate (2), or severe (3). There were no differences between the two groups when comparing the scores for TNF-alpha and IL-10. The tissue expression of TNF-alpha and IL-10, showed no significant differences between the two groups (Fig. 6).
DiscussionThe abnormal decrease in blood flow (hemorrhage, cardiac arrest, vascular obstruction, etc.) results in hypoxia and as a consequence in the development of intracellular acidosis, alterations in sodium and calcium intracellular concentration, degradation of sarcoplasmic phospholipids and of the cytoskeletal proteins, mitochondrial edema, and loss of the transmembrane potential, decreased glutathione and alpha-tocopherol, secretion of preconditioning trigger substances (adenosine, bradykinin, angiotensin, opioids, etc.) and expression of adhesion molecules, cytokines (i.e. TNF-alpha) and vasoactive agents (i.e. endothelin).23,24 Reperfusion results in vasoreactivity changes (endothelium dependent and non-dependent) with a subsequent imbalance between vasodilatation and vasoconstriction in all beds, endothelial dysfunction, and disruption of the expression and reactivity of substances such as ET-1 and NO.23,25 Furthermore, reperfusion promotes an initially local inflammatory response leading to the systemic release of cytokines, chemokines, complement and neutrophil activation, release of free oxygen radicals, necrosis and apoptosis.26,27 The severity of the lesion depends on the extension (one or more organs involvement) and the duration of hypoxia, as proven by Flores et al.28 several decades ago; they published a renal ischemia model showing that a blood flow occlusion beyond one hour was associated with areas of vascular obstruction due to cellular edema, despite reestablishing the blood flow. These authors also described how to reverse the “non-flow” using hypertonic solutions (mannitol, in this particular paper) that decreased the cellular edema and relieved the obstruction of the microcirculation.
Hypertonic saline solution (HSS) generates an osmotic gradient through the cell membranes that displaces fluid initially from the interstitial space, and then from the cells into the intravascular space.29 The movement of fluid from the cells contributes to reduce the capillary endothelial edema which improves microcirculation, particularly in case of shock.30 In models of isolated cardiac muscle, positive inotropic and lusitropic effects of the HSS, mediated by hyperosmolarity and the action of sodium on the Na+–Ca2+ exchanger have also been shown, maintaining the intracellular calcium homeostasis and its release from the sarcoplasmic reticulum.31 Moreover, the HSS modulates the reperfusion-associated inflammatory response, thanks to the attenuation of the PMNs activation, the decrease of the TNF-alpha serum levels, and the increase in the anti-inflammatory cytokines expression (IL-1, IL-10).25,32 The use of low volumes of HNN for resuscitation in hemorrhagic and septic shock, has characterized its role in attenuating the ischemia/reperfusion injury. Several papers have explored the mechanisms involved in the HSS effects, using HSS both prior to the ischemic insult and before reperfusion.33–35
In the experiment herein discussed, the HSS was infused prior to ischemia, in order to evaluate its role in the expression of cytokines, of vasoactive substances, and in the hemodynamic behavior following the recovery of the blood flow. This study showed that HSS does not change the inflammatory response resulting from 15min of ischemia, followed by 60min of reperfusion. Neither does it affect the hemodynamic response to aortic occlusion and to reperfusion. However, the group treated with hypertonic solution evidenced the impact of HSS on myocardial performance due to the significant increase of the systolic index one hour after reinitiating the aortic flow, to levels close to the baseline conditions. The impact of HSS on hemodynamic behavior was also associated with decreased vasodilatation, as shown by the changes in systemic vascular resistance.
The work by Li et al.6 and Gourdin et al.9 has shown that the occurrence of histological changes and inflammatory and anti-inflammatory serum cytokines are subject to the duration of ischemia and reperfusion. This experiment used short ischemia and reperfusion times (15min and 1h, respectively). The authors believe that this ischemia time was insufficient to give rise to any histological or serum changes in TNF-alpha and inflammatory interleukins; however, the finding of elevation in serum levels of ET-1 from time 1 (T1) in consistent with the analysis by Sánchez-Etayo et al.36 Changes in ET-1 may be observed from the first few minutes after the decrease in blood flow. In this experiment, ET-1 increased following ischemia in both groups. There are no differences between the two groups, probably because of the number of subjects. ET-1 levels increased permanently in a significant manner from T0 to T3 in the control group.
In an interesting clinical paper, Rizoli et al. showed the anti-inflammatory properties of HSS measuring other cytokines besides TNF-alpha.37 The trial includes blunt trauma patients with varying times between the lesion and admission of 44–255min, with at least one recorded episode of hypotension. The authors did a follow-up for the next 24h following resuscitation in the ER. Their findings show significant increases in anti-inflammatory cytokines with rising levels after the third hour of the infusion.
The limitations of this study on aortic occlusion and pre-treatment with HSS are the short ischemia and reperfusion times. Consequently, it was only possible to evidence changes in contractility, with no impact whatsoever on the modulation of inflammation.
In conclusion, pre-treatment with HSS within 15min prior to ischemia in the aortic flow suppression model does not change the expression of serum or tissue inflammation and anti-inflammation markers. HSS increases ventricular contractility after reperfusion.
FundingThis review is part of the joint effort within the framework of the Inter-Institutional Cooperation Agreement between S.C.A.R.E. and the Universidad Nacional de Colombia. Both institutions funded the research project entitled “Establishing the value of using hypertonic saline solution to modify the tissue ischemia/reperfusion injury: aortic occlusion porcine model”.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Escobar B, Guevara-Cruz OA, Navarro-Vargas JR, Giraldo-Fajardo AF, Dumar-Rodriguez JA, Borrero-Cortés C. Solución salina hipertónica para modificar la lesión tisular por isquemia/reperfusión: modelo porcino de oclusión de aorta. Rev Colomb Anestesiol. 2017;45:280–290.