Mycotoxins are secondary metabolites produced by fungal species that mainly belong to Aspergillus, Fusarium, Penicillium and Alternaria, which can grow in a variety of crops including cereals, oilseeds and fruits. Consequently, their prevalence in foods and by-products not only affects human and animal health but also causes important losses in both domestic and international markets. This review provides data about toxigenic fungal species and mycotoxin occurrence in different crops commonly grown in Argentina. This information will be relevant to establish adequate management strategies to reduce the impact of mycotoxins on human food and animal feed chains and to implement future legislation on the maximum permitted levels of these fungal metabolites.
Las micotoxinas son metabolitos secundarios producidos por diferentes especies fúngicas pertenecientes, principalmente, a los géneros Aspergillus, Fusarium, Penicillium y Alternaria. Dichos microorganismos pueden crecer en una gran variedad de cultivos, entre los que se incluyen cereales, oleaginosas y frutas. La presencia de micotoxinas en alimentos y subproductos no sólo afecta la salud humana y animal, sino que también causa pérdidas importantes en los mercados nacionales e internacionales. Esta revisión proporciona datos sobre la prevalencia de especies fúngicas toxigénicas y de micotoxinas en diferentes cultivos y productos cosechados en Argentina. Dicha información será relevante para establecer estrategias de manejo adecuadas para reducir la entrada de las micotoxinas en las cadenas alimentarias del hombre y de los animales, así como para establecer futuras legislaciones sobre los niveles máximos permitidos de dichos metabolitos.
Argentina is located in South America and due to its vast size and range of altitudes, has a large variety of agroclimatic areas. It is one of the most important food-producing countries worldwide, with almost 30 million hectares of cultivable soil. Agriculture, livestock and agroindustry are the main economic activities,which have traditionally supplied the country with 70–95% of its export earnings. The economy of Argentina is mainly focused on the import and export of cereals, oil grains, fruits, wine, tea, tobacco and cotton.50,66,67 Annually, 25–50% of worldwide harvested crops are contaminated with mycotoxins, which are secondary metabolites produced by several fungal species not only during crop development, but also in storage conditions. The fungal genera most commonly isolated from foods and by-products are Aspergillus,Penicillium, Fusarium and Alternaria. Different species belonging to these genera can contaminate several substrates and produce mycotoxins causing adverse health effects in consumers, in addition to economic losses in food-producing and food-exporting countries.1,40,95 Most mycotoxins showed immunosuppressive, hepatotoxic, nephrotoxic and neurotoxic effects and some of them are carcinogens.16,55 Data on the occurrence of toxigenic species and mycotoxins are relevant to determine the contamination risk both for humans and animals in different regions. The present review provides data about the occurrence of toxigenic fungal species and the most frequent mycotoxins detected in cereals, oilseeds, fruits and by-products from Argentina.
Classification of mycotoxinsThe most important mycotoxins based on their occurrence and toxicity are aflatoxins (AFs), fumonisins (FBs), zearalenone (ZEA), trichothecenes (TRCs), ochratoxin A (OTA) and patulin (PAT).55,93 Other toxicologically important mycotoxins, but less studied, are ergot alkaloids, Alternaria toxins, citrinin, cyclopiazonic acid, roquefortine C, mycophenolic acid, penitrems, verruculogen, griseofulvin, citreoviridin, among others .62,81
Aflatoxins and OTA are produced by a species belonging to the genus Aspergillus. Aflatoxins are mainly associated with Aspergillus flavus and Aspergillus parasiticus growth on a variety of food products, including cereals (e.g. maize, wheat, sorghum), peanuts, edible nuts, legumes and by-products.32 For OTA contamination in food, the most relevant producing Aspergillus species belong to section Nigri and Circundati.3 Within the section Nigri, Aspergillus carbonarius and Aspergillus niger have been identified as one of the main sources of OTA contamination and accumulation in grapes, raisins and wines.27 These species have also contributed to OTA contamination in coffee, cocoa, peanut and cereals.30,59,60,99
Fumonisins, ZEA and TRC are produced by several species of the genus Fusarium and wheat and maize are the most frequently contaminated crops. Fumonisins are mainly produced by several members of the Fusarium fujikuroi species complex (FFSC), including Fusarium verticillioides, Fusarium proliferatum, Fusarium nygamai, and Fusarium fujikuroi as well as by Fusarium oxysporum, which is not included in the FFSC.18 Zearalenone is a mycotoxin produced particularly by Fusarium graminearum sensu sticto but also by Fusarium culmorum, Fusarium cerealis, Fusarium equiseti, F. verticillioides, and Fusarium incarnatum.62 TRCs are divided into four types from A to D, type A and B being the most prevalent toxins found as natural contaminants in the food and feed chains. The most important type A-trichothecenes (A-TRC) are T-2 and HT-2 toxins. Among type B-trichothecenes (B-TRC), the most frequently occurring mycotoxin is deoxynivalenol (DON), along with its biosynthetic precursors 3-acetyldeoxynivalenol (3-ADON) and 15-acetyldeoxynivalenol (15-ADON). Type A-TRCs are produced mainly by Fusarium sporotrichioides and Fusarium langsethiae whereas DON by F. graminearum ss and F. culmorum. Nivalenol (NIV) co-occurs with Fusarenon-X (FUX) and DON, and is produced mainly by the F. graminearum species complex (FGSC), Fusarium crookwellense and Fusarium nivale.35,68
The most relevant Alternaria toxins are alternariol (AOH), alternariol monomethyl ether (AME), tenuazonic acid (TA), tentoxin and altenuene.37 Species within this genus are commonly isolated from wheat, sorghum and barley. In oilseeds such as sunflower and rapeseed, tomato, apples, citrus fruits and vegetables, the occurrence of Alternaria species has also been reported.73
Patulin is produced by different fungal species such as Penicillium expansum (Penicillium leucopus), Penicillium crustosum, Penicillium patulum (Penicillium urticae and Penicillium griseofulvum) and Aspergillus clavatus.92 Patulin-producing fungi have been isolated from various fruits and vegetables mainly from apple and apple-based commodities, and occasionally from other fruits such as pears, oranges, grapes and their sub-products.51–53,57,70,97,100,105 Patulin is highly soluble in water and highly stable in aqueous acid media; thus, it commonly contaminates fruit juices.50
“Emerging mycotoxins” are defined as “mycotoxins, which are neither routinely determined, nor legislatively regulated; however, the evidence of their incidence is rapidly increasing”. The main Fusarium mycotoxins included in this group are enniantins, beauvericin, moniliformin, fusaproliferin, fusaric acid and culmorin. Sterigmatocystin and emodin are produced by several Aspergillus species and mycophenolic acid is produced by fungi of the Penicillium species.8 Recently, some researchers have paid attention to their presence in foods and feeds, especially to enniatins and beauvericin. However, data on their toxicity and incidence are still limited, and further research on these metabolites is necessary to determine the toxicological risk due to their occurrence.
Toxigenic fungal species and natural occurrence of mycotoxins on different crops from ArgentinaSeveral fungal species are commonly isolated from a wide variety of crops commonly grown in Argentina. Their occurrence cause spoilage and reduction in food quality and safety during different stages of the chain production such as pre-harvest, post-harvest processing and transport. Thus, their presence in foods and by-products is considered a potential risk due to their ability to produce mycotoxins.
Wheat flour, bran and grainsAlternaria, Fusarium,Aspergillus and Penicillium species can be found as frequent fungal contaminants in wheat.11,89Alternaria was the predominant genus isolated from wheat in different agroecological regions from Argentina such as Entre Ríos and the southeast of Buenos Aires province, where Alternaria alternata is the most prevalent species followed by Alternaria infectoria.11,85 In contrast, Perello et al.82 observed a high incidence of A. infectoria in wheat grains collected from Buenos Aires area, which could be explained by changes in cropping systems in the last few years. The authors associated this species as the etiological agent of black point disease in wheat grains.
The main pathogen associated with Fusarium head blight (FHB) or scab worldwide is F. graminearum ss and, it is also the principal pathogen associated with FHB in wheat in Argentina.56,80 However, other Fusarium species have been isolated from bread (Triticum aestivum L.) and durum (Triticum turgidum L. var. durum) wheat grains during FHB non-outbreak years. Fusarium proliferatum, Fusariumsubglutinans and F. verticillioides species were recovered from bread wheat.86 Later, Palacios et al.77 reported the presence of F. proliferatum, F. oxysporum, F. subglutinans and F. equiseti in durum wheat.
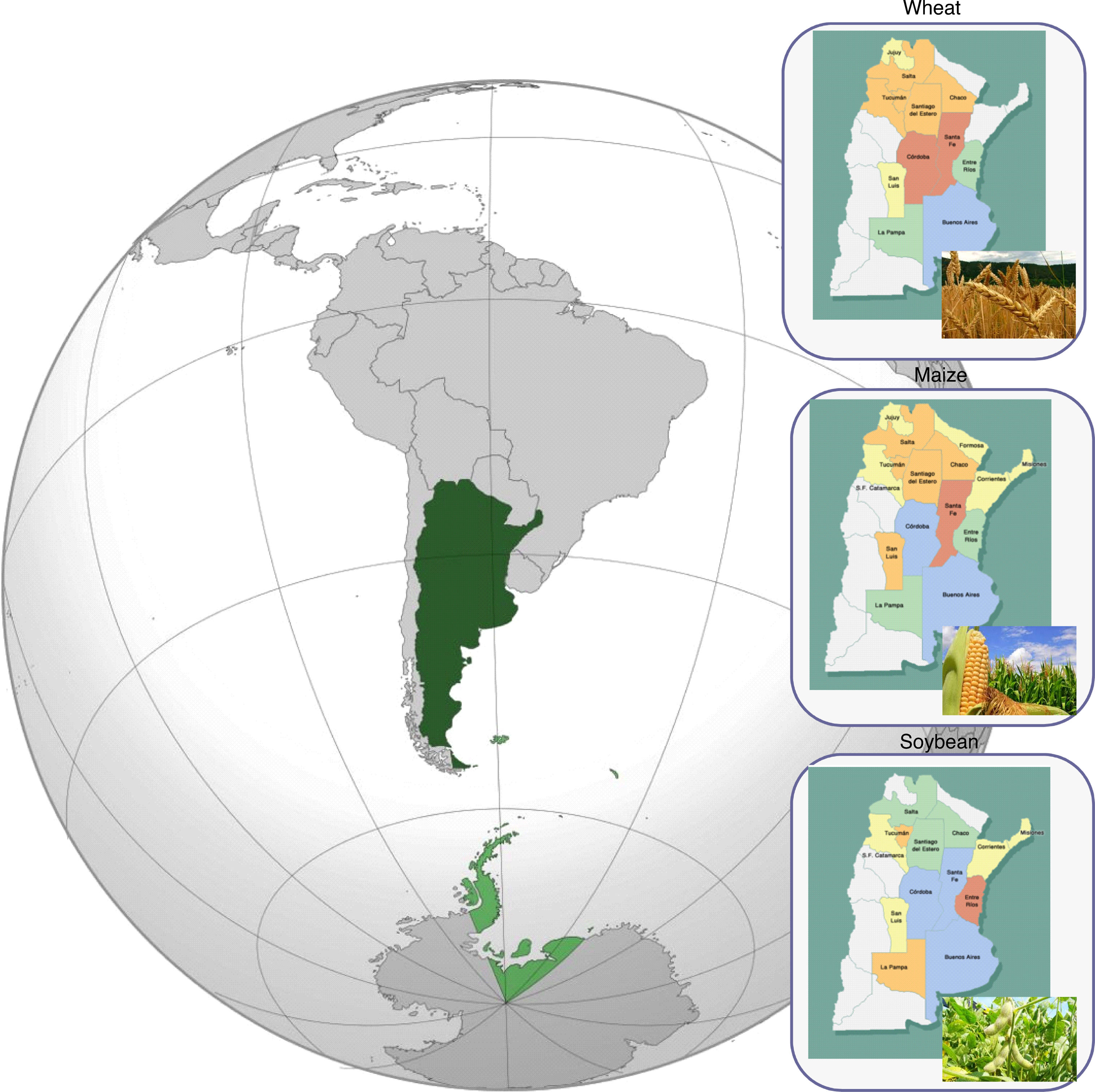
TRC natural occurrence on bread and durum wheat grains during FHB outbreak years was evaluated across the main wheat growing regions, including Santa Fe, Buenos Aires and Córdoba provinces (Figure 1). Type A-TRC, T-2 and HT-2 toxins were detected in 2 to 30% of the samples analyzed with levels ranging from 10 to 70 ng/g and from 10 to 30 ng/g for T-2 and HT-2, respectively. Among type B-TRC, DON was the predominant toxin detected in frequencies ranging from 50 to 85% of the samples, the maximum levels detected being higher than 10,000 ng/g.7,49,58,79,89 Palacios et al.78 detected not only DON but also the glycosylated form deoxynivalenol-3-glucoside (D3G) in 100 and 94% of durum wheat samples collected from three different geographic locations in the South of Buenos Aires province. The DON and D3G levels observed ranged from 50 to 9,400 ng/g and 50 to 850 ng/g, respectively. Moreover, the DON acetylated derivatives were detected but at lower frequency (49%). Recently, Cirio et al.29 reported natural DON contamination in wheat flour processed in different mills located in Buenos Aires, Santa Fe, Entre Ríos, Córdoba, and Tucumán provinces. Ninety-one percent of the samples were contaminated with DON and the mean level detected was 243,000 ng/g. Basilico et al.7 analyzed NIV occurrence in bread wheat harvested in Santa Fe province and detected NIV levels ranging from 100 to 1,000 ng/g in only 5% of the samples (Table 1).
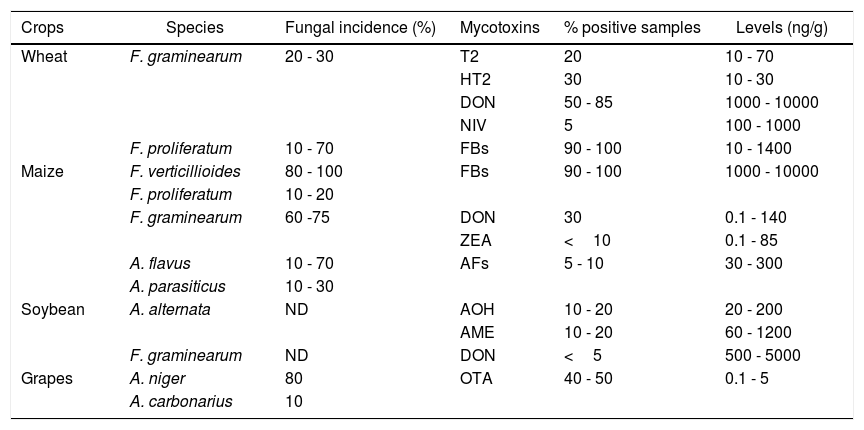
Fungal species and principal mycotoxins in the main crops cultivated in Argentina.
| Crops | Species | Fungal incidence (%) | Mycotoxins | % positive samples | Levels (ng/g) |
|---|---|---|---|---|---|
| Wheat | F. graminearum | 20 - 30 | T2 | 20 | 10 - 70 |
| HT2 | 30 | 10 - 30 | |||
| DON | 50 - 85 | 1000 - 10000 | |||
| NIV | 5 | 100 - 1000 | |||
| F. proliferatum | 10 - 70 | FBs | 90 - 100 | 10 - 1400 | |
| Maize | F. verticillioides | 80 - 100 | FBs | 90 - 100 | 1000 - 10000 |
| F. proliferatum | 10 - 20 | ||||
| F. graminearum | 60 -75 | DON | 30 | 0.1 - 140 | |
| ZEA | <10 | 0.1 - 85 | |||
| A. flavus | 10 - 70 | AFs | 5 - 10 | 30 - 300 | |
| A. parasiticus | 10 - 30 | ||||
| Soybean | A. alternata | ND | AOH | 10 - 20 | 20 - 200 |
| AME | 10 - 20 | 60 - 1200 | |||
| F. graminearum | ND | DON | <5 | 500 - 5000 | |
| Grapes | A. niger | 80 | OTA | 40 - 50 | 0.1 - 5 |
| A. carbonarius | 10 |
ND: No data.
Fumonisin contamination of durum wheat grain samples collected during two consecutive harvest seasons (2007 and 2008) in different commercial fields located in the South of Buenos Aires province was reported for the first time by Palacios et al.77 Data from this survey demonstrated that 97% of the samples collected during the 2007 harvest season showed total FB levels ranging from 10 to 1300 ng/g, while very low levels were detected in samples collected during the 2008 harvest season. The authors argued that these results could be explained by differences in rainfall during both evaluated harvest seasons. Later, Cendoya et al.17 reported FB occurrence not only in durum wheat grain samples, but also in bread wheat grain samples harvested in Buenos Aires province. From the samples, 93% were positive for FBs with levels ranging from 0.10 to 700 ng/g and from 0.10 to 1400 ng/g in bread and durum wheat, respectively. Recently FB contamination in flour wheat samples (68%) collected from different retail stores in Rio Cuarto city was observed, with levels ranging from 0.05 to 18.9 ng/g.18
Natural occurrence of AOH, AME and TA in 65 samples of whole, bran, and flour wheat obtained from a mill located in Gualeyguachú (Entre Ríos) was observed by Romero Bernal et al.90 The wheat grain samples showed low AOH and AME contamination in comparison with TA levels. AOH and AME were not detected in bran and flour samples. The mean levels found for TA in whole, bran and flour wheat were 19,200, 16,800 and 7,300 ng/g, respectively.
Maize flour and grainsThe genus Fusarium is commonly isolated from all maize-growing regions of Argentina. Studies on Fusarium species incidence carried out in both the main and the secondary (Northwest) maize production regions showed that F. verticillioides was the predominant species followed by F. proliferatum and F. graminearum ss.10,46,48,69,75,89Fusariumsubglutinans and Fusariumtemperatum occurrence was high (45 and 60%) in the secondary production region, where lower annual temperatures than in the other areas are recorded.41Penicillium incidence on maize grains ranged from 40 to 60% and the most prevalent species isolated were Penicilliumfuniculosum, Penicilliumcitrinum, Penicilliumdecumbens, Penicilliumoxalycum,Penicilliumbrevicompactum and Penicillium variable.46,48,60,75 The genus Aspergillus was also isolated from maize grains in levels that varied between 15 and 30%, A. flavus and A. parasiticus being the common observed species.13,36,60 Among other toxigenic species found in maize, A. alternata was observed in 5 to 10% of the samples.10,36,48,75,89 Other non-toxigenic fungal species belonging to Cladosporium, Curvularia, Diplodia, Epicoccum, Mucor, Nigrospora, Phoma, Rhizopus and Trichoderma genera were also isolated from this crop.10,36,48,89
Fumonisin B1 (FB1) is the main mycotoxin associated with maize. It was detected in high percentages of maize flour and grains (90 to 100%) collected in all maize growing regions and in different genotypes, with levels ranging from 1,000 to 10,000 ng/g.10,31,44,47,63,69,75,87,89,96,98 Deoxynivalenol and ZEA have been recovered from maize at lower levels than FB1; in 30% and<10% of the samples with levels ranging from 0.1 to 140 ng/g and from 0.1 to 85 ng/g, respectively.28,44,47,75,87,96 With regard to AFs, several studies demonstrated low incidence in maize (5 to 10%), with levels ranging from 30 to 300 ng/g. These studies showed that AF occurrence in maize is influenced by climate conditions.28,36,44,47,69,75,81,87,89,96
Although at present the natural occurrence of “emerging mycotoxins” in maize has not been evaluated in Argentina yet, the presence of F. subglutinans and F. temperatum have been reported, and both species have high potential to produce beauvericin, moniliformin and fusaproliferin.41,88 Possible synergic effects of these metabolites with other main mycotoxins such as DON and FBs have been observed.38,65 There is lack of knowledge concerning most of the emerging mycotoxins; thus, further studies will be necessary to obtain information about their natural occurrence and evaluate possible synergism with the main mycotoxins.
Soybean seeds, pods and flowersAlternaria and Fusarium are the most frequent genera isolated from soybean in Argentina followed by Sclerotinia, Phomopsis, Rhizoctonia, Cladosporium, Aspergillus, Chaetomium and Penicillium.4,9,11,43,45,91,103,106 With regard to Alternaria, A. alternata was the most common species found in soybean seed samples.4,11 Among Fusarium species, F. equiseti,Fusarium semitectum and FGSC were frequently isolated from soybean seeds and pods.6,24 A phylogenetic species recognition of the FGSC isolated from soybean seeds, pods and flowers samples was carried out in order to identify the species responsible for TRC production. The results showed evidence of the presence of four species within the F. graminearum species complex: F. graminearum ss, Fusarium cortaderiae, Fusarium meridionale and Fusarium boothii; F. graminearum ss being the most frequently identified fungal species .25
The natural occurrence of AOH and AME in 50 soybean seed samples harvested in Córdoba province was evaluated by Oviedo et al.74 Both mycotoxins were present in 60% of the samples whereas 16% and 14% were only contaminated with AOH and AME, respectively. Fifteen of the positive samples showed co-occurrence of both mycotoxins analyzed. Alternariol was detected at levels ranging from 25 to 211 ng/g, whereas AME was detected at concentrations ranging from 62 to 1,153 ng/g. Other studies on TRC natural contamination on soybean grains were conducted in samples also harvested in Cordoba province during the 2007/08 to 2013/14 harvest seasons.4,6,24,26 Data showed low DON levels in the sampled area, except during the 2013/14 season where a higher incidence and contamination with levels ranging from 0.7 to 4.3 ng/g were observed. These levels of contamination could be related to higher humidity periods during soybean grain filling and ripening during the 2013/14 harvest season. Although, none of the other mycotoxins such as AFs, ZEA, FBs and OTA were detected in soybean grains, the frequent presence of toxigenic species could indicate a risk of multiple mycotoxin contamination.45,106
Grapes and winesAlternaria and Aspergillus species were frequently isolated in Merlot, Malbec and Cabernet Sauvignon grape varieties collected from Mendoza wine grape growing region. Penicillium, Cladosporium, Fusarium, Trichoderma and Ulocladium species were isolated in lower frequency and were occasionally observed.20,61 In San Rafael wine grape growing region, the presence of four relevant genera in Malbec grape variety was observed. Among them, Alternaria was predominant (81%), followed by Cladosporium (7%) and alternatively by Aspergillus (4%) or Penicillium (3%). Moreover, Drechslera and other genera that belong to Mucoromycotina subphylum, were isolated in low levels (<5%). In this study, all Alternaria strains were identified as A. alternata.84 In another study, the incidence of Alternariaarborescens and Alternariatenuisima was also observed in Malbec grapes.101
Aspergillus section Nigri species were frequently isolated from different vineyards in Argentina.21–23,61,83 Studies carried out on red grape collected from Mendoza vineyards showed that species included within the A. niger aggregate were dominant and the main OTA producers.61,83 Subsequent studies on grapes harvested in different grape growing regions such as La Rioja - Chilecito, San Juan - Tulum Valley, Mendoza - Uco Valley, Mendoza North-East, Mendoza ZARM (high area near Mendoza River), Mendoza South and Neuquén - Río Negro), confirmed that A. niger aggregate species were dominant, followed by A. carbonarius and Aspergillus “uniseriate”. Aspergillus carbonarius showed the highest percentages of OTA-producer strains and also the highest toxin level produced, being mainly isolated from the warmer regions, such as La Rioja and San Juan.19 Using molecular tools, Aspergillus section Nigri species were grouped into four main clusters: A. carbonarius, Aspergillus tubingensis, A. niger “aggregate,” and A. “uniseriate.” The A. tubingensis cluster was the most prevalent group and was clearly separated from the A. niger “aggregate”.21 In another study, phylogenetic data corroborated the biodiversity of Aspergillus section Nigri populations. The sequencing data showed that the strains were grouped as A. carbonarius, A. tubingensis, A. niger, and Aspergillus japonicus. Moreover, Aspergillus homomorphus was identified and its isolation was relevant because it was the first time that it has been found in vineyards and in Argentina.22
Wine samples collected at manufacturers’ stock and retail markets from different regions of Argentina were analyzed to detect OTA occurrence, the results showing that none of the wine samples were contaminated.76 In another study, OTA contamination from grapes and wine samples collected in different grape-growing regions was evaluated.19 Although ochratoxigenic species were isolated from samples obtained from the regions analyzed, the OTA levels detected in grape and wine samples were low in most of the evaluated samples, ranging from 0.10 to 5.40 ng/g and from 0.01 to 4.82 ng/ml, respectively. Another study showed that imported wines, mainly from Argentina and France, were the most contaminated with OTA, with levels ranging from 0.44 to 2.77 ng/ml.33
Other foodstuffsAlthough peanuts (Arachis hypogaea L.), yerba mate (Ilex paraguariensis St. Hil.) and fruits are economically important crops in Argentina, few studies on toxigenic fungi and mycotoxin natural occurrence incidence have been conducted.
Studies on AF-producer species in peanuts harvested in the peanut-growing area of Córdoba province showed that A. flavus and A. parasiticus were frequently isolated.5,102 Magnoli et al.59 evaluated the occurrence of Aspergillus section Nigri in stored peanut grains, A. niger var. niger and A. niger var. awamori, followed by A. carbonarius and A. japonicus being the predominant isolated species. In another study, Magnoli et al. 59 analyzed stored peanut grains but during a 3-month period, in which, although A. carbonarius was isolated in a low percentage, the percentage of OTA-producer strains was high. Aflatoxin, cyclopiazonic acid and OTA contamination in peanuts was detected in levels ranging from 0.043 to 435 ng/g, from 493 to 4300 ng/g, from 0.5 to 170 ng/g, respectively.5,39,44,102
Patulin contamination in foods have been evaluated in different countries.2,34,54,64,71,104 In Argentina, Funes and Resnik42 analyzed PAT occurrence in solid and semisolid fruit by -products such as apple marmalade, jam, sweet and puree, and pear marmalade. The authors reported that 21.6% of the samples were contaminated with PAT (range 17–221 ng/g) and the highest contamination level was detected in apple puree with 50% of positive samples. Later, Oteiza et al.72 evaluated PAT contamination in fruit commodities (apple, apricot, grape, orange, peach, pear and pineapple) and different types of fruit by-products (concentrated cloudy juice, single strength cloudy juice, concentrated juice, concentrated pulp, single strength pulp and sulphited juice) collected between 2005 and 2013. Data showed that PAT was detected in 33.5% of the samples with levels ranging from 30,000 to 19,622,000 ng/ml. With regard to the effect of fruit commodity, the highest percentage of positive samples (40%) contaminated with PAT and the maximum concentration were detected in apple. The type of products was also an important factor that affected PAT contamination; the higher levels were detected in single strength cloudy juice and single strength pulp (67.4% and 54.1%, respectively). In grape apple and orange juice, the natural occurrence of Alternaria toxins has also been observed.12,94
In a study conducted by Castrillo et al.15, Aspergillus section Nigri incidence in different commercial brands of yerba mate was evaluated. Uniseriate species were more predominant than biseriate species. The percentage of isolated species was higher in milled yerba mate (without an aging period) than in elaborated yerba mate. In a previous study, the same authors performed a phylogenic analysis of Aspergillus section Nigri isolated from yerba mate. In contrast, the results showed that all isolated strains were included in A. niger and A. carbonarius clades.14 No studies about natural mycotoxin occurrence in yerba mate have been conducted yet.
ConclusionsDifferent toxigenic fungal species belonging to genera Fusarium, Aspergillus and Alternaria are isolated from the crops of greatest economic importance grown in Argentina. The isolation of these species was influenced by the type of crop and agro-ecological conditions. Mycotoxin occurrence was detected in all crops at variable levels, DON, TRC, FBs and OTA being the most frequently studied.
In Argentina the production of commodities increases annually and fungal contamination and mycotoxin occurrence in the food and feed chains represent a high risk to human and animal health, as well as considerable economic losses due to restrictions to the domestic and international markets. Studies on the occurrence of mycotoxins in different crops and by-products are important because they are reliable approaches to evaluate the potential exposure risk of the populations to these contaminants.
Conflict of interestThe authors declare that they have no conflicts of interest.
The present work received funding from the European Union's Horizon 2020 Research and innovation program under Grant Agreement No. 678781 (MycoKey).