Cryptococcosis is a fungal disease affecting more than one million people per year worldwide. Its main etiological agents are Cryptococcus neoformans species complex and Cryptococcus gattii species complex. Cryptococcal meningitis (CM) is considered an AIDS-defining condition. Rapid diagnosis by cryptococcal antigen assays, either the latex agglutination test (LA) or the lateral flow assay, is key to decreasing mortality due to cryptococcal disease. The aim of the study was to develop a latex agglutination reagent (LA-ANLIS) for the rapid and reliable diagnosis of cryptococcosis in Argentina. This reagent will be produced in order to supply the NMLN (National Mycology Laboratory Network). The evaluation of LA-ANLIS performance and its comparison with the Cryptococcus Antigen Latex Agglutination Test System (LA-IMMY) (Immuno-Mycologics, Inc., USA) were conducted in 94 samples of cerebrospinal fluid. LA-ANLIS and LA-IMMY compared exhibited 100% positive agreement and 97% negative agreement. LA-ANLIS showed 94% sensitivity and 97% specificity with the positive and negative predictive values of 94% and 97%, respectively. The LA-ANLIS is a reliable, reproducible and cost-effective reagent, especially useful in countries where the commercial kit is not generally available and must be obtained at a high cost. National production of reagents is the best choice for a reliable access to the rapid diagnosis of CM in Argentina.
La criptococosis es una enfermedad fúngica que afecta a más de un millón de personas por año en todo el mundo. Los principales agentes etiológicos pertenecen a los complejos de especies Cryptococcus neoformans y Cryptococcus gattii. La criptococosis meníngea (CM) se considera una enfermedad marcadora de sida. El diagnóstico rápido de esta enfermedad a través de la detección del antígeno de Cryptococcus, ya sea por aglutinación en partículas de látex o por inmunocromatografía, es clave para disminuir la mortalidad. El objetivo del presente estudio fue desarrollar un reactivo de aglutinación en partículas de látex para el diagnóstico rápido y certero de la CM en Argentina. Este reactivo (denominado en adelante LA-ANLIS) será producido para abastecer a la Red Nacional de Laboratorios de Micología. Se evaluó el desempeño del reactivo LA-ANLIS, y se realizó una comparación con el reactivo comercial Immuno-Mycologics, Inc. (en adelante, LA-IMMY) utilizando 94 muestras de líquido cefalorraquídeo. Hubo un 100% de acuerdo positivo y un 97% de acuerdo negativo entre los resultados obtenidos con los reactivos LA-ANLIS y LA-IMMY. El reactivo LA-ANLIS mostró una sensibilidad del 94% y una especificidad del 97%; los valores predictivos positivo y negativo fueron del 94 y del 97%, respectivamente. Se concluye que el LA-ANLIS es un reactivo confiable y rentable, que arroja resultados reproducibles, por lo que es especialmente útil en países donde los reactivos comerciales generalmente no están disponibles o sus costos son elevados. La producción nacional de reactivos es la mejor opción para asegurar el acceso de todos los hospitales al diagnóstico rápido de la CM en Argentina.
Cryptococcosis is a life-threatening systemic mycosis that affects humans among other vertebrates. Its main etiological agents are the related basidiomycetous yeasts belonging to Cryptococcus neoformans and Cryptococcus gattii species complexes. As noted, C. neoformans has worldwide distribution and is about eightfold more frequently isolated than C. gattii both in environmental and clinical samples (88.6% and 11.4% respectively)8. Moreover C. gattii distribution was considered to be restricted to tropical and subtropical regions until the occurrence of the ongoing outbreak on Vancouver Island in Canada and the Pacific Northwestern United States17,18.
Although cryptococcosis might occur in patients who are not apparently immunocompromised, most patients have a pre-existing condition or disease. The most frequent underlying conditions are human immunodeficiency virus (HIV) infection, extensive treatment with corticosteroids, organ transplantations, chronic leukemias and lymphomas, and sarcoidosis. Cryptococcal meningitis (CM) is considered an AIDS-defining condition, and is the most common fungal infection of the central nervous system and the third most frequent neurological complication in AIDS patients8,23.
In 2008, in the pre-ART (antiretroviral therapy) era the global annual incidence of cryptococcosis was estimated as 957900 cases per year. Since then, extensive ART expansion has occurred; AIDS-related deaths have reduced by 45% from 2.0 million to 1.1 million deaths24. Increasing access to ART has transformed the prognosis of HIV-infected patients in resource-limited settings. However, treatment coverage remains relatively low and HIV diagnosis occurs at a late stage. As a result, many patients continue to die of HIV-related opportunistic infections in the weeks prior to, or months following initiation of ART. The case fatality rate in patients with CM, the commonest presentation of HIV-related cryptococcal disease in adults, remains unacceptably high, particularly in sub-Saharan Africa, at between 35% and 65%, in contrast to 10% and 20% in most developed countries15. In Latin America, the lethality of CM has been reported to range from 13% to 73%, depending on the country, with many cases ranging between 30% and 60%11. A major reason for this high mortality is late diagnosis, largely only as a result of limited access to lumbar puncture and rapid diagnostic assays23,26.
Although in Argentina mycoses are not notifiable diseases, we collect the data of cases from the NMLN (National Mycology Laboratory Network). At present the NMLN is comprised of 160 laboratories all over the country. Thus, it was determined that cryptococcosis is the second deep mycosis diagnosed in our country with an increasing number of reported cases during the last decade, from 328 in 2004 to 499 in 2010, the estimated prevalence in 2010 being 1.23/100000 population9.
According to the Joint United Nations Program on HIV/AIDS (UNAIDS) there were 110000 people living with HIV in Argentina in 2013, only 70% were diagnosed with HIV and 47% were receiving ART3,12.
People at risk for cryptococcal disease are described as adults with a T CD4+ lymphocyte count of less than 100cells/μl of ART or on ART; however, they are either lost to follow-up or experience virologic failure. The importance of asymptomatic cryptococcal antigenemia as a precursor to symptomatic meningitis and death has been further defined. Cryptococcal antigenemia screening coupled with preemptive antifungal therapy has a proven survival benefit and has been incorporated into HIV national guidelines in many countries with high rates of cryptococcosis24.
The conventional approach to achieve a CM diagnosis requires a lumbar puncture with the use of an India ink test and a positive cryptococcal capsular antigen (CrAg) test or culture. One of the Guideline Development Group recommendations was the preferred use of a rapid CrAg assay, either the latex agglutination test (LA) or the lateral flow assay (LFA), in cerebrospinal fluid (CSF) or serum (depending on the lumbar puncture access). This recommendation was based on the fact that CrAg assays had a higher sensitivity and specificity, were easier to perform and were less dependent on technician skill than the India ink test25,26. Early diagnosis is key to decrease cryptococcal disease-related mortality; therefore, countries should prioritize reliable access to rapid diagnostic CrAg assays26.
There are commercial kits for cryptococcal antigen detection but they are imported from the USA; hence, they are very expensive for our country or are sometimes unavailable because of Customs-related constraints. As a result, some laboratories only perform culture and the India ink test in CSF. Therefore, the goal of the present study was to develop an immunological reagent for the rapid and reliable diagnosis of cryptococcosis in Argentina. This reagent (LA-ANLIS) will be produced in order to supply the NMLN.
Materials and methodsMicroorganismsStrains WM148 serotype A, UCLA 381-C serotype C and WM 628 serotype AD were gently provided by W. Meyer, University of Sydney, Australia; strains NIH 112B serotype B and NIH 52D serotype D were gently provided by E. Castañeda, Instituto Nacional de Salud de Colombia, and were preserved at −70±10°C in the culture collection (DMic) at the Mycology Department of the National Institute of Infectious Diseases of Argentina (INEI, ANLIS “Dr. Carlos G. Malbrán”).
SpecimensCerebrospinal fluid (CSF) samples were submitted to the Mycology Department by the following NMLN laboratories: Fundación para la Lucha contra las Enfermedades Neurológicas de la Infancia (Fleni), Dr. Orellana N.; Hospital de Clínicas “José de San Martín”, Dr. Fernández N.; Hospital General de Agudos “Dr. Juan A. Fernández”, Dr. Guelfand L.; Hospital Zonal General de Agudos “Dr. Diego Paroissien”, Mónaco L.; Hospital Interzonal Gral. de Agudos “Eva Perón”, Dr. Tuduri A. CSF samples were stored in the fridge at 5±3°C until use.
There were 94 CSF submitted samples that included 32 CSF from patients with CM confirmed by the India ink test, positive CrAg test or culture; 41 CSF from patients with non-infectious neurological diseases and 21 CSF samples from patients with a neurological infectious disease (Mycobacterium spp., Streptococcus spp., Staphylococcus spp., varicella-zoster virus, human cytomegalovirus, Trypanosoma cruzi, Toxoplasma gondii and Candida parapsilosis).
Preparation of C. neoformans glucuronoxylomannanGlucuronoxylomannan (GXM)was purified from different serotype strains: WM148 serotype A; NIH 112B serotype B; UCLA 381-C serotype C; NIH 52D serotype D; serotype AD WM 628, as reported by Cherniak et al.4
Neutral carbohydrate detection for each serotype was performed by the phenol-sulphuric acid method described by Masuko et al.20
GXM solution was diluted to 1μg/ml and was stored at −20±5°C.
Preparation of sensitized latex particles (SLPs)Anticapsular polyclonal antiserum was produced by the immunization of New Zealand White rabbits with intravenous injections of 3×108 formalin-killed C. neoformans cells, strain WM148 (Serotype A) at 4-day intervals2,13.
The agglutination titer was estimated by the direct agglutination method described by Ikeda et al.13 Immunoglobulins (Igs) from the cryptococcal antiserum were obtained by ion-exchange and affinity chromatography on DEAE Affi-Gel Blue gel (DEAE Affi-Gel® Blue Gel, Bio-Rad Laboratories). The Igs solution was quantified by directly measuring absorbance at 280nm. Several dilutions (range 0.1–1mg/ml) were prepared in sterile glycine buffered saline diluent (GBS) pH 8.2. The 0.15μm, 0.3μm and 0.8μm diameter latex beads (Ikertat polymers S.L) were used for SLP preparation. The latex particle suspension (10% p/v) was diluted 10-fold with Igs solution and was stirred at room temperature on a rotary shaker to 50rpm for 2h and later stored at 4°C overnight. Then, it was centrifuged at 13000×g for 30min. The supernatant was removed and the beads were resuspended up to 0.2% p/v in 1ml sterile GBS supplemented with 0.1% p/v albumin and 0.1% p/v sodium azide(diluent buffer)14. SLPs were evaluated by a titration procedure from the purified GXM solution of WM148 serotype A (1μg/ml).
Once the optimal conditions for SLP preparation were defined, a batch for the performance evaluation was prepared.
Latex agglutination testsCSF samples were centrifuged at 1000×g for 15min and the supernatant was boiled for 5min prior to testing.
Agglutination reactions were performed in triplicate by the following procedure: 25μl of test solution and 25μl of the SPLs were added with a pipette onto a black slide. They were then thoroughly mixed, and the slide was incubated for 5min at room temperature on a rotator set to 100rpm.
In addition, all tests were performed with a negative control (as serum bovine) and a positive control consisting of purified GXM.
The Cryptococcus Antigen Latex Agglutination Test System (Immuno-Mycologics, Inc., USA) was assayed according to the manufacturer's instructions.
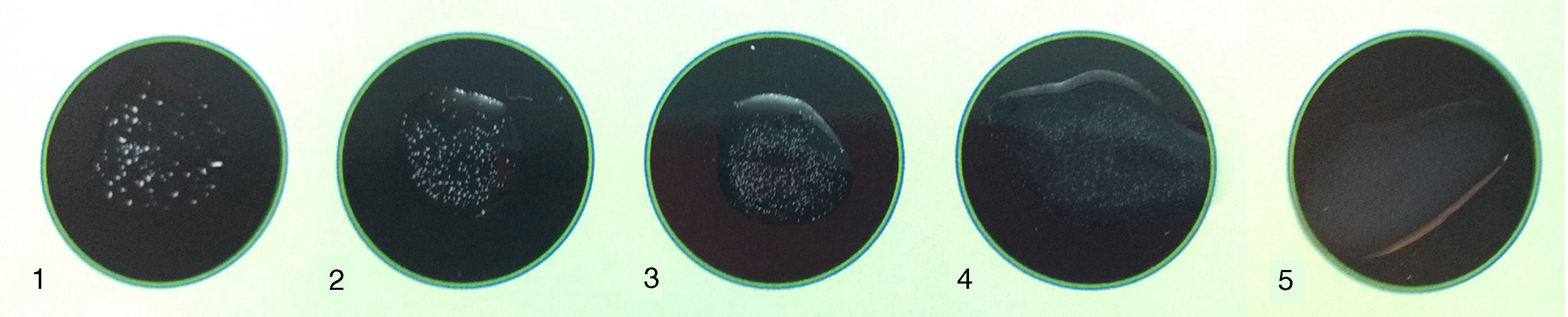
A four-point visual scale was graded from weak agglutination (1+) to strong agglutination (4+), which is exhibited pictorially in Figure 1.
Result interpretation of the agglutination test: (1) positive 4+ (heavy flocculent precipitates forming within 3min with clear background); (2) positive 3+ (heavy flocculent precipitates taking 5min to form with clear background); (3) positive 2+ (light flocculent precipitate against mostly clear background taking 5min to form); (4) positive 1+ (light flocculent precipitate against mostly clear background taking 10min to form); (5) negative (no precipitate, cloudy homogeneous background).
Undiluted specimens exhibiting no agglutination or a 1+ were considered negative. Any sample with a screening result graded from 2+ to 4+ was considered positive and underwent serial twofold dilutions using the diluent buffer. The titer was defined as the reciprocal of the highest dilution yielding a 2+ to 4+ agglutination reaction.
The LA-ANLIS, positive and negative control and diluent buffer were stored at 5±3°C until use.
Performance evaluation of LA-ANLISThe lower detection limits for the polysaccharide antigen of different serotypes were determined individually by a titration procedure from a solution of purified GXM at a concentration of 1μg/ml.
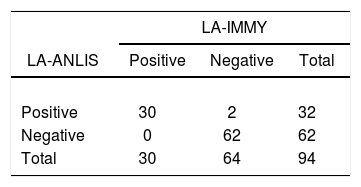
LA-ANLIS was evaluated using CSF samples by comparison with the Cryptococcus Antigen Latex Agglutination Test System (LA-IMMY). We estimated positive percent agreement (PPA) and negative percent agreement (NPA) with the 2×2 contingency table and their 95% score confidence limits (CL) were calculated by the Wilson's method known as “the score confidential interval” as described by the Clinical and Laboratory Standards Institute (CLSI)5.
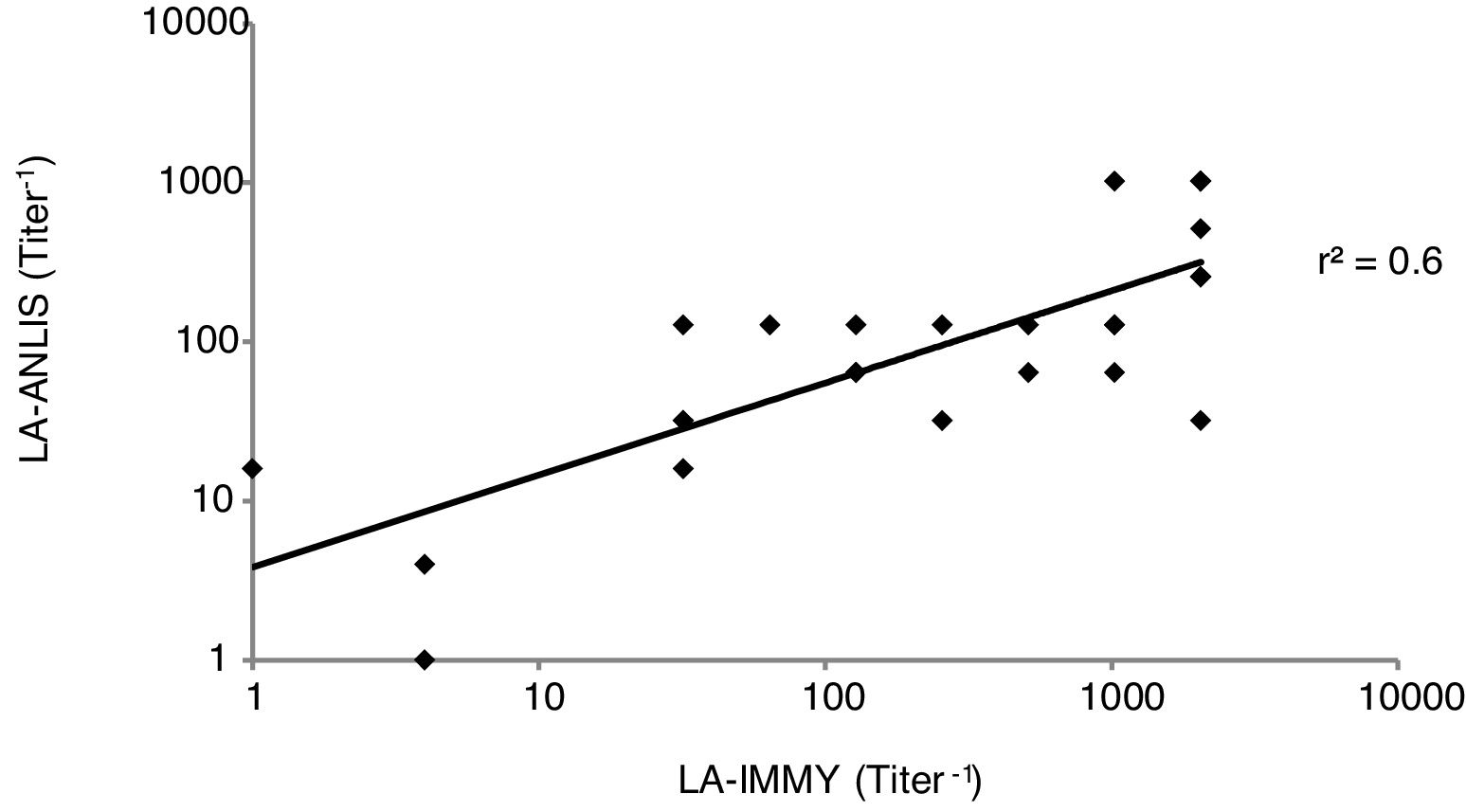
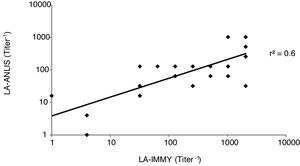
For titer comparison we analyzed each sample using both LA-ANLIS and LA-IMMY. For the analyzed results we established and evaluated a linear relationship between LA-ANLIS and LA-IMMY and calculated the correlation coefficient (r2) according to CLSI6.
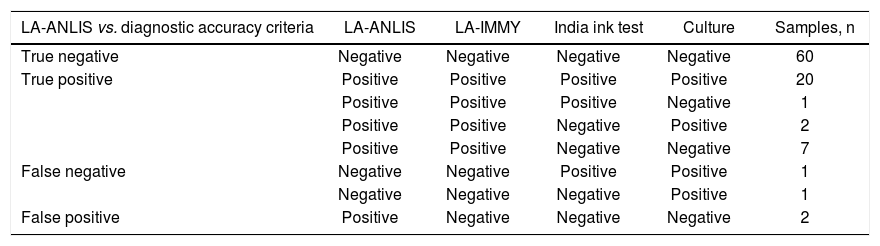
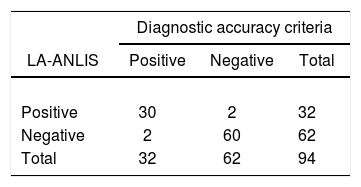
Moreover, the LA-ANLIS method was evaluated against diagnostic accuracy criteria using the previously mentioned samples. We established the diagnostic accuracy criteria as specimen that was positive on at least one of the methods tested (the India ink test, culture or LA-IMMY. We estimated sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) with the 2×2 contingency table and their 95% score CL were calculated by the Wilson's method5.
For the evaluation of stability, we conducted a classical design stability study at 5±3°C, in real time for four years (with four test points for each year). For this purpose, we used a titration procedure from a GXM purified solution7.
ResultsPreparation of sensitized latex particles (SLPs)Immunized rabbits demonstrated homologous agglutination titers between 1:16 and 1:1024. We selected 1:1024 serum for SLP preparation. Agglutinating activity was highest with 0.8μm diameter beads in the 0.04–0.06mg range of IgG per 1mg of beads with a lower detection limit of 15ng/ml of GXM from WM148 serotype A strain.
Performance evaluation of LA-ANLISClear agglutination activity was observed down to a dilution of 1:8 for serotype C, 1:16 for serotype B and AD and 1:32 for serotype A.
The comparison between LA-ANLIS and LA-IMMY was summarized in Table 1. Two samples that were negative with LA-IMMY, were positive with LA-ANLIS, but with low titer (titers 1 and 1:2). These two samples were negative for the direct mycological examination. LA-ANLIS exhibited 100% PPA (CL=89–100) and 97% NPA (CL=89–99) in LA-IMMY. The correlation of cryptococcal antigen titers of 29 specimens is shown in Figure 2. One positive sample could not be titrated because the volume was insufficient. We observed that r2 was 0.6.
The results of LA-ANLIS evaluated against diagnostic accuracy criteria were summarized in Tables 2 and 3. The two false positive samples were the same as previously described with a low titer. There were two samples that were false negative; one of them was negative for LA-IMMY and for LA-ANLIS but were positive for Indian ink and culture; the other sample had an agglutination activity of 1+, only with the non-diluted sample, with positive LA-IMMY and culture but negative for Indian Ink. We estimated a sensitivity of 94% (CL=80–98) and a specificity of 97% (CL=89–99). The predictable value of a positive test result was 94% and the predictive value of a negative result was 97%.
Performance analysis of the LA-ANLIS method.
| LA-ANLIS vs. diagnostic accuracy criteria | LA-ANLIS | LA-IMMY | India ink test | Culture | Samples, n |
|---|---|---|---|---|---|
| True negative | Negative | Negative | Negative | Negative | 60 |
| True positive | Positive | Positive | Positive | Positive | 20 |
| Positive | Positive | Positive | Negative | 1 | |
| Positive | Positive | Negative | Positive | 2 | |
| Positive | Positive | Negative | Negative | 7 | |
| False negative | Negative | Negative | Positive | Positive | 1 |
| Negative | Negative | Negative | Positive | 1 | |
| False positive | Positive | Negative | Negative | Negative | 2 |
In the evaluation of LA-ANLIS stability we observed false positive results in the negative control after three years.
DiscussionThe Cryptococcus antigen detection reagent LA-ANLIS was produced by coating polystyrene latex microspheres with C. neoformans-specific rabbit hyperimmune anti-serum. Experimental findings are consistent with the fact that C. neoformans GXM is the soluble antigen recognized by the test.
It is desirable to maximize the sensitivity of LA-ANLIS hyperimmune serum because high affinity IgG (determined by direct agglutination) conjugated to the latex particles results in the greatest sensitivity. Since carbohydrate antigens are often non-immunogenic or poorly immunogenic in animals, whole yeast is used for immunization16,19. Another factor for reagent sensitivity improvement was particle diameter; for visual slide agglutination the particle diameter range is 0.2–0.9μm21. In our experience the greatest sensitivity was obtained with a larger particle diameter (0.8μm). Efficient agglutination is dependent upon an optimum amount of IgG on the surface of the SLP22. Latex microspheres coated with anti-C. neoformans in the range of 0.02–0.06mg of IgG per 1mg of microspheres of 0.8μm diameter were found to be effective in LA-ANLIS. The IgG monolayer reported corresponds to an absorbed amount of a range of 3–15mg/m2 depending on the IgG configuration1,21, which is equivalent to 0.02–0.1mg of immunoglobulins per 1mg of microspheres of 0.8μm. This was in agreement with our results. The most serious problem in latex immunoagglutination assays is that the system can lose its colloidal stability after the antibody adsorption step. This low colloidal stability of latex–antibody complexes in the reaction medium may provoke the nonspecific agglutination of particles. A second inactive protein acting as a stabilizer was used post-treatment to increase colloidal stability21. We used bovine serum albumin (BSA) to emphasize the colloidal stability of antibody-covered particles. We obtained a stable reagent, and only after 3 years at 4°C it started to non-specifically agglutinate with bovine serum used as negative control.
The performance of LA-ANLIS determined as a sensitivity of 94%, a specificity of 97%, PPV 94% and NPV 97% was similar to other commercial kits25. LA-ANLIS as other CrAg detection assays is much more sensitive and specific than the India ink test (especially in patients with low CSF fungal burden), easier to perform, less dependent on technician skill and may also be used for screening or early diagnosis27. The diagnostic usefulness of the test was evident particularly in cases where cryptococcosis was indicated in spite of a negative direct microscopic examination of CSF and later demonstration of C. neoformans in culture.
Our data of 100% PPA and 97% NPA between LA-ANLIS and LA-IMMY commercial kit indicates that both are quite effective in detecting meningeal cryptococcal infection in human patients. A positive reaction of an untreated patient at titers of 1:4 or less is highly suggestive of cryptococcal infection but may be a false positive10 and should be confirmed by using other methods such as the India ink test, culture, other CrAg test or another sample. Titers of 1:8 or greater indicate active cryptococcosis has been reported by IMMY's manufacture. The two false negative reactions observed in this study could be caused by low titers or poorly encapsulated strains. A negative test does not exclude the possibility of cryptococcal meningitis, especially when patients have meningeal symptoms.
LA-ANLIS can be used to evaluate the response to therapy in the treated patient as LA-IMMY does. Antigen titration discrepancies between LA-IMMY and LA-ANLIS were observed. When antigen titration is being used to monitor therapy, all titrations should be performed using the same manufacturer's kit. It is also good practice to titer serial specimens simultaneously to minimize intra-laboratory variation. Declining titers indicate a positive response to therapy in the treated patient. Failure to decline indicates inadequate therapy. Our data indicates that LA kits from different manufacturers should not be used interchangeably for determining titers25.
LA-ANLIS is a reliable, reproducible and cost-effective reagent, especially useful in countries where the imported commercial kit is not easily available. National production of reagents is the best choice for reliable access to rapid diagnosis of cryptococcal meningitis in our country.
FundingThis work was supported by FOCANLIS 2009 (Fondos concursables de la Administración Nacional de Laboratorios e Institutos de Salud, 2009).
Conflict of interestThe authors declare that they have no conflicts of interest.
The authors would like to thank all institutions and biochemistry providing clinical samples. Thanks to Javier Espeche for language supervision.