Dark septate endophytes (DSE) are a heterogeneous group of fungi, mostly belonging to the Phylum Ascomycota, that are involved in a mutualistic symbiosis with plant roots. The aim of this study is to evaluate the behavior of two strains of DSE isolated from wheat roots of two cropping areas in the province of Buenos Aires, Argentina, against some agrochemicals. Of all the isolates obtained, two strains were identified as Alternaria alternata and Cochliobolus sp. These DSE were found to be tolerant to glyphosate, carbendazim and cypermethrin when evaluated at the recommended agronomic dose (AD), 2 AD and, in some cases, 10 AD. This work contributes to the study of the biology of this group of fungi and their tolerance in the presence of xenobiotics widely used in agriculture.
Los endófitos septados oscuros (DSE) son un grupo heterogéneo de hongos que participan de una simbiosis mutualista con raíces de plantas, perteneciendo principalmente al PhylumAscomycota. El objetivo de este estudio fue aislar DSE de raíces de trigo proveniente de dos áreas de cultivo de la provincia de Buenos Aires y evaluar el comportamiento de dos cepas de DSE aisladas de raíces de trigo frente a algunos agroquímicos en dos áreas de cultivo de la provincia de Buenos Aires. De todos los aislamientos obtenidos se seleccionaron dos cepas que se identificaron como Alternaria alternata y Cochliobolus sp. Se encontró que estos DSE son tolerantes al glifosato, el carbendazim y la cipermetrina, evaluados a las dosis agronómicas recomendadas (AD), a 2x AD y, en algunos casos, a 10x AD. Este trabajo contribuye al conocimiento de la biología de este grupo de hongos y su tolerancia a xenobióticos ampliamente utilizados en la agricultura.
Agricultural practices include the application of different pesticides to maintain crop performance by controlling insects, diseases and weeds. In nature, pesticide residues are subjected to physical, chemical and biochemical degradation processes, although they persist in the environment due to their high stability and water solubility22. The inadequate use of pesticides can affect microorganisms and their activity, causing alterations in the biological processes that impact on soil fertility and, consequently, on crop productivity8. Pesticides can reduce the production of microbial biomass; this change may cause alterations in the mineralization of organic matter, redox reactions, denitrification, nitrification, ammonification, and methanogenesis13. One of the most widely used pesticide is glyphosate, a potent, broad spectrum, non-selective, post-emergent herbicide that is capable of controlling 97% of the weeds. Glyphosate is generally used along with cypermethrin, an insecticide belonging to the family of pyrethroids and widely used in non-tillage systems. Among the fungicides, carbendazym is a systemic fungicide used for controlling a broad range of fungi affecting crops.
In the soil environment there are different types of microorganisms, such as fungi that participate actively in all the processes of transformation of organic matter. Fungal endophytes are organisms that colonize plant tissues during a specific period of their life cycle but cause no symptoms of tissue damage to their hosts29. Among this highly diverse group of endophytic fungi, dark septate endophytes (DSE) have received much attention in the recent years27,31. The genera Alternaria sp. and Curvularia sp. are widely known as pathogenic fungi in several cereals of commercial value1,40; however, species of the genera Alternaria and Curvularia (teleomorph: Cochliobolus) have been found to colonize grasses, playing a role as DSE fungi and promoting plant growth14,24. These DSE fungi are not thought to be pathogenic, as they are observed on healthy plants3.
Colonization patterns by DSE seem to be different according to the plant host and the cultivation system21. These fungi often inhabit oligotrophic soils that are associated with the roots of hundreds of plant species in all climate regions and major biome types33 as well as promoters of plant growth under greenhouse conditions27. Dark septate endophytes are able to hydrolyze major C, N and P polymers into usable subunits and therefore could provide N and P to the host plant5. Newsham21 performed a meta-analysis of independent studies to assess the inoculation of DSE in different crops, concluding that the contents of N and P in inoculated plant biomass were higher compared to non-inoculated plants. Conversely, another meta-analysis suggested negative to neutral effects of DSE inoculations18.
Wheat is one of the winter cereals of major importance in Latin America. Agricultural production is one of the fundamental economic activities of Argentina, which is among the main world producers of crops such as soybeans, wheat, sunflower and corn. In Argentina, the production area destined for the wheat crop was estimated in 5.95 million hectares, producing 18 million tons of the cereal19. Most studies have evaluated mycorrhizal and DSE root colonization in different hosts and Argentine regions17,28. Nevertheless, little information is available about the biology of DSE isolated from cereals27,31. The widespread use of pesticides has raised concerns about their potential impact on microbial communities and in this sense there are no studies evaluating the effects of agrochemicals on DSE. Therefore, the aim of this study is to isolate DSE from wheat roots and to assess the tolerance of isolated strains to different chemicals commonly used in agricultural practices.
Materials and methodsThirty wheat plants were randomly collected from two sites located in Arenales and Ferré in July 2011 (Buenos Aires Province, Argentina). The plants were at phenological stage Z5. Samples were transported to the laboratory and placed in a cold chamber at 4°C until their use. The roots were washed under tap water and sterilized superficially by immersing them in 70% ethanol for 2min followed by 1% sodium hypochlorite (NaOCl) for 3min and thoroughly rinsed five times with sterile distilled water. Subsequently, twenty five root pieces (5mm) of each plant were placed into drops of Gel-Gro (ICN Biochemicals, USA) with streptomycin and tetracycline hydrochloride 1% (Sigma–Aldrich, USA) in sterile Petri Plates 30 Plates30. The root pieces were incubated in the dark at 25°C and were periodically observed under a stereoscopic microscope (Nikon H550S, Japan). The segments of roots in which fungal growth was observed were placed on plates containing Malt Extract Agar (MEA) and cultured on such medium at 25°C, thereby obtaining several isolates. The endophytic nature of the thirty isolates was corroborated following the test of resynthesis (Koch's postulates) under greenhouse conditions31. Five isolates were identified following classical10,36 and molecular methodologies31. The strains were deposited at the Fungi Bank of the Facultad de Agronomía, Universidad de Buenos Aires27. Two isolates were selected for this assay due to their extracellular enzymatic production35, their phosphorus solubilization31 and their tolerance to different sodium salts32.
A culture of each strain grown on MEA for 7 days at 25°C was used as inoculum to evaluate their tolerance to pesticides. Subsequently, two successive picks were conducted on basal salt medium (BSM)15 with the purpose of causing them a nutritional stress (starvation). The BSM containing the herbicide (glyphosate), the fungicide (carbendazim) or the insecticide (cypermethrin) was used in an agronomic common dose (AD), 2 AD and 10 AD. Doses per Petri dish were 0.20ppm, 0.40ppm and 2ppm for the herbicide; 18.12ppm, 36.25ppm and 181.25ppm for the fungicide; and 2.02ppm, 4.04ppm and 20.2ppm for the insecticide respectively. The control treatment was BSM medium plus sucrose (2%). The inoculum was standardized using 5mm-diameter disks obtained from the zone with active growth of a colony with 7 days of growth for all strains and all tested media. Each agrochemical dose and its respective control were tested in quintuplicate. Fungal growth was assessed periodically from the third day by measuring the colony diameter at 12 days. Fungal growth rates were analyzed using the following formula26: Growth rate=(Fd−Id)/(Ft−), where: Fd is the final diameter (cm), Id is the initial diameter (cm), Ft is the final time (days) and It is the initial time (days).
Moreover, the inhibition percentage was calculated for each fungi and each agrochemical at 6 and 12 days using the following formula: Inhibition (%)=(Cd−Td)/Cd×100, where: Cd is the mean colony diameter in the control treatment (cm) and Td is the mean colony diameter in the agrochemical treatment (cm).
Statistical analysisA two-way analysis of variance (ANOVA) with DSE strains (A. alternata and Cochliobolus sp.) and agrochemical type (4 levels: 0; AD; 2 AD; 10 AD) as factors was performed from the data obtained on growth parameters and effective concentration (EC 50) using the statistical software Infostat Professional version 2.0. Normality and homogeneity of variance were tested. Comparisons were made using the Tukey's test with p<0.05.
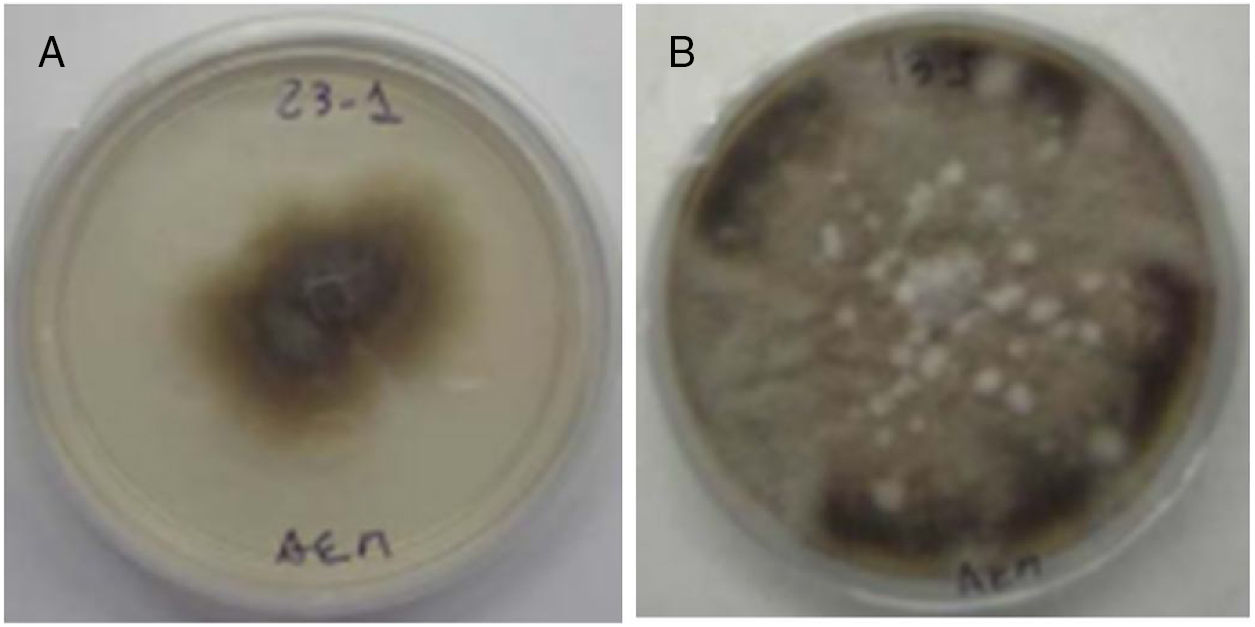
ResultsThe physicochemical properties of the soil (Typic Argiudoll) were: organic carbon 1.7%, available phosphorous 15.0mg/kg (Kurtz and Bray No. 1 method), pH 6.2, electrical conductivity 0.5d/Sm clay 27.5%, silt 50.5% and sand 22%. Figure 1 shows the morphology of the DSE colony of A. alternata and Cochliobolus sp.
The strain Cochliobolus sp. reached a diameter of 75mm of the colony after 12 days of growth while A. alternata completed the culture dish (90mm) after 9 days (data not shown). There was significant change in the kinetics of growth of the strains by adding the different kinds of agrochemicals to the medium. The results from the two-way analysis of variance showed that there were significant interactions between the DSE strains and the agrochemicals in growth rate (p<0.0001). Therefore, this interaction was further analyzed. Table 1 shows that the growth of A. alternata was significantly faster than that of Cochliobolus sp. in the control treatment and was less affected than Cochliobolus sp. when the agrochemicals were added.
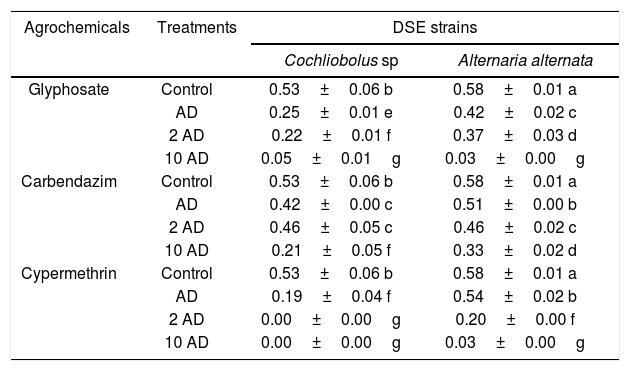
Growth rate of Cochliobolus sp. and Alternariaalternata (DSE strains). Comparison between DSE strains A. alternata and Cochliobolus sp. and agrochemical type
| Agrochemicals | Treatments | DSE strains | |
|---|---|---|---|
| Cochliobolus sp | Alternaria alternata | ||
| Glyphosate | Control | 0.53±0.06 b | 0.58±0.01 a |
| AD | 0.25±0.01 e | 0.42±0.02 c | |
| 2 AD | 0.22±0.01 f | 0.37±0.03 d | |
| 10 AD | 0.05±0.01g | 0.03±0.00g | |
| Carbendazim | Control | 0.53±0.06 b | 0.58±0.01 a |
| AD | 0.42±0.00 c | 0.51±0.00 b | |
| 2 AD | 0.46±0.05 c | 0.46±0.02 c | |
| 10 AD | 0.21±0.05 f | 0.33±0.02 d | |
| Cypermethrin | Control | 0.53±0.06 b | 0.58±0.01 a |
| AD | 0.19±0.04 f | 0.54±0.02 b | |
| 2 AD | 0.00±0.00g | 0.20±0.00 f | |
| 10 AD | 0.00±0.00g | 0.03±0.00g | |
4 levels of agrochemicals: 0; AD (agronomic dose); 2 AD; 10 AD. Means±SD (n=5). Different letters differ significantly (p<0.05).
The presence of glyphosate reduced the growth rate of both DSE strains. A. alternata showed significant differences in AD and 2 AD regarding Cochliobolus sp.; however, when the dose was increased to 10 AD, no differences were found between both strains.
The fungicide carbendazim was the most tolerated agrochemical by the DSE strains. The growth rate of A. alternata was reduced by the addition of carbendazim as its dose increased in the solid media. No differences were found in 2 AD between both fungi (Table 1). On the other hand, the insecticide (cypermethrin) was the most toxic agrochemical on growth rate. A. alternata reduced its growth rate as the dose of cypermethrin increased in the media, while a dose equal to or greater than 2 AD inhibited the growth of Cochliobolus sp. (Table 1).
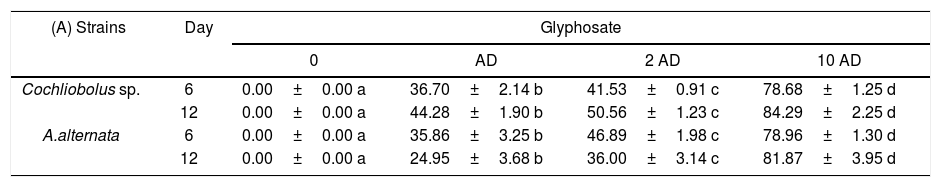
The percentages of growth inhibition are shown in Table 2A–C. The herbicide (Table 2A) increased this parameter (p<0.05) in all the agrochemicals and doses tested for both fungi and on both days evaluated (day 6 and day 12). The fungicide increased the growth inhibition percentage of Cochliobolus sp.; however, an induction of the growth of A.alternata was observed when the fungicide was added at AD and 2 AD evaluated at 6 days. In these doses, at the end of the growth (day 12) no significant differences were detected with respect to the control treatment. For both evaluation days, the addition of carbendazim at 10 AD generated a significant increase (p<0.05) in the inhibition percentage (Table 2B).
Inhibition of growth rate of Cochliobolus sp. and Alternaria sp. (DSE strains) growing at different concentrations of: (A) glyphosate, (B) carbendazim and (C) cypermethrin
| (A) Strains | Day | Glyphosate | |||
|---|---|---|---|---|---|
| 0 | AD | 2 AD | 10 AD | ||
| Cochliobolus sp. | 6 | 0.00±0.00 a | 36.70±2.14 b | 41.53±0.91 c | 78.68±1.25 d |
| 12 | 0.00±0.00 a | 44.28±1.90 b | 50.56±1.23 c | 84.29±2.25 d | |
| A.alternata | 6 | 0.00±0.00 a | 35.86±3.25 b | 46.89±1.98 c | 78.96±1.30 d |
| 12 | 0.00±0.00 a | 24.95±3.68 b | 36.00±3.14 c | 81.87±3.95 d | |
| (B) Strains | Day | Carbendazim | |||
|---|---|---|---|---|---|
| 0 | AD | 2 AD | 10 AD | ||
| Cochliobolus sp. | 6 | 0.00±0.00 a | 6.37±3.42 b | 10.76±3.59 b | 35.16±4.32 c |
| 12 | 0.00±0.00 a | 11.70±1.21 b | 12.37±4.28 b | 49.67±2.06 c | |
| A.alternata | 6 | 0.00±0.00 b | −4.31±1.05 a | −2.82±1.96 a | 36.03±2.14 c |
| 12 | 0.00±0.00 a | 0.00±0.00 a | 0.00±0.00 a | 47.62±2.18 b | |
| (C) Strains | Day | Cypermethrin | |||
|---|---|---|---|---|---|
| 0 | AD | 2 AD | 10 AD | ||
| Cochliobolus sp. | 6 | 0.00±0.00 a | 59.12±3.24 b | 84.61±0.00 c | 84.61±0.00 c |
| 12 | 0.00±0.00 a | 59.87±4.48 b | 90.60±0.00 c | 90.60±0.00 c | |
| A. alternata | 6 | 0.00±0.00 a | 26.03±0.94 b | 64.24±2.79 c | 79.82±0.77 d |
| 12 | 0.00±0.00 a | 6.50±3.73 b | 62.12±2.74 c | 83.12±0.00 d | |
Each value represents the mean value±SD (n=5). Different letters differ significantly (p<0.05).
Table 2C shows the percentages of growth inhibition generated by the insecticide. The DSE Cochliobolus sp. exhibited significant inhibition when the AD was placed in the culture medium (p<0.05); however, no changes were detected between 2 AD and 10 AD. On the contrary, A. alternata recorded increases in inhibition in all the doses studied (p<0.05).
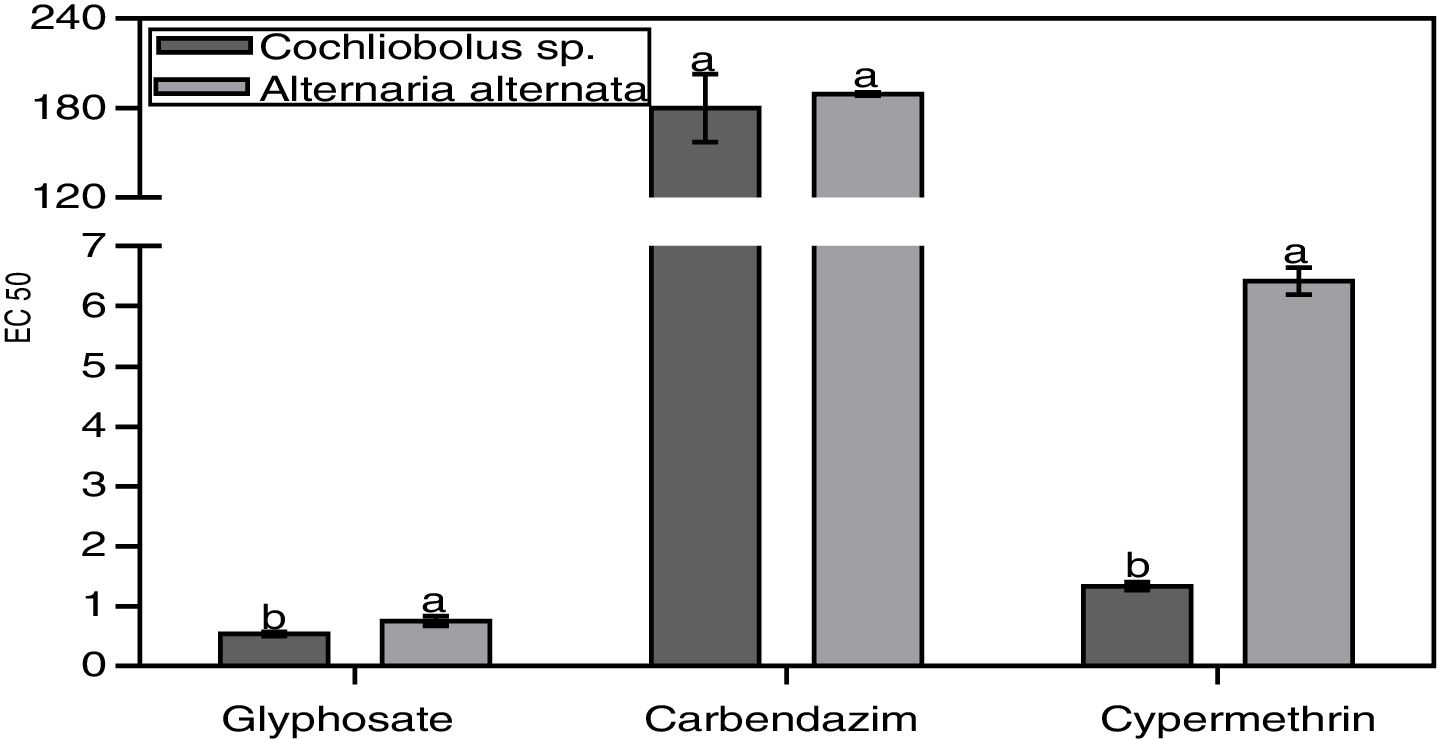
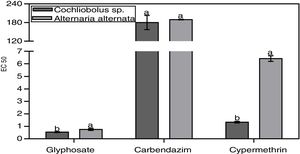
The concentrations of the agrochemicals that caused a 50% reduction in fungal growth compared to the control (effective concentration: EC 50) varied depending on the agrochemical type (Fig. 2). In all the agrochemicals analyzed, the fungus A. alternata was the most tolerant, presenting higher EC 50. The insecticide was the agrochemical that caused the greatest effect on DSE growth, followed by the herbicide. The fungicide proved to be the least toxic agrochemical for these fungi (Fig. 2). Cochliobolus sp. did not differ significantly from A. alternata in the EC 50 against carbendazim.
DiscussionThis study evaluates the tolerance of two DSE fungi to different agrochemicals. The tested DSE fungi were isolates of A.alternata and Cochliobolus sp. It is known that these fungi are grass pathogens; however, several studies have shown that they can also be found in the roots as DSE as non-pathogenic fungi3,37. Therefore, the fungal species classified as non-pathogenic cause disease symptoms in certain host plants but are neutral or beneficial in other hosts.
Moreover, although pesticides are designed to be as specific as possible, they have effects on non-target fungal organisms. In accordance with our results, glyphosate (using equivalent herbicide concentrations) had an in vitro toxic effect on culturable mycobiota (Aspergillus section Flavi) strains from agricultural soils6. Likewise, other authors12 found a reduction of microbial biomass at a higher glyphosate concentration and a temporary inhibitory effect at recommended field doses. Furthermore, effects of glyphosate on soil microbial communities and its mineralization in soils after various applications of this herbicide have been found16. A recent study11 also demonstrated behavioral differences between glyphosate and its major metabolite, AMPA, related to the physical properties of saturated hydraulic conductivity (Ks) and soil moisture. Other authors found that glyphosate affects arbuscular mycorrhizal fungi due to a reduction in spore viability, root colonization and arbuscule percentage9. However, DSEs are more tolerant to different types of stress than other soil fungi23. This could be due to the fact that the chemical compounds act effectively on the mycelium, while spores and chlamydospores are more resistant to these compounds due to their low activity given their water content and the presence of a large amount of unsaturated fatty acids4.
There is much less research on the effect of carbendazim on fungi. However, this fungicide has detrimental effects to fungal and bacterial biocontrol agents such as Trichoderma harzianum, Trichoderma virens, Bacillus subtilis and Pseudomonas fluorescens20. In this research, we found that DSE strains have the potential to tolerate high concentrations (181.25ppm, 10 AD) of fungicide; these results are in accordance with those of others authors2. One study showed that the type of fungicide applied to the soil must be seriously considered as the authors detected negative effects on Rhizophagus fasciculatus (arbuscular mycorrhizal fungi) with benomyl (systemic benzimidazole); however, they found no effects when captan (non-systemic fungicide)7 was applied. Nevertheless, our results showed high tolerance of DSE fungi against carbendazim, which is a derivative of benomyl. Bipolaris tetramera and A. alternata have potential to tolerate and degrade 100ppm of carbendazim in vitro38. In accordance with that article, we found negative inhibition values in agronomic doses, 2 AD and no differences compared with control. These results would indicate an Alternaria sp. promotion growth when grown with the addition of carbendazim.
In the case of cypermethrin, there are studies that demonstrate that this insecticide caused a negative effect on soil enzymatic activities and microbial diversity34. Our DSE strains showed low cypermethrin tolerance, high inhibition percentage and low EC50. Nevertheless, a study revealed that when the pesticide is applied on pepper plants there is an increase in bacterial abundance and a change in the composition of the community of the Firmicutes phylum to Bacteroidetes and γ-Proteobacteria phyla39. Other authors showed the potential of Aspergillus niger, Aspergillus terreus, Monilochaetes and Fusarium strains in the biodegradation of 50–150ppm of this pyrethroid25.
This research has shown that agrochemicals commonly used in agriculture have detrimental effects on the growth of DSE fungi, even when these agrochemicals are used at recommended doses; however, these negative effects vary according to the agrochemical considered. Moreover, this paper makes evident that DSE response to this type of toxin depends on the studied species of DSE fungus. This research is a contribution to a better understanding of the behavior of DSE against xenobiotics, evaluating the tolerance and/or their use as a source of nutrients.
ConclusionThe DSE A. alternata tolerates all three agrochemicals at the tested doses while Cochliobolus sp. is glyphosate and carbendazim-tolerant in all three doses but shows no tolerance to cypermethrin when the agronomic dose is twofold. In general, there is a decrease in the growth of the strains when increasing the dose of the agrochemicals studied.
Conflict of interestThe authors declare that they have no conflicts of interest.
The present research was financially supported by the Agencia Nacional de Promoción Científica y Tecnológica, Project PICT 2016:1136 and the Universidad de Buenos Aires, UBACYT 068/2011, Facultad de Agronomía, Argentina. We thank Ing. Raul Silvio Lavado the critical review of this manuscript.