In Argentina, despite the important studies conducted on the prevalence of infection and the antibiotic resistance of Helicobacter pylori, there are no reports simultaneously analyzing a profile of virulence factors of the bacterium and polymorphisms in cytokine genes in patients with different alterations in the gastric mucosa (including intestinal metaplasia, IM). Our aim was to evaluate H. pylori genotypes in 132 adult patients with chronic gastritis presenting three different histological findings (inactive chronic gastritis, active chronic gastritis IM− and active chronic gastritis IM+) along with SNP-174 G>C in the IL-6 gene. cagA, vacA and babA2 genes were analyzed by multiplex PCR. The -174 G>C SNP IL-6 gene was analyzed by PCR-RFLP. Patients with active chronic gastritis IM+ showed the highest proportion of the cagA(+)/IL-6GG, cagA(+)/vacAm1s1/IL-6GG and cagA(+)/vacAm1s1/babA2(+)/IL-6GG combinations (p<0.05). There was 4-5 times greater probability of finding patients presenting the GG genotype for SNP-174 G>C IL-6, which in turn were infected with the most virulent H. pylori genotypes -cagA(+), cagA(+)/vacAm1s1 and cagA(+)/vacAm1s1/babA2- in the ACGIM+ group in comparison to the ICG group. Our results provide regional data to the idea that the transition towards severe alterations in the gastric mucosa would be the result of a balance between specific factors of H. pylori and inherent host factors. This fact can be useful to identify patients at greater risk and to select those individuals requiring appropriate eradication treatment to prevent progression to gastric cancer.
En Argentina, a pesar de los importantes estudios realizados sobre la prevalencia de infección y la resistencia a antibióticos de Helicobacter pylori, no existen reportes que analicen simultáneamente un perfil de factores de virulencia de la bacteria y polimorfismos en genes de citoquinas en pacientes con diferentes alteraciones en la mucosa gástrica (incluida la metaplasia intestinal [MI]). Nuestro objetivo fue evaluar genotipos de H. pylori en 132 pacientes adultos con gastritis crónica, con tres diferentes hallazgos histológicos (gastritis crónica inactiva [GCI], gastritis crónica activa [MI−] y gastritis crónica activa [MI+]), junto con el SNP-174 G>C en el gen de IL- 6. Los genes cagA, vacA y babA2 se analizaron mediante PCR multiplex. El SNP-174 G>C IL-6 se analizó mediante PCR-RFLP. Los pacientes con gastritis crónica activa MI+ mostraron la mayor proporción de combinaciones cagA(+)/IL-6GG, cagA(+)/vacAm1s1/IL-6GG y cagA(+)/vacAm1s1/babA2(+)/IL-6GG (p<0,05). Hubo 4-5 veces mayor probabilidad de encontrar pacientes con el genotipo GG en SNP-174 G>C IL-6 y a su vez infectados con los genotipos más virulentos de H. pylori—cagA(+), cagA(+)/vacAm1s1 y cagA(+)/vacAm1s1/babA2—en el grupo gastritis crónica activa MI+ en comparación con el grupo GCI. Nuestros resultados aportan datos regionales a la idea de que la transición hacia alteraciones más graves en la mucosa gástrica resultaría de un equilibrio entre factores específicos de H. pylori y factores inherentes al huésped. Esto puede ser útil para identificar pacientes con mayor riesgo y seleccionar aquellos individuos que requieran un apropiado tratamiento de erradicación para prevenir la progresión al cáncer gástrico.
Helicobacter pylori (H. pylori) is a gastric pathogen widely recognized as a causative agent of gastritis worldwide. Furthermore, H. pylori is an important etiological agent for a multi-step cascade which, starting from chronic gastritis, can evolve to pre-neoplastic lesions (such as intestinal metaplasia), dysplasia and up to gastric adenocarcinoma10,15,17,46,52. In fact, in 1994 it has been classified as a Class I carcinogen by the International Agency for Research on Cancer18. In addition, gastric cancer remains the 3rd leading cause of cancer-related death and the leading cause of infection-related cancer worldwide12. Epidemiology of gastric cancer shows a high variability: there are countries presenting high prevalence (e.g., Korea, Japan, China, Colombia, Chile) whereas others show low prevalence rates (generally, developed countries such as USA, Canada and Australia). Infection rates in Argentina are found at intermediate values, with the highest values as well as the highest mortality rate from stomach cancer reported in the southern provinces11,12,25,27,37.
It has been mentioned that the cascade of events that would lead to adenocarcinoma seems to be triggered – among other causes – by bacterial-specific virulence factors (such as the cagA, vacA, babA2 gene) as well as by factors such as polymorphisms in cytokine genes9,21,43,50. In this sense, greater mucosal Interleukin 6 (IL-6) levels in early gastric cancer with active H. pylori infection than those without H. pylori infection49, have been observed. In addition, individuals with the G allele at position -174 in the promoter sequence of IL-6 have been shown to produce higher levels of IL-613. More recently, the importance of IL-6 mediated stromal–epithelial cell interaction in gastric tumorigenesis has been suggested22.
The abovementioned Correa's cascade is often referred to follow a linear progression; nevertheless, most patients present little or no change over time. On the contrary, other patients exhibiting a dynamic process alternating regression and/or progression of lesions in their gastric mucosa and even rapid progression bypassing some of the putative steps is not an infrequent finding. It has been suggested that intestinal metaplasia (IM) is a “point of no return”, at which eradication is less effective at causing regression and indeed does not change the risk of progression in certain patients: once IM is established, eradication of H. pylori only partially achieves a successful reduction of the risk of progression to adenocarcinoma and the genetic damage to gastric stem cells becomes irreversible23.
In Argentina, despite the important studies carried out in relation to the prevalence of infection and resistance of H. pylori to the different antibiotics used for its eradication4,19,20,34,35,48,53 there are no reports simultaneously analyzing a profile of virulence factors of the bacterium and polymorphisms in cytokine genes of the host in patients with chronic gastritis showing different histopathological findings in the gastric mucosa (including pre-neoplastic lesions such as intestinal metaplasia). Hence, our aim was to begin an evaluation of H. pylori genotypes in adult patients residing in the north-central region of the Province of Santa Fe (Argentina) with different degrees of chronic gastritis, along with SNP-174 G>C in the promoter sequence of the IL-6 gene.
Material and methodsPatients and samples. Adult dyspeptic patients (n=270) attending the Gastroenterology Service of “Dr. José María Cullen” Hospital (Santa Fe, Argentina) were enrolled in the study after informed consent was obtained. All of them underwent upper gastrointestinal endoscopy. The study was approved by the Research Ethics Advisory Committee of the Facultad de Bioquímica y Ciencias Biológicas (Universidad Nacional del Litoral) as well as by the Ethics Committee of the “Hospital Dr. J.M. Cullen”. Patients who had received antibiotic therapy and/or PPIs in the last 15 days were excluded from the study. Both biopsy sampling and histological analyses were carried out following the modified Sydney standardized protocol. Activity of gastritis was defined by the presence of neutrophils in the gastric mucosa. Two additional samples were taken from antrum and corpus for the rapid urease test and molecular detection of H. pylori.
DNA purification and H. pylori molecular detection. DNA purification was carried out following a procedure using Proteinase K as previously described4. DNA integrity was evaluated by agarose gel electrophoresis; DNA yields and purity were assessed by absorbance analysis at 260/280nm. Purified DNA was stored at −20°C until use. H. pylori was studied in gastric biopsies by nested PCR targeting the hsp60 gene, using previously reported conditions20.
H. pylori cagA, vacA and babA2 gene analysis. cagA, vacA and babA2 genes were analyzed by multiplex PCR using sequence specific primers4.
Analysis of -174 G>C single nucleotide polymorphism (SNP) in the IL-6 gene. The SNP-174 G>C (rs1800795) in the promoter sequence of the IL-6 gene was analyzed by PCR-RFLP according to Guzman-Guzman et al16. As a control, the same SNPs were also analyzed in a group of H. pylori (−) patients. In all cases, the -174 G>C IL-6 polymorphism was in Hardy–Weinberg equilibrium, which was similar to that recently reported by Strauss et al.45, when evaluating the distribution of IL-6 genotypes in seronegative and seropositive Argentine individuals for Chagas disease
Agarose gel electrophoresis. PCR products were analyzed by electrophoresis in 2% agarose gels (D1 Agarose, Biodynamics, Argentina). All in vitro amplification reactions included an internal control (human beta-globin gene). PCR-RFLP fragments were resolved in 3% agarose gels (LE Agarose, Inbio Highway, Argentina). All gels were prepared in the presence of a fluorescent stain and photographed under UV light.
Statistical analysis. The association between categorical variables was evaluated using the Chi-square test and Odds ratio. A p-value <0.05 was considered statistically significant. A Hardy–Weinberg equilibrium analysis was carried out to evaluate the -174 G>C polymorphism in the IL-6 gene.
ResultsThe analysis was performed on samples from 132 patients with a histological diagnosis of chronic gastritis and with positive results for H. pylori by in vitro amplification of the hsp60 gene plus histological analysis and/or rapid urease test (in other words, if a positive result was obtained by 2 out of 3 tests).
The age range of patients was 18–65 years, with a mean value of 41.7 years (median=41). With regard to gender, 68.1% (90) and 31.8% (42) were women and men, respectively, in a 2.14:1 ratio.
When analyzing the H. pylori genotypes present, the presence of the cagA gene was detected in 34% of all patients with chronic gastritis, while the babaA2 gene was present in 53% of the samples analyzed.
All patients presented the vacA gene in its different variants: 67% corresponded to the m1s1 variant, followed by the m2s2 and m2s1 variants, in 25% and 8%, respectively; the presence of the vacAS2M1 variant was not detected. The cagA(+)/vacA m1s1 combination (reported as one of the most virulent) was found in 32% of the patients, while the genotype called “triple virulent” cagA(+)/vacAm1s1/babA2 (+) was present in 18% of patients.
Taking into account the alterations in the gastric mucosa, the total number of patients was reclassified into 3 groups (a) patients with inactive chronic gastritis (ICG, n=43; age: mean, 41.4; range=18–65), (b) patients with active chronic gastritis without intestinal metaplasia (ACGIM−, n=69; age: mean, 40.3; range=22–60) and (c) patients with active chronic gastritis with intestinal metaplasia (ACGIMI+, n=20; age: mean, 46.3; range=28–64). No significant differences were found in relation to the age of the different groups (p>0.05).
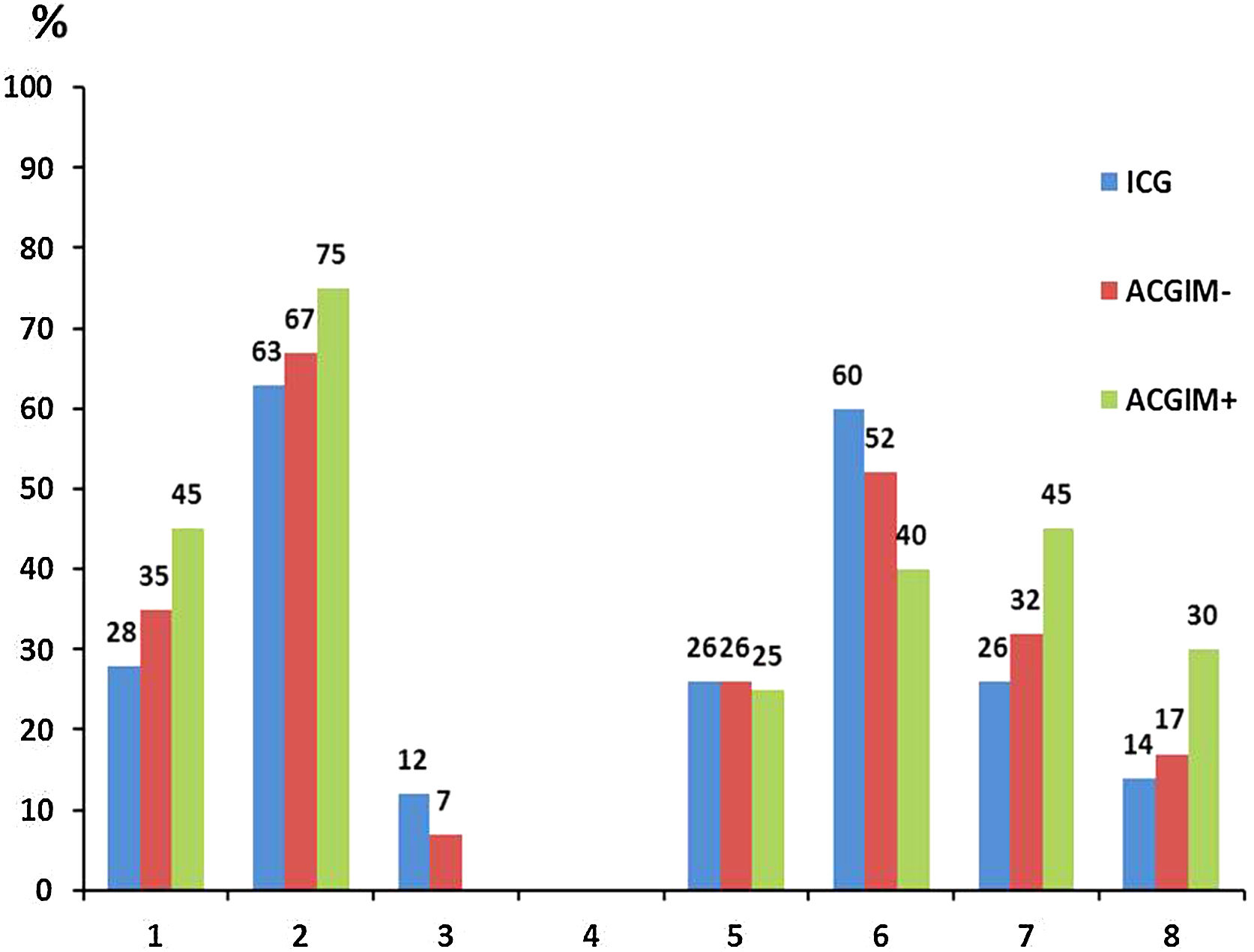
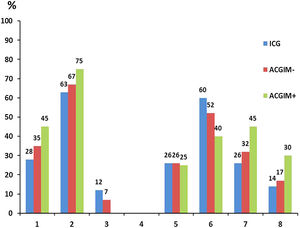
When analyzing the distribution of H. pylori genotypes in the 3 above-mentioned groups, although there were no significant differences between them, interestingly the group of ACGIM+ patients exhibited the highest proportion of genotypes cagA(+), vacAs1m1 and the combination cagA(+)/vacAm1s1 as well as the highest proportion of the “triple virulent” genotype cagA(+)/vacAm1s1/babA2.
On the contrary, although the ICG group showed the highest frequency of babA2 (+) strains, it also had the lowest frequency of cagA(+), vacAs1m1 genotypes and the cagA(+)/vacAm1s1 and cagA(+) combinations/vacAm1s1/babA2 (Fig. 1).
Then, the -174 G>C polymorphism in the promoter sequence of the IL-6 gene was analyzed in each group. Based on our results, the ACGIM+ group showed the highest proportion of both the high-expression GG haplotype and the G allele, with significant differences compared to the ICG group (p<0.05).
Finally, the -174 G>C polymorphism in the IL-6 gene was analyzed in conjunction with the H. pylori genotypes present in each group. In this case, in the group of patients with ACGIM+ we found the highest proportion of cagA(+)/IL-6 GG, cagA(+)/vacAm1s1/IL-6GG and cagA(+)/vacAm1s1/babA2 (+)/IL-6GG combinations, with significant differences both with the ICG group and with the patients with ACGIM− (p<0.05) (Table 1).
Distribution of genotypes/alleles of IL-6 rs1800795 polimorphism and combinations of H. pylori virulence factors/GG IL-6 rs1800795 genotype in patients with different types of chronic gastritis.
| ICG (n=43) | ACGIM− (n=69) | ACGIM+ (n=20) | |
|---|---|---|---|
| SNP IL-6 GG | 27 (62.8%)(*) | 49 (71%) | 17 (85%)(*) |
| SNP IL-6 GC | 14 (32.6%) | 18 (26.1%) | 3 (15%) |
| SNP IL-6 CC | 2 (4.6%) | 2 (2.9%) | 0 (0%) |
| SNP IL-6 Alelo G | 68 (79%)(*) | 116 (84%) | 37 (92.5%)(*) |
| SNP IL-6 Alelo C | 18 (21%) | 22 (16%) | 3 (7.5%) |
| cagA(+)/IL-6 GG | 7 (16%)(*) | 16 (23%)(**) | 9 (45%)(*)(**) |
| cagA(+)/vacAS1M1/IL-6 GG | 7 (16%)(*) | 16 (23%)(**) | 9 (45%)(*)(**) |
| cagA(+)/vacAS1M1/babA2(+)/GG | 3 (7%)(*) | 9 (13%)(**) | 6 (30%)(*)(**) |
ICG, inactive chronic gastritis; ACGIM−, active chronic gastritis without intestinal metaplasia; ACGIM+, active chronic gastritis with intestinal metaplasia. (*)(**) Significant differences (p<0.05).
Furthermore, there was 4–5 times greater probability of finding patients exhibiting the GG genotype for SNP -174 G>C IL-6, which in turn were infected with the most virulent H. pylori genotypes – cagA(+),cagA(+)/vacAm1s1 and cagA(+)/vacAm1s1/babA2 – in the ACGIM+ group in comparison to the patients of the ICG group (Table 2).
Association of combinations of H. pylori virulence factors and GG IL-6 rs1800795 genotype in patients with different types of chronic gastritis.
| Group | OR | CI 95% | p | |
|---|---|---|---|---|
| ICG | 1 | N.D. | N.D. | |
| cagA(+)/GG | ACGIM− | 1.55 | 0.58 to 4.15 | 0.3809 |
| ACGIM+ | 4.21 | 1.27 to 13.92 | 0.0186 | |
| cagA(+)/vacAm1s1/GG | ICG | 1 | N.D. | N.D. |
| ACGIM− | 1.55 | 0.58 to 4.15 | 0.3809 | |
| ACGIM+ | 4.21 | 1.27 to 13.92 | 0.0186 | |
| cagA(+)/vacAm1s1/babA2(+)/GG | ICG | 1 | N.D. | N.D. |
| ACGIM− | 2.00 | 0.51 to 7.84 | 0.3201 | |
| ACGIM+ | 5.71 | 1.25 to 25.96 | 0.0240 |
ICG, inactive chronic gastritis; ACGIM−, active chronic gastritis without intestinal metaplasia; ACGIM+, active chronic gastritis with intestinal metaplasia. OR: Odds ratio. CI: confidence interval, ICG group was taken into account as a reference. p-values in boldface type indicate statistical significance.
The association between H. pylori infection and gastric cancer is a well-known clinical event. Moreover, H. pylori is recognized as one of the factors that would contribute to the development of the cascade of events described by Correa10, which begins with chronic gastritis followed by the development of pre-neoplastic lesions (such as intestinal metaplasia) leading to adenocarcinoma32. On the other hand, different articles have shown important differences in relation to the infecting H. pylori genotypes as well as the epidemiology of gastric cancer around the world and even within the same country5,8,38,47,55. Furthermore, different studies have explored the role of polymorphisms in interleukins associated with pre-neoplastic lesions in patients infected with H.pylori42,54.
In this context, our country has a vast surface area and although important studies in relation to the prevalence of infection as well as resistance to antibiotics have been published4,19,20,34,35,48,53, to our knowledge there are no studies that simultaneously analyze: (a) the profile of virulence factors of the bacterium, (b) the polymorphisms in the cytokine genes of patients with chronic gastritis and (c) the histopathological findings in the gastric mucosa, including pre-neoplastic lesions such as intestinal metaplasia. Taking this into account, our aim was to begin a study focused on these variables in adult patients with chronic gastritis from the north-central region of Santa Fe province (Argentina).
Based on our results, the frequency of the best-studied virulence factors (cagA, vacA and babA2) in patients with chronic gastritis is related to previous studies in Argentina6,24,26,28,31,48. However, some differences can be highlighted: the frequency of the cagA gene found in our study (34%) is similar to that reported in the western region (30–40.8%)28,48; however, lower than the data for Buenos Aires city (71–84%)6,24,26. On the other hand, as reported by other authors in our country, the vacAm1s1 variant was predominant in the group of patients with chronic gastritis studied6,24,28,48. With respect to the virulence factor babA2, we found a considerably higher proportion of patients carrying this gene than that reported by Medina et al.31, who found 9.7% in gastric biopsies of patients from another geographical region of our country. This could represent a real difference in the circulating genotypes or might be due to different experimental conditions, fundamentally given by the sequence of primers used for the amplification of the babA2 gene, as recently indicated44, as well as by the presence of allelic variations in the babA2 gene owing to microevolution phenomena described during colonization in some patients29. This microheterogeneity has been reported previously in interesting studies by Matteo et al.30 and Armitano et al.1 where the cag pathogenicity island (cagPAI), vacA, bab, oipA, dupA status and the presence of several genes from the genomic plasticity zone were studied to analyze intra-host variation in patients with chronic gastritis and peptic ulcer disease. In these studies, an inter-niche variability was proven for most of the genes analyzed in the cagPAI, for the genes oipA, dupA and for those in the plasticity region and, in a lesser extent, for the bab gene. However, such significant variability was not observed for the cagA and vacA genes. Matteo et al.30 found that 37/40 patients had isolates with the same profile for the vacA gene whereas Armitano et al.1, identified that 24/28 and 25/28 patients had H. pylori isolates with the same status for the cagA and vacA genes respectively, in the different niches studied.
In the next analysis, when classifying the patients into groups according to the histological findings (inactive chronic gastritis, active chronic gastritis IM− and active chronic gastritis IM+), as expected, we observed a trend towards a higher proportion of patients carrying the most virulent genotypes in patients with active chronic gastritis IM+, although differences were not statistically significant. However, when the analysis of the -174 G>C SNP in the IL-6 gene was included, we found a higher proportion of the IL-6 GG genotype in patients infected with both cagA(+) H. pylori strains as well as with the combinations cagA(+)/vacAs1m1 and cagA+/vacAs1m1/babA2 (+) in the active chronic gastritis IM+ group in relation to the other two groups. In this case, differences were significant in patients with inactive chronic gastritis as well as in patients with active chronic gastritis that did not exhibit intestinal metaplasia.
Previous studies were conducted to analyze the impact of polymorphisms at position -174 of the IL-6 promoter in the development of gastric pathologies in the presence of H. pylori, with contradictory results. Similarly to our study, Gatti et al.14 found an association between the GG alleles of IL-6 (-174 base) and the presence of gastric adenocarcinoma, independently of the tumor type (diffuse or intestinal). Similarly, a study conducted in Portugal reported a higher frequency of the IL-6 -174C/G genotype in the gastric cancer cases analyzed40. Attar et al.2 also found a high frequency of the G allele and the GG genotype in patients compared to control subjects, suggesting that the polymorphism could influence the susceptibility to gastric cancer. On the other hand, although our findings differ from those by Ramis et al.36 and Santos et al.41 who found no correlation between polymorphisms in IL-6 and a higher risk of gastric carcinoma, they are consistent with the possibility of finding greater lesions in the gastric mucosa of those patients infected with more virulent strains of H. pylori with a potential higher local inflammatory response triggered by a greater expression of pro-inflammatory cytokines such as IL-6. In this sense, individuals with the G allele at position -174 have been shown to produce higher levels of IL-613. Furthermore, mucosal IL-6 levels were also greater in early gastric cancer with active H. pylori infection than without H. pylori infection, and the levels decreased dramatically after the eradication of infection49. In a similar way, a positive correlation between mucosal IL-6 mRNA expression level and virulence factors of H. pylori in Iranian adult patients with chronic gastritis has been recently reported3. In addition, Kinoshita et al.22, among other authors, suggested the importance of IL-6 mediated stromal–epithelial cell interaction in gastric tumorigenesis.
Due to the fact that Latin America is a region with one of the highest mortality rates due to gastric cancer in the world7 and since it has been mentioned that, in some individuals, intestinal metaplasia might be a “point of no return” to the development of gastric adenocarcinoma, we consider relevant to report the first analysis in our region (Santa Fe, Argentina) of H. pylori genotypes and IL-6 SNP in patients with different types of gastritis, including pre-neoplastic lesions such as intestinal metaplasia. It should be noted that our analysis not only included the presence/absence of H. pylori associated to gastritis and pre-neoplastic alterations but also the identification of H. pylori genotypes along with the -174 G>C polymorphism on the IL-6 gene in each patient.
These results obtained so far for our region would be in accordance with the idea that the transition towards more severe alterations during H. pylori infection would result from a balance between specific factors of the bacterium and host factors that modulate the inflammatory response during infection of the gastric mucosa.
In this sense, a recent review by Rudnicka et al.39 analyzed results from large-scale epidemiological studies, literature meta-analyses as well as from clinical and animal model studies focusing on the search of host immune system regulators (such SNPs in cytokines/growth factors) together with H. pylori virulence factors that facilitate the development of pre-cancerous and cancerous lesions, in the context of environmental and geographical determinants. Among the bacterial determinants related to gastric cancer development, they highlighted the infection with cagA-positive (particularly with a high number of EPIYA-C phosphorylation motifs) and vacA-positive isolates (in particular s1/m1 allele strains). They also reported that the combined genotyping of bacterial and host determinants suggests that the accumulation of polymorphisms favoring host and bacterial features increases the risk for precancerous and cancerous lesions in patients and they also suggested that biological determinants are urgently needed to predict the clinical course of infection in individuals with confirmed H. pylori infection.
Furthermore, a whole-genome analysis was recently used to study 723 H. pylori genomes from different regions of the world in order to compare American (from 14 geographical sites across America, from Canada to Argentina) versus non-American populations33. In this study, genetic variants that were more common in the Americas than in the populations from other continents were identified, mainly resulting in non-synonymous mutations in functionally relevant regions of virulence genes (among them, cagA, vacA and babA genes), in domains known to interact with molecules of the host. Based on previous reports, it was suggested that the progression to serious clinical diseases, such as gastric cancer, is associated with the carriage of specific genotypes of the bacterium, in particular those that carry genes linked with virulence, concluding that the differences found between American and non-American H. pylori genomes could reflect a co-evolutionary process potentially contributing to the high risk of gastric cancer in the region.
To date, our work has aimed at analyzing the presence or absence of the most studied genes in H. pylori (cagA, vacA and babA2) and their association with the severity of gastric lesions without considering variations in their structure (such as those due to the presence of different types/number of EPIYA sequences), additional polymorphisms in the cagA gene or the integrity of cag PAI which mediates efficient CagA translocation, important for the commencement of pathogenicity51. Hence, our efforts will be devoted to increasing both the spectrum of genes of H. pylori (including variations in their structure) as well as on the host in a greater number of individuals. This could be useful to identify patients at risk of developing more aggressive alterations in the gastric mucosa who will require appropriate eradication treatments and a more rigorous follow-up to prevent progression to gastric cancer.
FundingThis work was supported by a grant from the Universidad Nacional del Litoral, Santa Fe (Argentina) (CAI+D 2020 N° 50520190100198LI).
Conflict of interestThe authors declare that they have no conflicts of interest.
Authors thank to the Servicio de Anatomía Patológica of the Hospital "Dr. J.M. Cullen‟ for the histopathological analysis as well as to the Universidad Nacional del Litoral for funding this research. Authors also acknowledge Facundo Zalazar for language assistance.