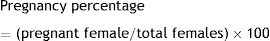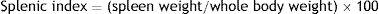Leishmaniasis is a group of parasitic zoonotic diseases caused by intracellular protozoans belonging to the genus Leishmania. Little is known about the effects that this parasitosis may have on the reproductive parameters and pregnancy of infected humans and pets. This study aimed to evaluate the influence of chronic cutaneous leishmaniasis caused by Leishmania (Leishmania) amazonensis on reproductive and fetal parameters using a female murine model. A control group of female BALB/c mice and a group infected with L. (L.) amazonensis were mated with healthy males. Clinical parameters were monitored during the pre-mating and gestational periods. Female mice were euthanized on day 19 of gestation, when the fetuses were weighed and their length measured and embryonic resorptions and fetal death were recorded. We observed five fetal deaths and three embryonic resorptions in the infected group. Furthermore, there was a decrease in fertility in the infected group (26.32%). The weight of the offspring from infected mothers was lower than that in the control group (1.019±0.035g and 1.163±0.032g, p<0.01). Fetal length was reduced in the infected group (3.71±0.05cm in the control group and 3.40±0.06cm in the infected group p<0.001). This study shows that cutaneous leishmaniasis caused by L. (L.) amazonensis impairs reproductive and fetal parameters in mice.
La leishmaniasis comprende un grupo de enfermedades zoonóticas parasitarias causadas por protozoos intracelulares pertenecientes al género Leishmania. Poco se conoce sobre los efectos que esta parasitosis puede tener sobre los parámetros reproductivos y la gestación en humanos y en otras especies infectadas. El objetivo de este estudio fue determinar la influencia de la leishmaniasis cutánea crónica, causada por Leishmania (Leishmania) amazonensis, en parámetros reproductivos y fetales. Se apareó un grupo control y un grupo infectado de ratones hembra BALB/c (previamente inoculado con L. (L.) amazonensis) con machos sanos. Se analizaron parámetros clínicos durante los períodos pregestacional y gestacional. Las hembras fueron eutanasiadas en el día 19 de gestación, momento en el cual se pesaron y midieron los fetos y se registraron las reabsorciones embrionarias y las muertes fetales. Se observaron 5 muertes fetales y 3 reabsorciones embrionarias en el grupo infectado. Además, hubo una disminución en la fertilidad de este último grupo (26,32%). Por otra parte, el peso de la descendencia de madres infectadas fue menor que el del grupo control (1,019±0,035g y 1,163±0,032g, respectivamente, p<0,01). Por último, la longitud fetal se redujo en el grupo infectado (3,71±0,05cm en el grupo control y 3,40±0,06cm en el grupo infectado, p<0,001). Este estudio muestra que la leishmaniasis cutánea causada por L. (L.) amazonensis afecta los parámetros reproductivos y fetales en ratones.
Leishmaniasis is a worldwide anthropozoonotic disease caused by an intracellular protozoan of the genus Leishmania3. It involves more than 30 species, 21 of which are pathogenic to humans2. This disease is currently considered a growing public health concern worldwide2 due to the recent increase in the number of cases: between 12 and 15 million people infected in 98 countries, with 0.7–1 million cases estimated yearly9. In Argentina, the transmission of leishmaniasis has become more severe, and the number of cases has increased since 198041. Although the Northern region of the country is the endemic area, new cases have been detected in non-endemic regions. The reason behind this increase is climatic change, which causes the dissemination of transmitting vectors and the transfer of species acting as reservoirs, making this pathology very difficult to control30. This disease presents different clinical forms and can compromise the skin, mucosa, and viscera, depending on parasite and host factors. The most common form of the disease is cutaneous, caused in Argentina by three species: Leishmania (Viannia) guyanensis, Leishmania (V.) braziliensis, and Leishmania (Leishmania) amazonensis By contrast, Leishmania (Leishmania) infantum causes the visceral clinical form and has higher morbidity and mortality rates1,27. The predominant mode of transmission is the bite of infected sandflies, although new means of dissemination have been described recently: organ transplants, blood transfusions, contaminated cutting objects, and sexual and vertical forms5,22.
Some of the main damage caused by the new means of transmission, specifically in the vertical and sexual forms, are the dissemination of the infectious agents to the offspring or alterations during the gestational period. These alterations could culminate in embryonic resorptions, fetal deaths, abortions, and congenital malformations, which could lead to an increase in perinatal mortality31,33. These complications also occur in other infectious diseases transmitted by vectors, such as Zika and Chagas diseases11,32. The transmission of visceral leishmaniasis to the offspring has been widely reported18,20,26,34,38,39 but little is known about the influence of visceral and cutaneous leishmaniasis on maternal reproductive parameters. Thus, the behavior of the disease during pregnancy and fetal development remains unclear37,39.
To date, no reports have shown the effects of L. (L.) amazonensis infection on reproductive and fetal parameters or clinical features during pregnancy. Therefore, this study aimed to evaluate the influence of chronic cutaneous leishmaniasis caused by L. (L.) amazonensis on reproductive and fetal parameters using a female murine model.
Materials and methodsStudy designIn this study, we used an analytical, experimental, and controlled methodological design.
ParasitesPromastigotes of L. (L.) amazonensis (MHOM/VE/84/MEL) were grown at 23°C in Novy-MacNeal-Nicolle medium (NNN) (Invitrogen, Massachusetts, USA) with Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 20% heat-inactivated fetal bovine serum (FBS), 2mM l-glutamine, 100U/ml penicillin, and 100mg/ml streptomycin (Life Technologies, San Diego, CA, USA) for 4 days and then transferred to supplemented RPMI 1640 medium10. Infectivity was maintained by serial passage through mice.
AnimalsEight-week-old female BALB/c mice were used in this study. They were kept in standard conditions, with barriers and controlled light cycle and temperature. Water and food were provided ad libitum. All animals were treated in conformity with the Guiding Principles for the Care and Use of Animals of the US National Institute of Health.
Experimental protocolThirty-nine females were distributed randomly in two groups: uninfected group (n=12) and mice infected with 1×106 promastigotes of L. (L.) amazonensis (n=27) by the intradermal route on the right footpad (RFP).
Footpad swelling was measured weekly using a digital caliper (SCHWYZ, ED-10P, Switzerland) up to 10 weeks after infection. The value for uninfected footpads was subtracted from each infected footpad to estimate lesion size. A digital precision balance (Sartorius, Göttingen, Germany) was used to determine body weight.
Ten weeks after the start of the infection, a polygamous mating system was performed16. In total, 19 infected and 8 control female mice were mated with healthy mice. The remaining females (4 control and 8 infected) were employed to estimate the splenic index and antibody response.
Each female was checked daily for 7 days to evaluate the presence of a mucous plug6. Day 1 of pregnancy was determined when the mucous plug was detected and, at that moment, each female was transferred to the cage with the initial group.
Pregnant females were weighed every day until their euthanasia. Gestation was interrupted on day 19 of gestation, when the females were euthanized and the spleen was removed.
At necropsy, maternal and offspring data were recorded. The number of pregnant females in both groups was counted to correlate the pregnancy with the infection. Therefore, the pregnancy percentage was calculated from pregnant females with regard to the total in each group according to the following formula:
Two independent experiments were carried out with the same number of animals per repetition.
Fetal parametersThe total number of living and dead fetuses, implantation sites, and embryonic resorptions in each group were counted, and the weight and length of the fetuses were measured.
Two different intrauterine deaths were established: embryonic resorptions and fetal deaths. Embryonic resorptions were established as the difference between total implantation sites and the number of concepti because embryonic resorption is defined as prenatal death followed by degeneration and complete resorption of the conceptus43. By contrast, pink and perfused fetuses were considered viable28, while the rest and those showing maceration were considered fetal death43.
The frequency of intrauterine death/pregnant female was calculated. Similarly, the percentage of females that presented embryonic resorptions and/or fetal deaths were calculated and defined as intrauterine death rate (%)28.
Splenic indexThe splenic index is used as an indicator of splenomegaly, which is an immune response to the systemic infection by Leishmania. This index was determined through the following formula using non-pregnant females47:
Purification of DNA and PCR analysisDNA from all the spleens from pregnant mice (n=5 infected and n=4 control) and L. (L.) amazonensis promastigotes (as positive control) was obtained using the modified phenol-chloroform method19. DNA samples were rehydrated with 50μl of ultrapure water and stored at −20°C. DNA concentration and purity were determined by spectrophotometric determination at A260 and A280 (Genway Genova).
Detection of Leishmania DNA was carried out by Polymerase Chain Reaction (PCR) with the described primers36 forward, 5′-CCTATTTTACACCAACCCCCAGT-3′ [JW11] and reverse, 5′-GGGTAGGGGCGTTCTGCGAAA-3′ [JW12], which amplify a 120-bp fragment of the minicircle kDNA. PCR was performed in accordance to the project described35 under the following conditions: extracted DNA (50ng) was mixed with a solution containing 1× PCR buffer, 2.5mM MgCl2, 200μM each dNTP, 50pmol of each primer, and 0.0625 U of Taq polymerase (Invitrogen) in a 20μl final volume. After initial denaturation (8s at 95°C), 40 cycles of denaturation for 10s at 95°C, annealing for 10s at 56°C, and elongation for 8s at 72°C were carried out.
This reaction was performed in duplicate for both the uninfected control and the infected pregnant mice samples, as well as for the positive controls [i.e., L. (L.) amazonensis cultures] and the negative controls (i.e., samples without DNA) in a Rotor-Gene™ 6000 instrument (Corbett Life Science, Mortlake, Australia).
PCR products (3 of 4 control and 3 of 5 infected mice) and the molecular weight marker (100-bp Plus DNA ladder, Trans®, Beijing, China) were loaded on a 2.5% agarose gel and run at 85V for 20min. Images were collected using the ChemiDoc™ XRS System (BioRad®, California, USA).
Antibody responseSerum samples from non-pregnant females diluted in 1:500 were used to determine antibody response by the enzyme-linked immunosorbent assay (ELISA) following the protocol described42. Briefly, 96-well microtitration plates (MaxiSorp; Nalge-Nunc International, Pittsburgh, USA) were coated with 3μg/well of total L. (L.) amazonensis antigens in PBS overnight at 4°C. Plates were incubated with 5% non-fat dry milk in PBS at room temperature for 2h to block nonspecific bindings. After washing with PBS containing 0.05% Tween-20 (Sigma Aldrich, St Louis, USA), the plates were incubated at 37°C for 1h with 1:500 dilutions of mice sera. Then, the plates were washed and incubated with horseradish peroxidase-conjugated goat antimouse IgG (Thermo Scientific, Pittsburgh, USA) diluted 1:10000. Finally, a color reaction was developed by the addition of 100μl/well of substrate solution 3,3′,5,5′-tetramethylbenzidine (Sigma Aldrich, St Louis, USA) for 30min.
Statistical analysisDifferences between groups were tested for significance by two-way analysis of variance (ANOVA), the Fisher's test, and two-tailed Student's t-tests, depending on the case. A p<0.05 was considered statistically significant. The data shown represent the mean values and standard error of the mean (SEM). The software used for all statistical analyses was GraphPad Prism version 5.01 for Windows (GraphPad Software, California, USA).
Ethical considerationsAll applicable international and institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were under the ethical standards of the institution or practice at which the studies were conducted (Institutional Animal Care and Use Committee of the School of Medical Science, Universidad Nacional de Cuyo, protocol approval no. 65/2015).
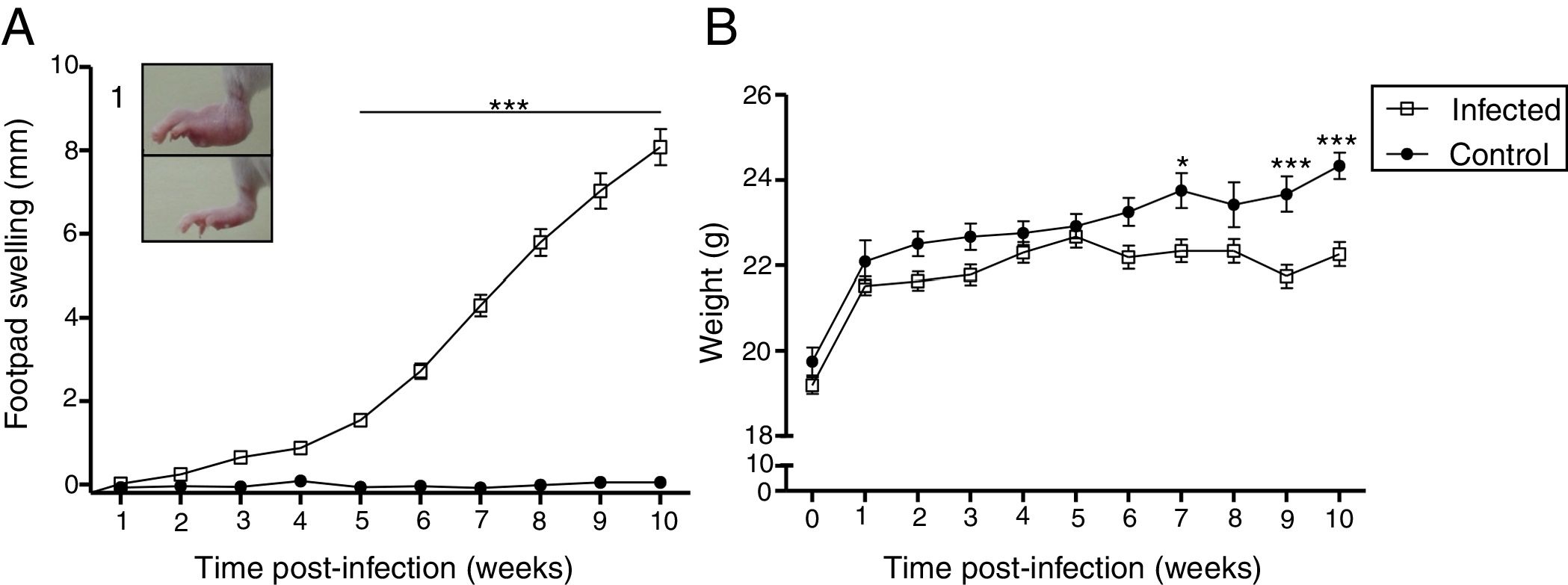
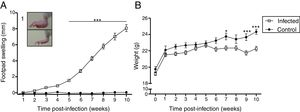
ResultsCourse of L. (L.) amazonensis infection in the pre-mating stageWe evaluated the course of the infection in the pre-mating stage weekly by measuring footpad swelling and body weight for 10 weeks (Fig. 1). There was an increase in footpad swelling in the infected group from the 5th week post-infection (p<0.01), when most of the lesions were nodular or nodule-crusted. Body weight was lower in the infected group: This difference was statistically significant in the 7th week and from the 9th week after the infection.
Clinical features induced by L. (L.) amazonensis infection. Footpad swelling (A) and weight (B) were measured weekly for 10 weeks. Each point represents average thickness of the footpad (A) and weight (B) after infection. These parameters were measured in all females from both groups (n=12 from the control and n=27 from the infected group). Photograph of RFP (1). *p<0.05; **p<0.01p<0.001 by two-way ANOVA.
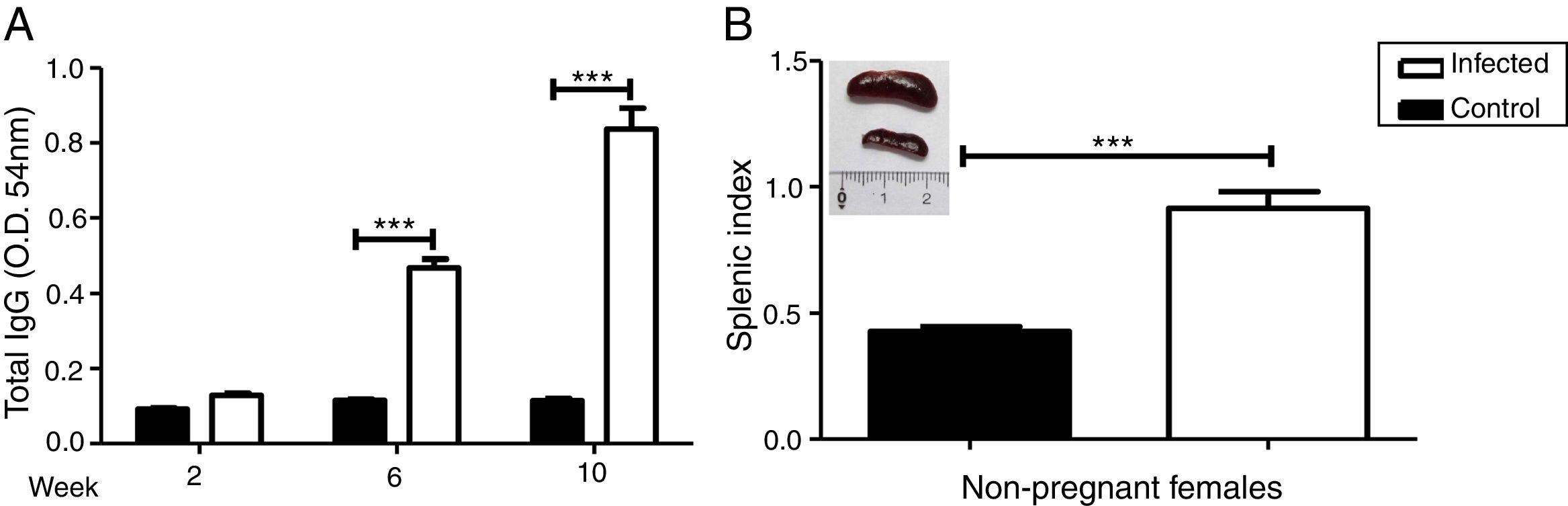
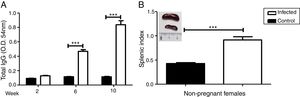
We evaluated the specific antibody response against Leishmania and splenomegaly when the animals were euthanized to analyze the systemic effect of infection. Antibody response was measured by ELISA (Fig. 2A) and splenomegaly by the splenic index (Fig. 2B).
Increase in antibody response and splenic index due to L. (L.) amazonensis infection in pre-mating females. Antibody response to infection was measured three times after infection (A). Splenic index and spleen image of both groups were evaluated 10 weeks after infection (B). These parameters were measured in non-pregnant females (n=4 control and n=8 infected mice). Values represent mean±SEM of optic density values (O.D.) (A) and of splenic index (B). ***p<0.001 by t-test.
There was a statistically significant increase in antibody response in the infected group from the 6th week after the infection (p<0.001). The results of the splenic index showed that the infected group developed splenomegaly with a statistical difference between groups (p<0.001); splenomegaly could even be observed macroscopically (Fig. 2B).
Thus, we confirmed the course of L. (L.) amazonensis infection by evaluation of different parameters such as footpad swelling, pre-mating weight, and antibody response.
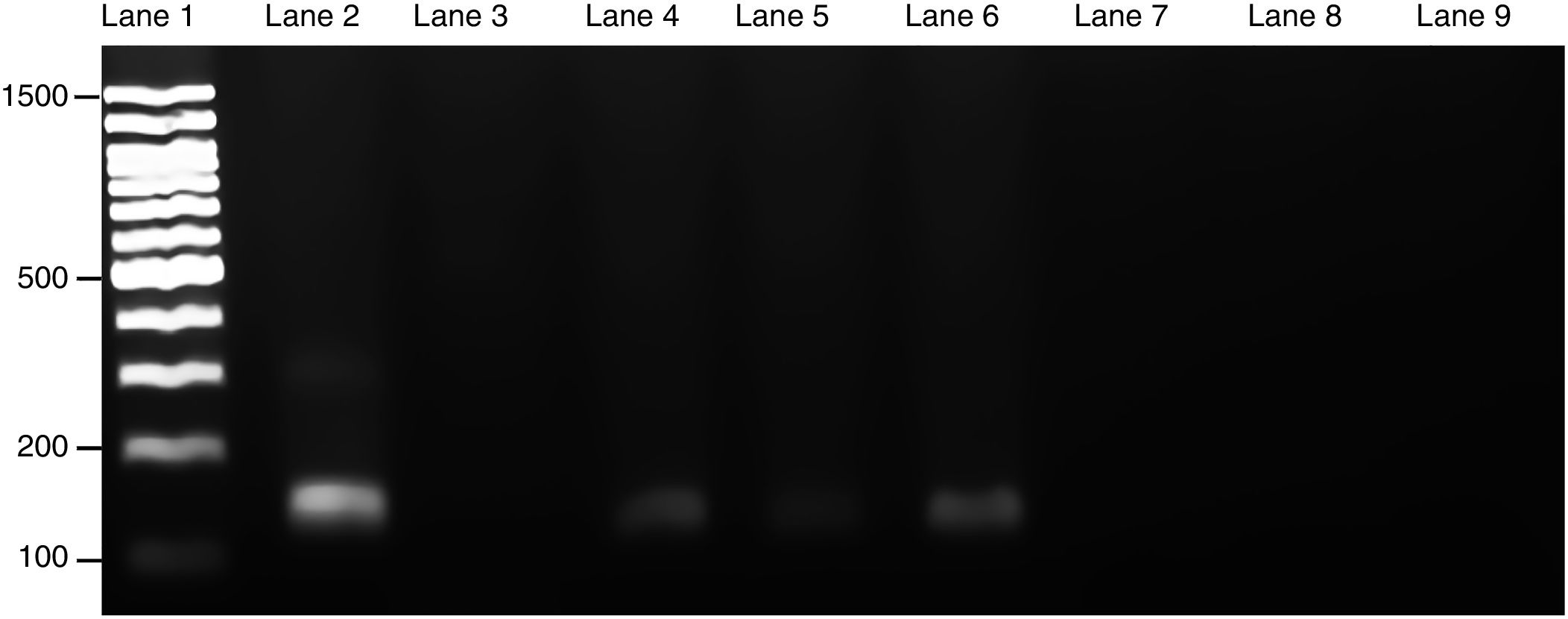
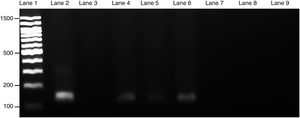
Course of maternal L. (L.) amazonensis infection in the pregnancy stageParasite invasion was confirmed by PCR, which demonstrated that spleens from infected pregnant mothers tested positive for the presence of Leishmania parasite, exhibiting a 120-bp amplification product (Fig. 3). Furthermore, the spleens from the healthy mothers tested negative for Leishmania.
Presence of L. (L.) amazonensis in maternal spleen was confirmed by PCR. Three PCR products from each pregnant female group were used to make an electrophoresis gel. Lanes: 1: 100-bp molecular weight; 2: in vitro culture of L. (L.) amazonensis; 3: non-template control; 4–6: infected maternal spleen; 7–9: non-infected maternal spleen. The size of the product was 120-bp.
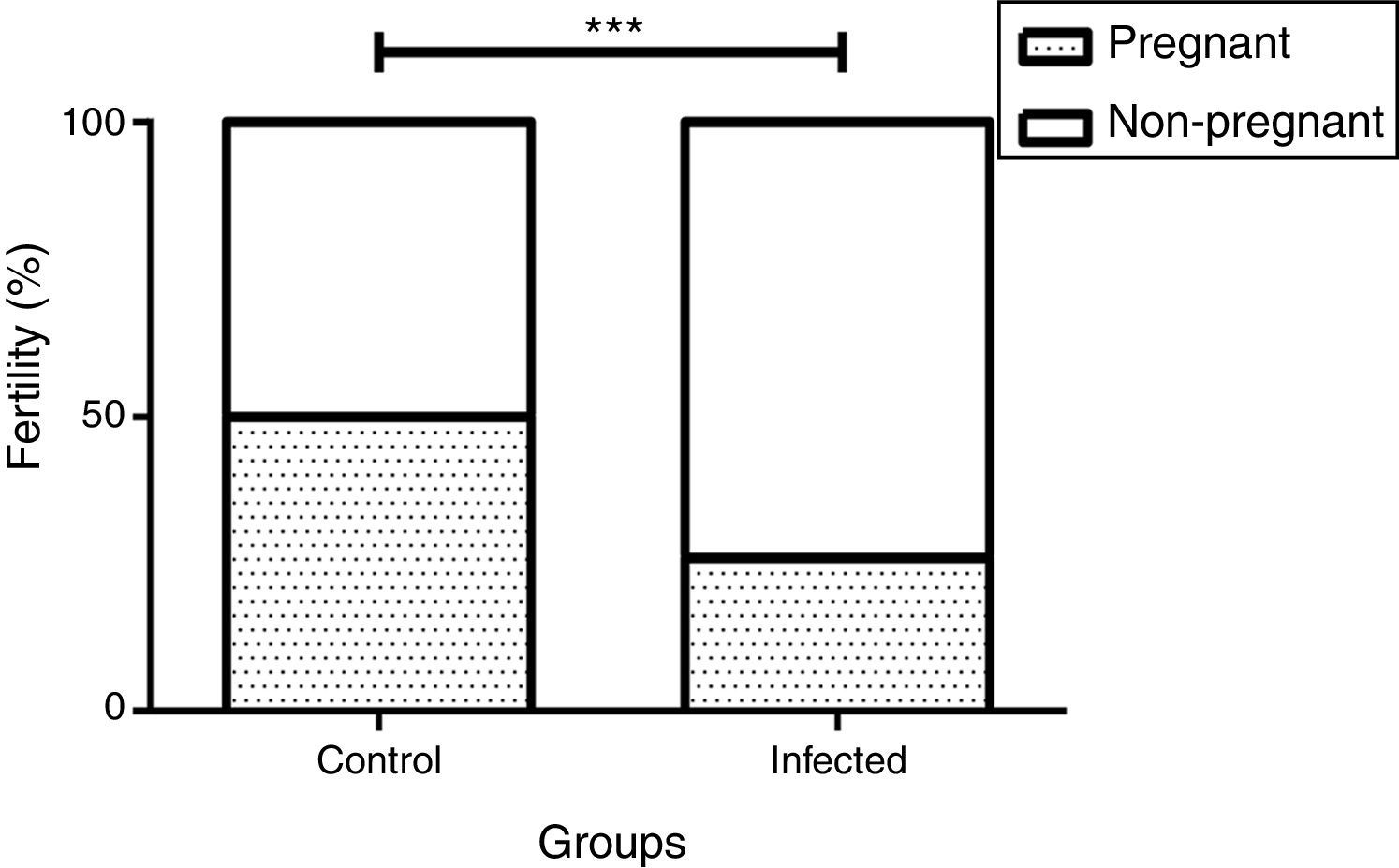
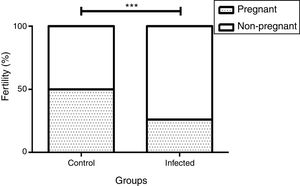
We observed a correlation between the pregnancy percentage and the infection by L. (L.) amazonensis: There was a statistically significant decrease in the percentage of infected pregnant females relative to the uninfected control group (26% and 50%, p<0.001 by the Fisher's correlation test) (Fig. 4). In this way, we observed that there were fewer pregnant females in the infected than in the control group (5 out of 19 and 4 out of 8, respectively). Finally, no differences were observed in female weight between the groups during the pregnancy stage (data not shown).
Influence of infection by L. (L.) amazonensis on female mice fertility rate. Fertility rate was calculated based on pregnant and non-pregnant females of each group after mating (n=8 control and n=19 infected mice). Bar graph represents the correlation between pregnancy result and infection, ***p<0.001 through Fisher's test.
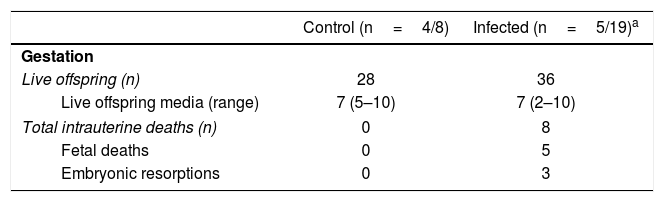
The total number of live offsprings and the media of fetuses by female were similar between the groups (seven in each group). However, it should be noted that we observed five fetal deaths and three embryonic resorptions only in the infected group (Table 1). Two of 5 (40%) infected pregnant females exhibited intrauterine deaths with a frequency of 9.09% and 77.78% of intrauterine deaths in each one.
Gestational features in cutaneous leishmaniasis.
| Control (n=4/8) | Infected (n=5/19)a | |
|---|---|---|
| Gestation | ||
| Live offspring (n) | 28 | 36 |
| Live offspring media (range) | 7 (5–10) | 7 (2–10) |
| Total intrauterine deaths (n) | 0 | 8 |
| Fetal deaths | 0 | 5 |
| Embryonic resorptions | 0 | 3 |
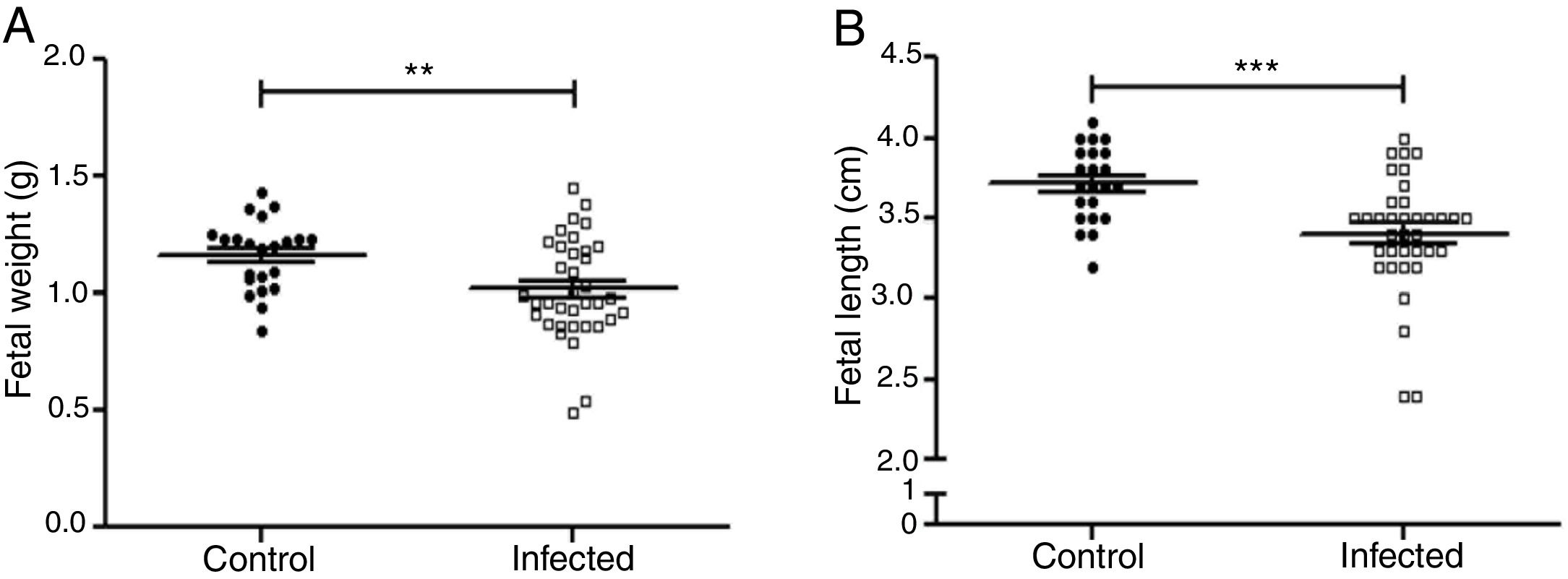
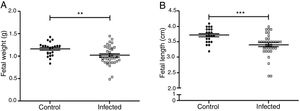
Fetal parameters were evaluated for weight and length (Fig. 5A and B). Fetal weight was significantly lower in the infected group than in the control group (1.019±0.035g and 1.163±0.032g, p<0.01). Fetal length was significantly reduced in the infected group as compared to the control group (3.40±0.06cm and 3.71±0.05cm, p<0.001).
Influence of infection by L. (L.) amazonensis on fetal parameters in mice. Fetal weight (a) and length (b) were determined (n=28 control, and n=36 offsping from infected females). Statistically significant differences can be observed; **p<0.01; ***p<0.001 by using t-test. Values represent mean±SEM.
Different clinical parameters were analyzed in females infected by L. (L.) amazonensis, such as pre-mating footpad swelling, body weight, antibody response, splenic index, and mortality. We observed a weight decrease in the infected pre-mating group (Fig. 1B). This result is in agreement with that of another study, which analyzed the infection by species causing visceral leishmaniasis, such as L. (L.)donovani29; however, weight decrease was not observed in cutaneous leishmaniasis caused by Leishmania (Leishmania) mexicana5. Moreover, there were no deaths in female mice with cutaneous leishmaniasis caused by L. (L.) amazonensis, as opposed to visceral leishmaniasis17.
Previous studies indicate that advanced clinical leishmaniasis is characterized by increased production of anti-Leishmania antibodies and severe splenomegaly in the case of visceral leishmaniasis8. In accordance with these findings, our results showed an increase in anti-Leishmania IgG antibody levels over time (Fig. 2A), indicating an ascending course of the disease. Moreover, the increase in the splenic index (Fig. 2B) confirms that splenomegaly was generated as a response to a systemic infection.
For cutaneous Leishmania species, it is usually thought that the parasite is limited to skin infections. However, the presence of cutaneous L. (L.) amazonensis species in internal organs has previously been identified in murine models of infection13. The presence of parasites in the spleen of the infected pregnant group (Fig. 3) confirms that L. (L.) amazonensis also reaches internal organs.
Infectious diseases have developed new forms of transmission that could play a fundamental role in the pathogenesis of the infection. In the case of visceral leishmaniasis, a large number of studies have reported the transmission to the offspring by different species26,34,39. However, there are no reports of natural vertical transmission of cutaneous leishmaniasis, and few experimental assays have been conducted with species that produce this type of pathology5,37.
It has been shown that pregnancy impairs resistance of C57BL/6 mice to L. (L.) major infection; thus, pregnant C57BL/6 mice developed larger cutaneous lesions23. This demonstrates that infection is exacerbated during pregnancy due to the relative immune tolerance induced in the middle of this stage. Furthermore, pregnancy with splenomegaly is associated with an increased risk to the fetus, with complications like intrauterine growth restriction44. Therefore, parasites that reach the spleen could also colonize other organs, such as the placenta, or the fetuses, inducing clinical manifestations of the disease in the offspring20,37.
Our results showed a strong influence of infection by L. (L.) amazonensis on the reproductive parameters in BALB/c, in which fertility depletion was observed (Fig. 4). This result is inconsistent with other studies, such as the findings for L. (L.) mexicana, which did not reduce fertility in female BALB/c5. Therefore, female fertility depletion has not been documented using any other species of Leishmania. This decrease in the fertility rate could be associated with different factors, such as immune imbalance45. It is well known that the success of pregnancy depends on a Th1-like cellular immune response in the pre-implantation period. However, the change to an immunotolerant profile (Th2 and Treg-like response accompanied by an inhibition of IFN-γ production) is necessary for fetal development25. Specifically, the increase in anti-Leishmania IgG antibody levels (Fig. 2A) observed in our infected female mice confirms that a chronic L. (L.) amazonensis infection induces a high humoral immune response, in accordance with other studies, where a Th2-like immune profile was demonstrated12,15. This type of immune response in a pre-implantation period could explain the observed results regarding the reproductive and fetal parameters.
Fetal complications such as deaths, embryonic resorptions (Table 1), body length, and weight reduction of the offspring were observed in the L. (L.) amazonensis infected group (Fig. 5). These complications have been documented for other species of Leishmania, like Leishmania (Leishmania) mexicana, Leishmania (Leishmania) major and L. (V.)braziliensis5,33,38. As for fetal weight, a reduction was also observed in the first report of vertical transmission of human visceral leishmaniasis by Leishmania (Leishmania) donovani infection26 and in experimental murine cutaneous leishmaniasis by L. (L.) mexicana and L. (L.)major4,45. Both fetal weight and length reduction are associated with intrauterine growth restriction24. On the one hand, these reductions can be attributed to infected mothers with splenomegaly44. The infection can also decrease the ingestion and absorption of food as well as cause placental lesions that affect the transfer of nutrients to the fetus40. On the other hand, these reductions can also be attributed to an imbalance in the maternal cellular response.
However, if the maternal response to Leishmania infection is associated with a Th2/Th17 profile, the response may contribute to uncontrolled infection leading to severe maternal disease (Th2) and to increased hostility to pregnancy, resulting in fetal deaths and embryonic resorptions (Th17)7.
Therefore, our results allowed us to show the relationship between intrauterine growth restriction and L. (L.) amazonensis infection in a murine model. This relationship has also been observed when natural and experimental infections by Zika and Trypanosoma cruzi occur14,21,40,46.
ConclusionTaken together, our results demonstrate that cutaneous leishmaniasis caused by L. (L.) amazonensis infection impairs reproductive and fetal parameters in mice, generating (1) fertility depletion; (2) intrauterine growth restriction; and (3) fetal deaths and embryonic resorptions.
The results of the present study highlight the importance of considering cutaneous leishmaniasis by L. (L.) amazonensis as a disease that affects reproduction as well as fetal health. We consider that these findings should be taken into account for the treatment of human cases although further studies should be conducted to evaluate the effects of cutaneous leishmaniasis on human pregnancy.
Conflict of interestThe authors declare that they have no conflicts of interest.
Acknowledgments and fundingThis study was supported by Universidad Nacional de Cuyo (UNCuyo SeCTyP J063), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) (PIP 11220150100210Co 2015-2017), Agencia Nacional de Promoción Científica y Tecnológica (AGENCIA) (PICT No. 2015-3157), Universidad del Aconcagua (CIUDA 2017-2019) and Fundación Alberto Roemmers (2017–2019).
The authors are grateful to Dr Flavia Alejandra Bruna for her help in image editing.