Thermoacidophiles can exist in a state of dormancy both in moderate temperatures and even in cold conditions in heap leaching. Sulphide mineral ores such as chalcopyrite produce sulfuric acid when exposed to the air and water. The produced sulfuric acid leads to the decrease of pH and exothermic reactions in heap leaching causing the temperature to increase up to 55°C and the activation of thermoacidophilic microorganisms. The aim of the present study was to isolate indigenous extreme thermoacidophilic microorganisms at ambient temperature from Sarcheshmeh Copper Complex, to adapt them to the high pulp density of a chalcopyrite concentrate, and to determine their efficiency in chalcopyrite bioleaching in order to recover copper. In this study samples were collected at ambient temperature from Sarcheshmeh Copper Complex in Iran. Mixed samples were inoculated into the culture medium for enrichment of the microorganisms. Pure cultures from these enrichments were obtained by subculture of liquid culture to solid media. Morphological observation was performed under the scanning electron microscope. Isolates were adapted to 30% (w/v) pulp density. For the bioleaching test, the experiments were designed with DX7 software. Bioleaching experiments were carried out in Erlenmeyer flasks and a stirred tank reactor. The highest copper recovery in Erlenmeyer flasks was 39.46% with pulp 15%, inoculums 20%, size particle 90μm and 160rpm. The lowest recovery was 3.81% with pulp 20%, inoculums 20%, size particle 40μm and 140rpm after 28 days. In the reactor, copper recovery was 32.38%. Bioleaching residues were analyzed by the X-ray diffraction (XRD) method. The results showed no jarosite (KFe3(SO4)2(OH)6) had formed in the bioleaching experiments. It seems that the antagonistic reactions among various species and a great number of planktonic cells in Erlenmeyer flasks and the stirred tank reactor are the reasons for the low recovery of copper in our study.
Los microorganismos termoacidófilos pueden estar en estado latente tanto a temperatura moderada como baja, en lixiviación en pilas. Los minerales sulfurosos, como la calcopirita, producen ácido sulfúrico cuando se exponen al aire y al agua. El ácido sulfúrico producido conduce a la disminución del pH y a reacciones exotérmicas durante la lixiviación en pilas, lo que hace que la temperatura aumente hasta 55°C y se activen los microorganismos termoacidófilos. El objetivo del presente estudio fue aislar del complejo de cobre Sarcheshmeh (Irán) microorganismos termoacidófilos extremos que proliferan a temperatura ambiente e investigar su adaptación a la alta densidad de pulpa del concentrado de calcopirita, así como su eficiencia para biolixiviarese mineral, con el objeto de recuperar el cobre. Se recogieron muestras a temperatura ambiente del citado complejo, y luego muestras mixtas se inocularon en un medio de cultivo de enriquecimiento. A partir de estos enriquecimientos, mediante el subcultivo del cultivo líquido a medio sólido, se obtuvieron cultivos puros. La observación morfológica se realizó bajo microscopio electrónico de barrido. Los aislados estaban adaptados al 30% p/v de densidad de pulpa. Para la prueba de biolixiviación, los experimentos fueron diseñados con el software DX7. Los experimentos de biolixiviación se llevaron a cabo en Erlenmeyers y en un reactor tanque con agitación. La mayor recuperación de cobre en los Erlenmeyers fue del 39,46% y se obtuvo con la pulpa al 15%, un inóculo del 20%, un tamaño de partícula de 90μm y una agitación de 160rpm. La menor recuperación fue del 3,81% y se obtuvo con la pulpa al 20%, un inóculo del 20%, un tamaño de partícula de 40μm y una agitación de 140rpm, después 28 días. En el reactor, la recuperación del cobre fue del 32,38%. El análisis de difracción de rayos X (XRD) no mostró que se formara jarosita (KFe3[SO4]2[OH]6) en los experimentos de biolixiviación. Dicha técnica sirve para determinar la estructura cristalina de una sustancia desconocida. Al parecer, las reacciones antagónicas entre las diversas especies y el mayor número de células planctónicas en los Erlenmeyers y en el reactor fueron las causas de la baja recuperación de cobre observada en este estudio.
Chalcopyrite (CuFeS2), is the main copper-containing sulfide mineral and the most persistent of the copper sulfides in the lithosphere8,13,17,18,35,37,38,40. Moreover, it is economically the most important resource of copper19,38. In the pyrometallurgical processes used for chalcopyrite, more than 90% copper recovery can be achieved; however, serious pollution to the environment is always produced such as sulfur dioxide and arsenic trioxide2,37. The use of microorganisms to extract copper from chalcopyrite has several advantages such as easy operation, obvious economic benefits, being more environmentally friendly than pyrometallurgical processes8,10,41. At first, it was believed that the most important microorganisms involved in the leaching of chalcopyrite were the mesophilic acidophiles9,25. However, they have been less successful in oxidizing chalcopyrite mostly due to slow kinetics, poor extraction, long leaching time and passivation of the mineral by ferric precipitates and/or sulfur. For this reason, their industrial use is still a major challenge in the field of biohydrometallurgy12,14,38. In order to overcome these problems, various methods proposed by researchers include the control of redox levels via the addition of catalysts such as silver and bismuth, ultra-fine grinding of the concentrate prior to bioleaching and the addition of chloride1,4,12,13. It seems that thermophilic microbes are much more efficient in increasing the rate of chemical ferric oxidation of chalcopyrite and other minerals2. Therefore, another way to overcome this problem is to use thermophilic microorganism strains instead of mesophilic microorganisms2,14,24,40. This approach has garnered much attention in recent years and has been assessed greatly in batch tests at laboratory scale13.
In recent years, new species of thermophilic microorganisms with greater capacity for solution of metal sulfides and more resistance to different toxic metal cations have been isolated3,14,26. However, all thermoacidophilic microorganisms do not confer the same degree of effectiveness, and vary from one species to another2. Therefore, it is imperative to find new species capable of giving high dissolution percentages and tolerating higher pulp density. Although several researchers have reported that pulp densities higher than 15–20% (w/v) are injurious to cell viability and oxidizing activity, both work with mesophiles and thermophiles2. Moreover, chalcopyrite concentrates produced from distinctive ores may contain different concentrations of contaminants such as metals and organic chemical materials. Mostly, the presence of various concentrations of metals and chemical materials is highly undesirable for bioleaching and may prevent the growth of standard microorganisms17. Autochthonous microbes are not only more resistant than metals and chemical materials, but can also multiply for several generations within the copper sulfide ore and may effectively increase the extraction of copper during bioleaching. In addition, the isolation of such microbes would eliminate the necessity of using standard laboratory cultures, specifically for large-scale studies in industrial processing of mineral sulfide ores or concentrates18,24.
The aim of this study was to isolate indigenous extremely thermoacidophilic microorganisms at ambient temperature from Sarcheshmeh Copper Complex in Iran, determine their efficiency in the bioleaching of chalcopyrite for the recovery of copper and answer the key question of whether the above mentioned microorganisms can tolerate higher pulp density than other extreme thermophilic microorganisms or not.
Material and methodsSite description and samplesSamples were collected at the Sarcheshmeh Copper Complex. The mine is located 160km southwest of Kerman city in Iran and has been continuously exploited for more than 40 years. Sample collection was carried out in January 2015. Ambient temperature was about 6°C. Eight samples were collected from mine soil at the surface layer (0–10cm). Pregnant leach solution (PLS) samples were taken from the pond located under heap number one. Five samples were also taken from running waters around the mine. All samples were collected in several plastic bags and carried to the laboratory with no temperature control within a day. All samples were mixed in one bucket. pH value was 2.4 and was measured by analyzer (Metrohm model 827).
Culture mediumMixed samples were inoculated into the culture medium for enrichment and cultured in autoclaved Acidianus brierleyi medium [DSMZ (http://dsmz.de/media/media150.htm)]. Experiments were performed in 500ml Erlenmeyer flasks. Each flask contained 150ml culture medium and 50ml mixed samples.
Initial pH was adjusted to 1.98 with Merck sulfuric acid. Cultures were run in a thermostatic rotary agitator (Innova, New Brunswick Scientific Innova 4200 incubator shaker) at 70°C and 125rpm. Water loss by evaporation was compensated by the daily addition of distilled water. ORP (oxidation–reduction potential=Eh) using Eh meter (WTW model 325, Electrode SenTix ORP 0–100/3mol/l KCl) and pH value was monitored in successive intervals.
Isolation of microorganismThe mixed culture was subcultured based on growth rate at 7–18 day intervals in the new medium with the above condition in an Erlenmeyer flask. Cell density and morphology of the microorganism in the solution were analyzed according to the methods described by Keeling et al.18 and Zeng et al.37 The pure culture was obtained after four successive subcultures in one month under aerobic condition. The redox potential and pH of each culture were monitored and adjusted as described above.
Growth on solid mediaTo ensure that a pure culture was achieved, pure cultures from these enrichments were obtained by subculture of 0.1ml liquid culture to solid media solidified with 0.6% Gelrite in aerobic condition. The solid medium was prepared with the above composition; only sulfur was replaced by sodium thiosulfate.
Morphological observationThe morphology of the isolated culture grown on Gelrite was observed under a Scanning Electron Microscope PHENOM Pro X (Max Magnification: 45000×, Resolution: 25nm, Detectors: BSD, SED and SDD, working voltage: 5kV, 10kV and 15kV).
Concentrate preparation and chemical analysisA representative sample of chalcopyrite concentrate was obtained from the Sarcheshmeh Copper Complex of Iran. Sampling took two months. The samples were placed in the laboratory on a sheet to dry. The dried samples were rolled and sifted successively until a sample for testing could be obtained. The d90 of the sample achieved 90μm using a cyclosizer. This concentrate was then pulverized to a particle size of d90=40μm. The chemical analysis of the concentrate was performed using X-ray fluorescence (XRF) and X-ray diffraction (XRD) methods.
Adaptation of microbes to chalcopyrite prior to leaching testsThe adaptation of isolates to the chalcopyrite concentrate was carried out according to the procedures described by Astudillo and Acevedo2. Fifteen transfers were done aerobically at 7 day-intervals, at 70°C for about four months onto the abovementioned medium in an orbital incubator shaker agitated at 125–175rpm. Pulp densities were 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22, 24, 26, 28 and 30% (w/v). This adapted population was used as inoculum to new cultures of increasing pulp density.
Bioleaching experimentsExperiment designThe experiments were designed with DX7 software23. Four parameters, inoculum percentage, pulp density, agitation and size particle of concentrate, were considered in this investigation and consequently, 31 experiments were carried out using the following levels: inoculum percentage of bacteria (5, 10, 20%); pulp density (10, 15, 20%); agitation (120, 140, 160rpm); particle size (90, 40μm).
Shake flaskBioleaching experiments were carried out in autoclaved 500ml Erlenmeyer flasks. The total volume of each flask was 200ml containing medium, adapted culture containing about 1.0×107cells/ml and pulp density. Erlenmeyer flasks were placed in a rotary shaking incubator at 70°C. Control experiments were conducted without inoculum.
The evaporated water in the medium was supplemented with sterilized deionized water and culture medium. Evaporation losses were determined by weighing all flasks before and after sampling. The leaching experiments lasted for 28 days. The experiment was accomplished at 160, 120 and 140rpm respectively.
Stirred tank reactorThe best result obtained in the shake flask was tested in the stirred tank reactor for better recovery of copper. Bioleaching experiments were carried out in a 1l glass reactor at 70°C. Oxygen was introduced, also into the base of the reactor, at an approximate rate of 1l/min (Fig. 1).
AnalysesDuring the experiment, pH and ORP values were measured. Aliquots of the solution were sampled, and the concentration of Cu2+, Fe2+, total iron and acid in the solution was measured in successive intervals. Copper and total iron concentrations in the solution were determined by atomic absorption spectrophotometer; variant AA220. At the end of the bioleaching tests, solids were removed by filtration, dried and their chemical composition was analyzed by XRF and XRD. XRD was used for jarosite examination. The acid consumption was compensated by sulfuric acid to keep pH value around 2 in the early stages. All analyses were performed in the central laboratory of Sarcheshmeh Copper Complex.
StatisticsThe statistical analysis of the bioleaching and control experiments in the shake flask were performed using One-Sample t-test. p≤0.05 was accepted as statistically significant.
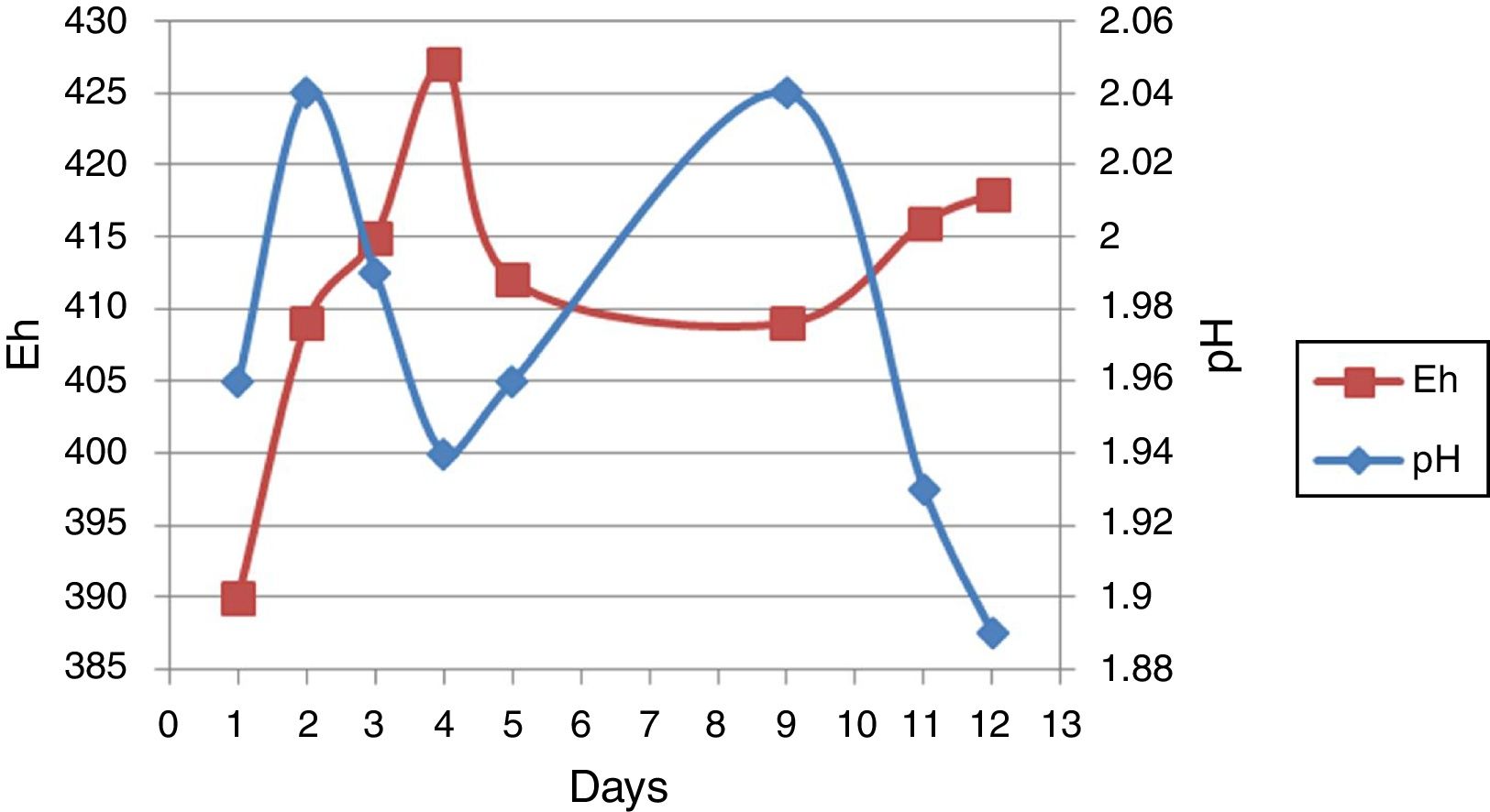
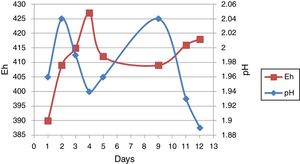
Result and discussionMeasure of pH and EhpH and Eh-related curve before bioleaching over 12 days from one of the sub-cultures was shown in Figure 2. pH increased from an initial value of 1.96–2.4 after 2 days of incubation, followed by a decrease up to the 5th day. The curve was followed by an increase and then a decrease. Eh increased from 390mV initially on the first day to 427mV after 4 days of incubation. This curve was followed by an increase and a decrease.
Growth on solid mediaAfter incubation at 70°C for about 30 days, very small colonies were observed. In order to prevent the plate from drying out, the plastic lacuna was used. Inoculated plates were inspected daily for microbial growth and water evaporation.
Growth in anaerobic and aerobic conditionsWhen the colonies grown on Gelrite were cultured in liquid media, growth was poor and the number of microbes did not increase over time. Our results showed that in addition to the compounds used to culture the microorganisms, mine soil should also be analyzed to provide trace elements for growth. If possible, trace elements should be identified to enhance microorganism growth, because the microorganisms grow very well as long as they are in the presence of their natural ingredients.
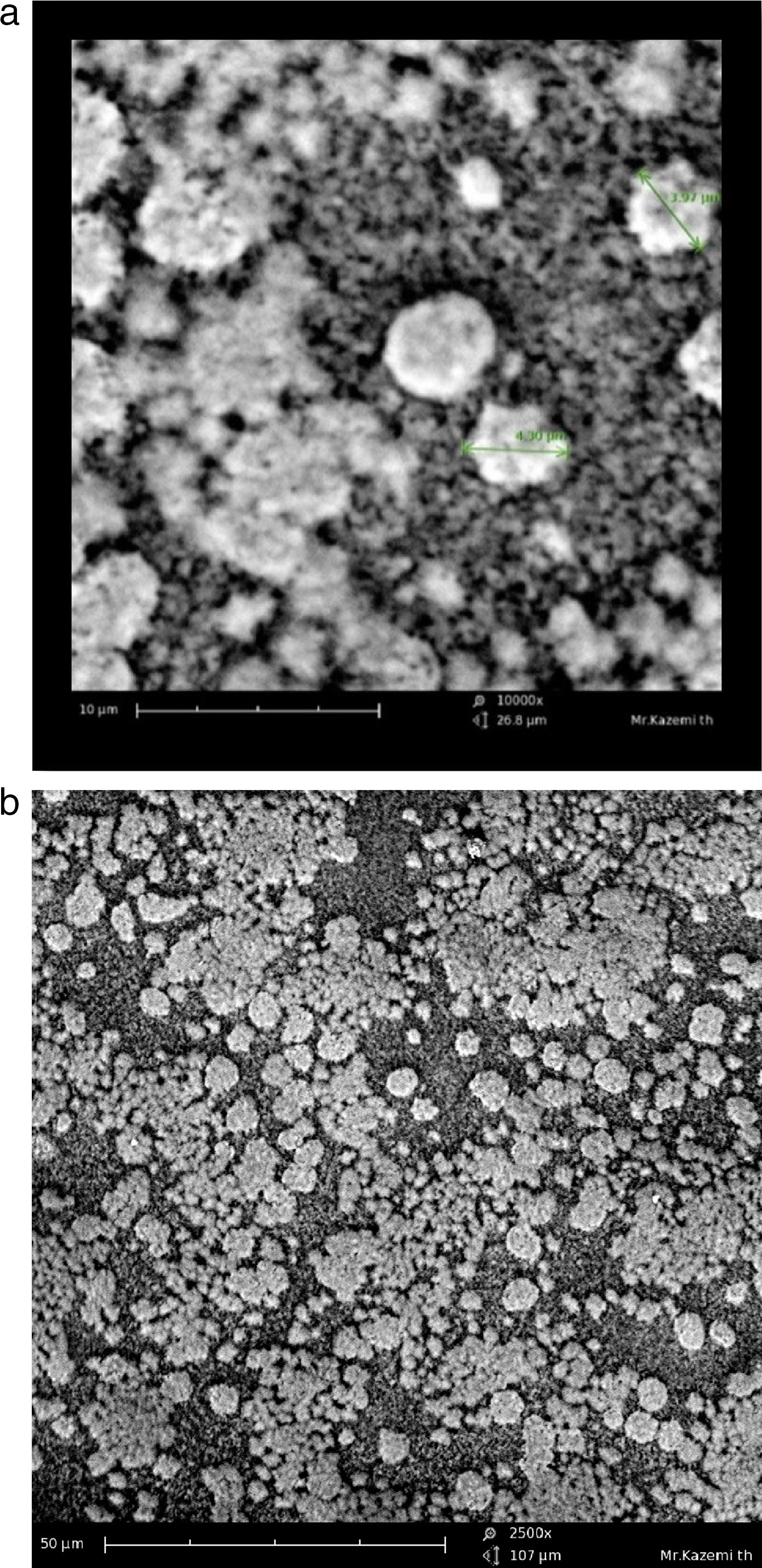
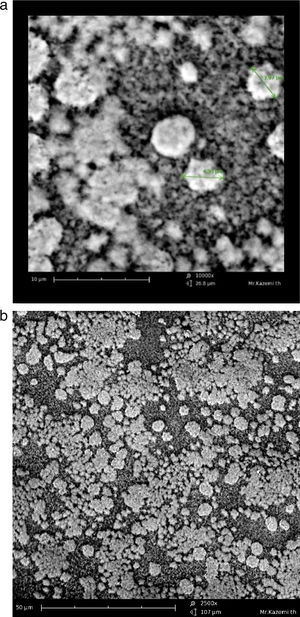
Morphological observationThe cell morphology in the scanning electron microscope was similar to Sulfolobus-like microorganisms, with lobed irregular cocci, variable shape and size (Fig. 3). The isolate was motile.
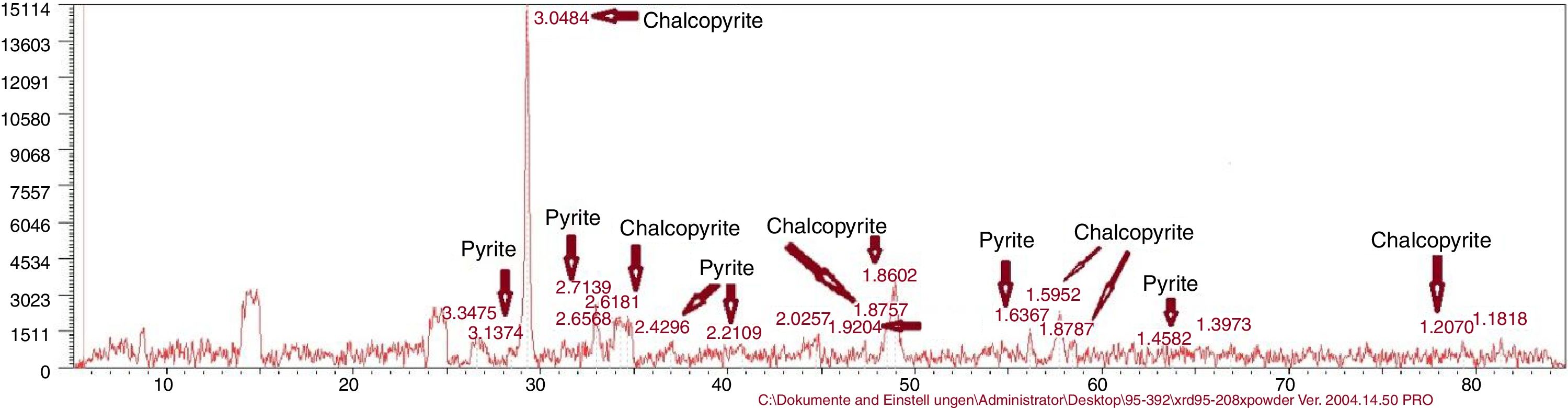
Chemical composition of chalcopyrite concentrateThe chemical composition of the concentrate with X-ray fluorescence (XRF) analysis is presented in Table 1. X-ray diffraction (XRD) analysis reported chalcopyrite 95.5% and pyrite 4.4%.
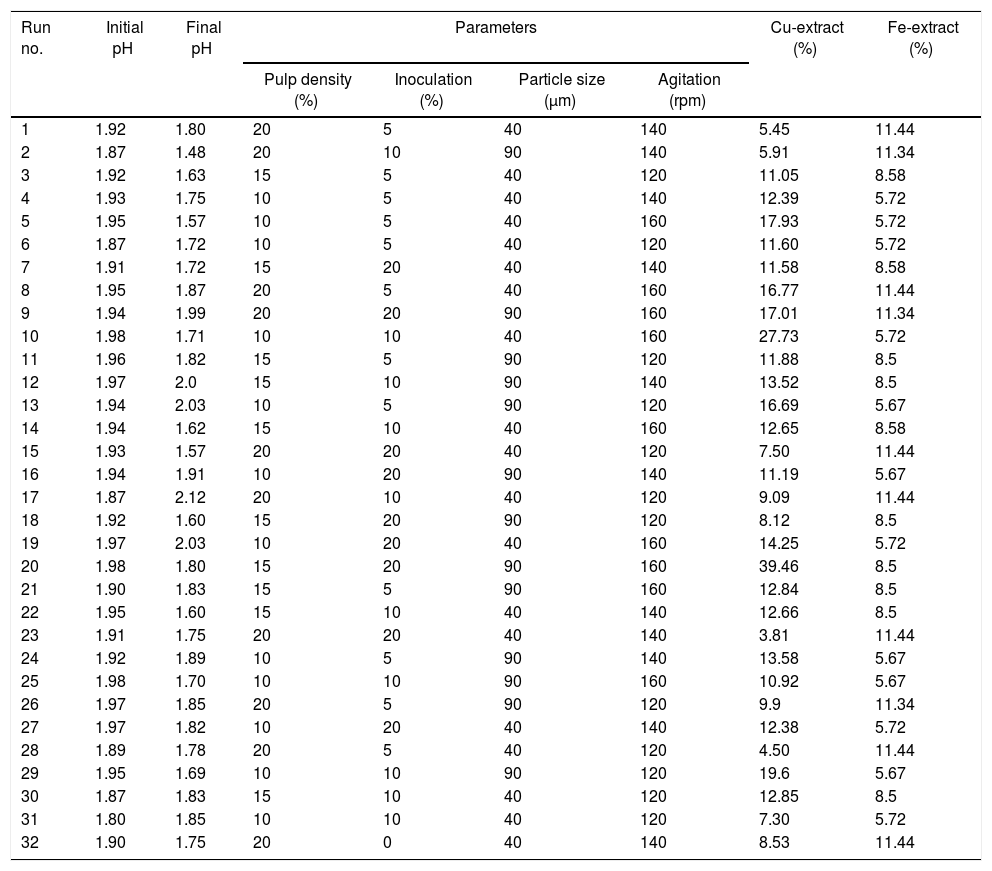
Bioleaching experiments and copper recovery in shake flaskAccording to the residue analysis, the result of the experiment design based on four selected control parameters and their application in the study are shown in Table 2. The results showed that different runs had different percentage recovery of copper after 28 days. As it is seen, the highest recovery is run 20 with pulp 15%, inoculums 20%, particle size 90μm and 160rpm with 39.46% recovery and the lowest recovery is run 23 with pulp 20%, inoculums 20%, particle size 40μm and 140rpm with 3.81% recovery. Run 32 is chemical leaching (control).
Design of experiments based on four selected control parameters
| Run no. | Initial pH | Final pH | Parameters | Cu-extract (%) | Fe-extract (%) | |||
|---|---|---|---|---|---|---|---|---|
| Pulp density (%) | Inoculation (%) | Particle size (μm) | Agitation (rpm) | |||||
| 1 | 1.92 | 1.80 | 20 | 5 | 40 | 140 | 5.45 | 11.44 |
| 2 | 1.87 | 1.48 | 20 | 10 | 90 | 140 | 5.91 | 11.34 |
| 3 | 1.92 | 1.63 | 15 | 5 | 40 | 120 | 11.05 | 8.58 |
| 4 | 1.93 | 1.75 | 10 | 5 | 40 | 140 | 12.39 | 5.72 |
| 5 | 1.95 | 1.57 | 10 | 5 | 40 | 160 | 17.93 | 5.72 |
| 6 | 1.87 | 1.72 | 10 | 5 | 40 | 120 | 11.60 | 5.72 |
| 7 | 1.91 | 1.72 | 15 | 20 | 40 | 140 | 11.58 | 8.58 |
| 8 | 1.95 | 1.87 | 20 | 5 | 40 | 160 | 16.77 | 11.44 |
| 9 | 1.94 | 1.99 | 20 | 20 | 90 | 160 | 17.01 | 11.34 |
| 10 | 1.98 | 1.71 | 10 | 10 | 40 | 160 | 27.73 | 5.72 |
| 11 | 1.96 | 1.82 | 15 | 5 | 90 | 120 | 11.88 | 8.5 |
| 12 | 1.97 | 2.0 | 15 | 10 | 90 | 140 | 13.52 | 8.5 |
| 13 | 1.94 | 2.03 | 10 | 5 | 90 | 120 | 16.69 | 5.67 |
| 14 | 1.94 | 1.62 | 15 | 10 | 40 | 160 | 12.65 | 8.58 |
| 15 | 1.93 | 1.57 | 20 | 20 | 40 | 120 | 7.50 | 11.44 |
| 16 | 1.94 | 1.91 | 10 | 20 | 90 | 140 | 11.19 | 5.67 |
| 17 | 1.87 | 2.12 | 20 | 10 | 40 | 120 | 9.09 | 11.44 |
| 18 | 1.92 | 1.60 | 15 | 20 | 90 | 120 | 8.12 | 8.5 |
| 19 | 1.97 | 2.03 | 10 | 20 | 40 | 160 | 14.25 | 5.72 |
| 20 | 1.98 | 1.80 | 15 | 20 | 90 | 160 | 39.46 | 8.5 |
| 21 | 1.90 | 1.83 | 15 | 5 | 90 | 160 | 12.84 | 8.5 |
| 22 | 1.95 | 1.60 | 15 | 10 | 40 | 140 | 12.66 | 8.5 |
| 23 | 1.91 | 1.75 | 20 | 20 | 40 | 140 | 3.81 | 11.44 |
| 24 | 1.92 | 1.89 | 10 | 5 | 90 | 140 | 13.58 | 5.67 |
| 25 | 1.98 | 1.70 | 10 | 10 | 90 | 160 | 10.92 | 5.67 |
| 26 | 1.97 | 1.85 | 20 | 5 | 90 | 120 | 9.9 | 11.34 |
| 27 | 1.97 | 1.82 | 10 | 20 | 40 | 140 | 12.38 | 5.72 |
| 28 | 1.89 | 1.78 | 20 | 5 | 40 | 120 | 4.50 | 11.44 |
| 29 | 1.95 | 1.69 | 10 | 10 | 90 | 120 | 19.6 | 5.67 |
| 30 | 1.87 | 1.83 | 15 | 10 | 40 | 120 | 12.85 | 8.5 |
| 31 | 1.80 | 1.85 | 10 | 10 | 40 | 120 | 7.30 | 5.72 |
| 32 | 1.90 | 1.75 | 20 | 0 | 40 | 140 | 8.53 | 11.44 |
The results of the statistical analysis in relation to the extracted copper in different processes of bioleaching test (31 stage) relative to the control stage (chemical leaching) showed that the mean extracted copper in bioleaching test were 12.83. The results of One-Sample t-test showed that there is a significant difference between bioleaching and chemical leaching (p=0.001).
The aim of bioleaching processes is the biodegradation of ore or concentrate using organisms which do so effectively. Those microorganisms which can degrade ores more effectively are the ones with the fastest growth and the fastest doubling time. Therefore, there is a strong positive natural selection for this type of microorganisms, which would become the microbial dominant community in bioleaching environment while the others would be eliminated or washed away. In this study, the chalcopyrite concentrate did not sterilize so that the microbial community could contribute to the bioleaching like the mineral processes. Some studies like the present study have shown at laboratory scale that extreme thermoacidophilic culturing contains Sulfolobus-like microorganisms and can be successfully effective in chalcopyrite concentrate bioleaching6,7,28. In this study, the mixed microorganisms were confirmed by microscopic (Fig. 3) and metagenomic (results not shown) techniques.
Most of the work on thermophilic microorganisms has been confined to rather low pulp densities of less than 5%18,21,30. Similar to Guo and Rubio, in this work, thermophilic microorganisms can be adapted to 30% pulp density15,29. In some papers it was mentioned that the cell wall of extreme thermoacidophiles is not as rigid as that for the mesophilic species, therefore they are demolished when exposed to disturbance at high pulp densities30,36. In another article, the cell wall structure of thermophilic microorganisms was reported to lack peptidoglycan and cell membranes, which are much more flexible than those of mesophilic microorganisms. This means that this type of microorganism can only endure low pulp densities, which is one of the principal problems for its industrial use14,34. These statements are not correct. Firstly, because all cells have cell membrane and secondly, because these very specialized membrane lipids present in thermoacidophiles are critical for survival in extremes of both pH and temperature16,26.
Some researchers propose that very tiny concentrates may not be necessary to obtain acceptable copper extraction rates and levels13,32,33. Similar to these statements, we also take the best result in 90μm particle size in run 20 and 10. Conversely, some researchers believe that the thin particle size of the used ore assists growth, by preparing better accessibility for the microorganisms to the mineral sulfides27.
It seems that the attachment of the microorganisms to the surface of the chalcopyrite particles is more important than particle size, because, the highest copper dissolution is achieved when the inoculated microorganisms are able to access the chalcopyrite surface and adhere to it via extracellular polymeric substances (EPS)11,36,38. Similar to Plumb et al., in our result, the greatest number of cells that were observed in each of the flask cultures were planktonic cells27. This issue is one of the reasons we did not achieve greater recovery. Similar to Zeng et al., we recommend, the attached microorganisms on the chacopyrite surface be detected by atomic force microscope (AFM)39.
Our result shows that the highest recovery was at 160rpm and the lowest was at 140rpm. By increasing agitation, recovery also increased and the reason why the 140rpm had the lowest recovery was due to the successive subculturing of isolates from 160 and 120rpm. The successive subculturing decreased its ability to bioleach over time. Hence, it is recommended that at each stage of bioleaching previous adapted microbes that are well grown should be used18. In this survey, the number of microorganisms was high during 28 days with initial inoculums between 2.4×107 and 4.8×107cells/ml. Keeling et al. kept their microorganisms alive, without additional nutrients or growth supplements for over 90 days18. The microorganisms used in this study were also able to remain alive for more than 28 days; however, if bioleaching was too long, there would be no benefit for industrial recovery.
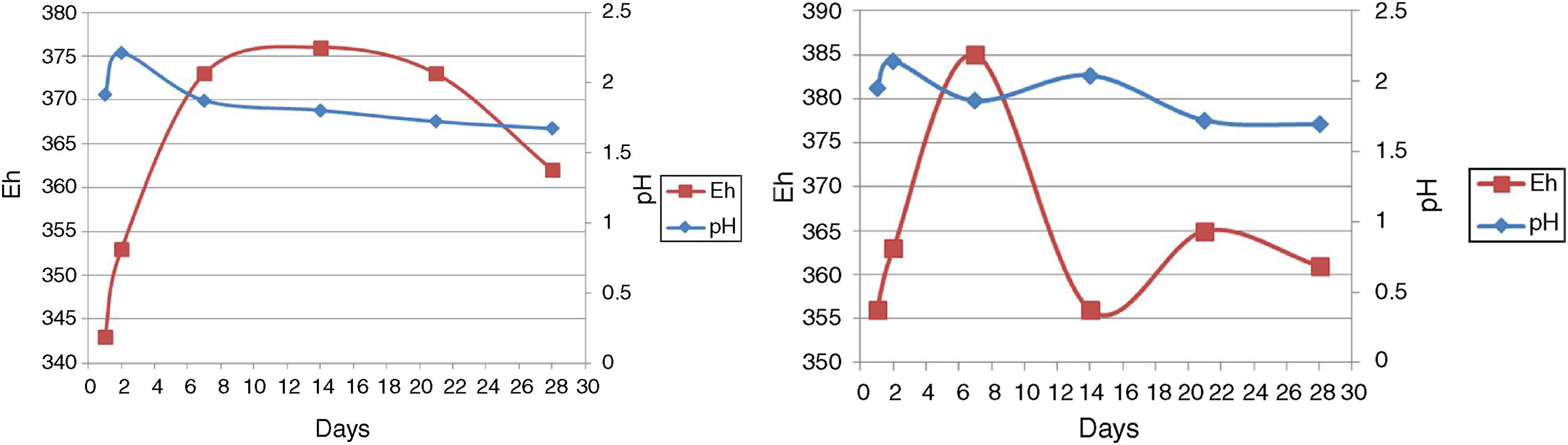
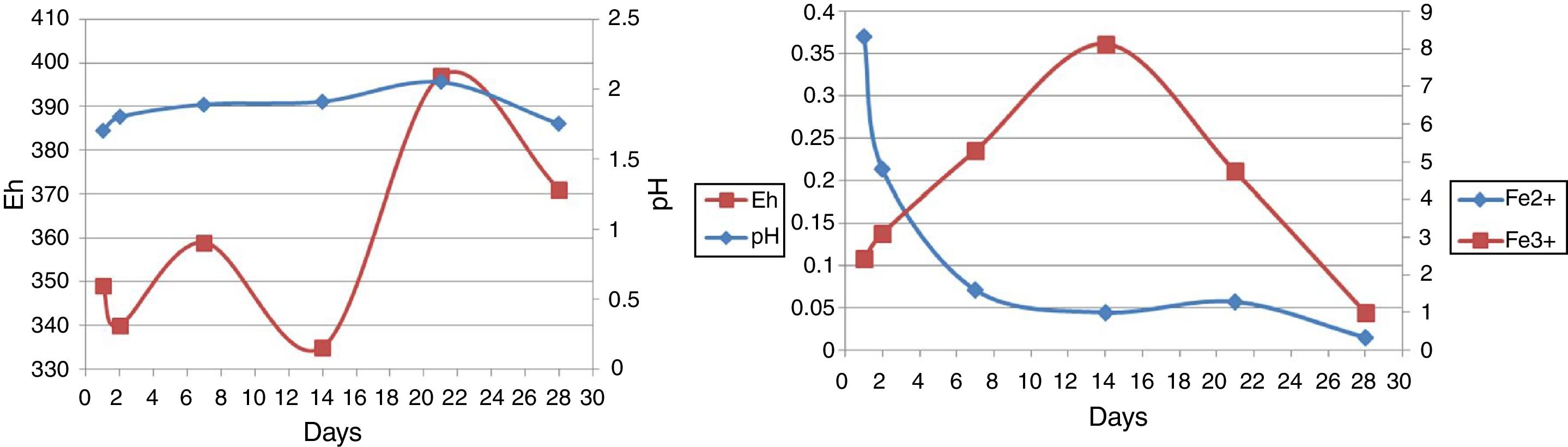
pH and EhAs seen in Figure 4, run 20 and run 23 both represent the increase and decrease in pH. However, there are more fluctuations in run 23, although run 23 at 14–28 days and run 20 at 2–28 days are associated with reduced pH. As a result of redox potential, run 23 is also associated with more fluctuations, as can be seen in Figure 4.
In the bioleaching experiments, all microorganisms utilized were acidophilic. Hence, maintaining the pH in the correct range was the most important factor for the extractive process to yield copper, iron and other elements22,31. Similar to the study of Zhou et al., an initial pH increase was seen in the early days, then the pH started to decrease, but it was not continuous41. Our result indicated the lowest copper recovery when pH was below 1.57 and above 2.12 (results not shown). Therefore, at a pH value lower than 1.57, a slight growth was observed and it was the end of the bioleaching process.
In run 20, the redox potential value showed an increase of 353–376mV from the beginning to the end of the bioleaching process. No correlation was seen between the decrease in the pH value and the increase in redox potential. Our result shows that the increase in redox potential does not reflect an increase in the concentration of Cu and Fe in solution, because the passivation of chalcopyrite occurs at elevated potentials4. Furthermore, similar to Sandstrom et al., the redox potential was relatively indifferent to changes in retention time20,30. Therefore, ORP value does not increase over time.
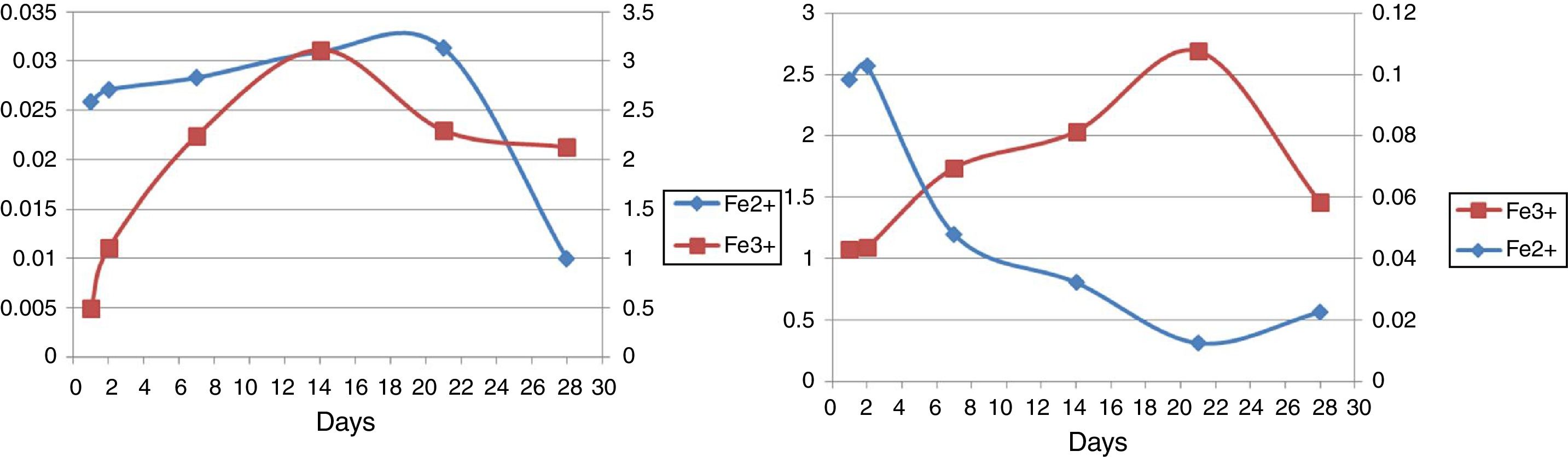
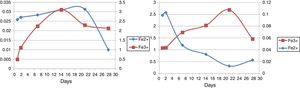
Ferrous and ferric concentrationsAs has been shown in Table 2, in run 20 and run 23 the total extracted iron is 8.5% and 11.44% respectively. Initial ferric iron concentrations were low in both runs. Ferric iron concentration in run 20 started to increase from the second day to 21 days later, but decreased from the 21st to 28th days. Moreover, in run 23, the ferric iron concentration increased in 14 days and decreased from the 21st to 28th day. Ferrous iron concentration in run 20 started to increase from the first day to the second day, but decreased from the 2nd to the 21st day. From the 21st to 28th days, the iron concentration increased slightly. In run 23, the ferrous iron concentration also increased from the beginning to day 21st and from the 21st to 28th days the amount significantly decreased (Fig. 5).
Chemical leaching (control)The pH of control increased gradually until the 21st day and then decreased to 28th days (Fig. 6, left). Changes in soluble ferric and ferrous iron concentration in leachates are shown in Figure 6, right. A mixture of thymol with methanol was added to the control flasks to prevent microbial growth; however, due to evaporation at high temperatures, chalcopyrite was sterilized in the oven at 150°C for 2h instead. In chemical leaching with the conversion of ferrous iron to ferric iron, from the 1st to the 14th day, the amount of ferrous iron decreased and the amount of ferric iron increased. From the 14th to 21st day due to the lowering of oxygen and accumulation of ferric iron, the converting process from ferrous iron to ferric iron decreases and results in the increasing of ferrous iron from the 14th to the 21st day. The decrease of ferrous iron and ferric iron from the 21st and 28th day shows the termination of leaching reactions. The high oxidation–reduction potential in chemical leaching relative to bioleaching is due to the higher production of ferric iron (8g/l) in chemical leaching. In chemical leaching the presence of ferric iron is necessary to trigger the leaching reactions as in bioleaching5.
Reactor leachingBioleaching of chalcopyrite concentrate by extreme thermoacidophiles was performed at 15% pulp density for 28 days in a stirred tank reactor (Run 20). pH value in the reactor was between 1.74 and 2.17 and Eh value was between 298 and 419. Copper recovery was 32.38% (result not shown). The result of the tested solutions cannot be trusted due to a device error, a sampling error due to water evaporation, hence no report was filed. If the tests are not done as soon as the specimens arrive at the laboratory, Fe2+ becomes Fe3+. Therefore, the residual results are more reliable. As has been shown in Table 3 and Figure 7, the XRD result showed no jarosite formed in the bioleaching experiments.
Based on the results obtained in this research, the following conclusions could be summarized:
It is not surprising that extreme thermoacidophilic microorganisms can be separated from the environments at ambient temperature. Hyperthermophilic enzymes are inactive in an acidic environment and low temperature and these microorganisms are inactive in these environments. Although mineral oxidation is an exothermic process, most of the bioleaching microorganisms grow and activate in a temperature higher than ambient temperature.
As the pH, temperature and aeration system are under control under laboratory condition, the shake flask environment and the reactor are highly homogeneous. However, the solution metals and metalloids increase and can have a significant effect on the variation and number of indigenous microbial species. It has been argued in some studies that the microbial population is completely archaea at a temperature above 70°C and consists of species of the order Sulfolobales. Sulfolobus and Acidianus are example of Sulfolobales. It has been observed in some studies that antagonistic reactions between Acidianus sp. (DSMZ 29038) and Sulfolobus metallicus inhibit bioleaching to some extent. The antagonistic effects have been observed during pre-colonization tests. Releasing some antagonistic compounds by S. metallicus has also been observed. It seems that quorum sensing signals and chemotactic responses also have a significant role in bioleaching. We use an unsterilized concentrate for bioleaching in these studies, because the sterilization of chalcopyrite concentrate is not cost effective in industry. It seems that the antagonistic relationship among various species of the order Sulfolobales is another reason for the low recovery of copper in our study.
The survival of the microorganism inside the ore depends on the expression of metal resistance genes. The horizontal transition of such genes in the environment has been well documented. Therefore, the adaptability of indigenous microorganisms with chalcopyrite concentrate in various percentages before bioleaching seems unnecessary. As bioleaching starts, metal concentration in leachate increases and species resistant to the high concentration of metals survive at the end of the bioleaching process. These microorganisms contain resistant and adaptable mechanisms toward metals such as active efflux or trapping of the metal ions by metal chaperones, gene duplications, the presence of genomic islands (GI), passive mechanisms for pH and cations homeostasis in acidic condition, as well as an inorganic polyphosphate (polyP)-driven metal resistance mechanism.
Authors’ contributionAll authors had equal role in design, work, statistical analysis, and manuscript writing.
Conflict of interestThe authors declare no conflict of interest.
This research was supported by the Sarcheshmeh Copper Complex of Iran. We would like to thank Mr. Ahmad Moguei nejad (Head of Biohydrometallurgy Laboratory).