In the present work, the biosynthesis of silver-nanoparticles (AgNP) was evaluated using the aqueous extract from Justicia spicigera. The obtained silver nanoparticles were characterized using UV–visible spectroscopic techniques, energy dispersive X-ray spectrometers (EDS), zeta potential and dynamic light scattering. The antimicrobial activity of biosynthesized AgNP was tested against foodborne bacteria (Bacillus cereus, Klebsiella pneumoniae and Enterobacter aerogenes) and phytopathogenic fungi (Colletotrichum sp., Fusarium solani, Alternaria alternata and Macrophomina phaseolina). The elemental profile of synthesized nanoparticles using J. spicigera shows higher counts at 3keV due to silver, confirming the formation of silver nanoparticles. Scanning electron microscopy (SEM) analysis showed a particle size between 86 and 100nm with spherical morphology. AgNP showed effective antibacterial and antifungal activity against the tested organisms principally with B. cereus, K. pneumoniae, E. aerogenes, A. alternata and M. phaseolina. Therefore, further studies are needed to confirm the potential of AgNP from J. spicigera in the control of indicator organisms under field conditions.
En el presente trabajo se evaluó la biosíntesis de nanopartículas de plata (AgNP) en presencia de una sal de plata y extractos acuosos de Justicia spicigera. Las nanopartículas así obtenidas fueron caracterizadas mediante técnicas espectroscópicas UV-visibles, espectrómetros de rayos X de energía dispersiva (EDS), potencial zeta y dispersión de luz dinámica. La actividad antimicrobiana de las AgNP biosintetizadas se probó frente a diversas bacterias transmitidas por alimentos (Bacillus cereus, Klebsiella pneumoniae y Enterobacter aerogenes) y hongos fitopatógenos (Colletotrichum sp., Fusarium solani, Alternaria alternata y Macrophomina phaseolina). El perfil elemental de las nanopartículas sintetizadas utilizando el extracto de J. spicigera mostró valores altos a 3keV, lo que confirma la formación de nanopartículas de plata. El análisis por microscopía electrónica de barrido (SEM) reveló un tamaño de partícula entre 86 y 100nm, con morfología esférica. Las AgNP mostraron una actividad antibacteriana y antifúngica efectiva contra los organismos evaluados, principalmente contra B. cereus, K. pneumoniae, E. aerogenes, A. alternata y M. phaseolina. Se necesitan más estudios para confirmar el potencial de las AgNP derivadas de J. spicigera en el control de organismos indicadores en condiciones de campo.
Silver has been used as an antimicrobial agent for centuries; the recent resurgence of interest for this element particularly focuses on the increasing threat of antibiotic resistance caused by the abuse of antibiotics11. However, there are some limitations in using Ag ions or Ag salts as antimicrobial agents. Probable reasons include bacterial resistance to silver18. Nevertheless, this type of limitation can be eliminated by using silver in nano form. In this sense, the silver nanoparticles (AgNP) may penetrate inside the cell causing damage by interacting with phosphorus and sulfur-containing compounds such as DNA and protein16. Currently, various chemical and physical methods are known for the preparation of silver and other metal nanoparticles. However, these methods are very costly and hazardous for the environment13. In this sense, green nanotechnology has great importance due to the elimination of harmful reagents and the effective synthesis of expected products in an economical manner. In recent years, the use of plants as source of bioactive compounds for the reduction of silver nanoparticles has been highlighted, because of its eco-friendly, economical protocol and for providing a single step technique for the biosynthesis of nanoparticles12. The family Acanthaceae, is characteristic of tropical regions of the world and its widespread distribution includes ecosystems in Asia, Africa, and the Americas7. In Mexico, Justicia spicigera (common name, muicle or muitle) is a plant that has been used as a natural pigment source for dying fabrics and crafts17. Nowadays, J. spicigera has been the focus of scientific interest mainly because of its significant content of phenolic compounds that contribute to its antioxidant and antidiabetic activity making this plant an interesting alternative for possible biotechnological applications5. However, studies about the use of J. spicigera for the phytosynthesis of AgNP as a green chemistry method are scarce. In the present study, we report the easy synthesis of AgNP by an environmentally friendly procedure involving the in situ reduction of Ag by J. spicigera extracts and the evaluation of their antimicrobial activity against bacterial and fungal strains.
Material and methodsBiosynthesis of silver nanoparticles (AgNP) from Justicia spicigeraTo prepare AgNP from J. spicigera, leaves of this plant were collected in Chiapas, Mexico. Leaves were cut into small pieces and dried using an electric oven for 10h at 50°C. Then 20g of dried leaves of J. spicigera were mixed with 200ml of deionized water and heated at 60°C for 30min. About 50ml of aqueous J. spicigera leaf extract were then centrifuged at 1792g (Thermo Scientific Sorvall Legend Micro 17 with dual Row 24×1.5/2.0ml Rotor) for 25min to remove particulate matter and to obtain clear solutions which were then refrigerated to 4°C for further use. For AgNP synthesis 10ml of aqueous J. spicigera leaf extract were added into 40ml of aqueous solution of 10mM silver nitrate and heated to 60°C for 15min. Finally, a brown solution was formed, which stands as a preliminary identification of the formation of AgNP3. The AgNP were purified by centrifugation at 11200×g (Thermo Scientific Sorvall Legend Micro 17 with dual Row 24×1.5/2.0ml Rotor) for 15min and the precipitate was thoroughly washed with sterile distilled water to get rid of any unwanted impurities and transferred to a freeze dryer. The obtained powder was used in antimicrobial assays.
Characterization of AgNPTo determine the bioreduction of silver ions and the production of AgNP, the absorption spectra of the solutions, J. spicigera: silver nitrate, were scanned in UV–visible (vis) spectra, between wavelengths of 350–500nm in a spectrophotometer (DR6000™ UV VIS Spectrophotometer, USA), having a resolution of 1nm and using double distilled water as a blank reference.
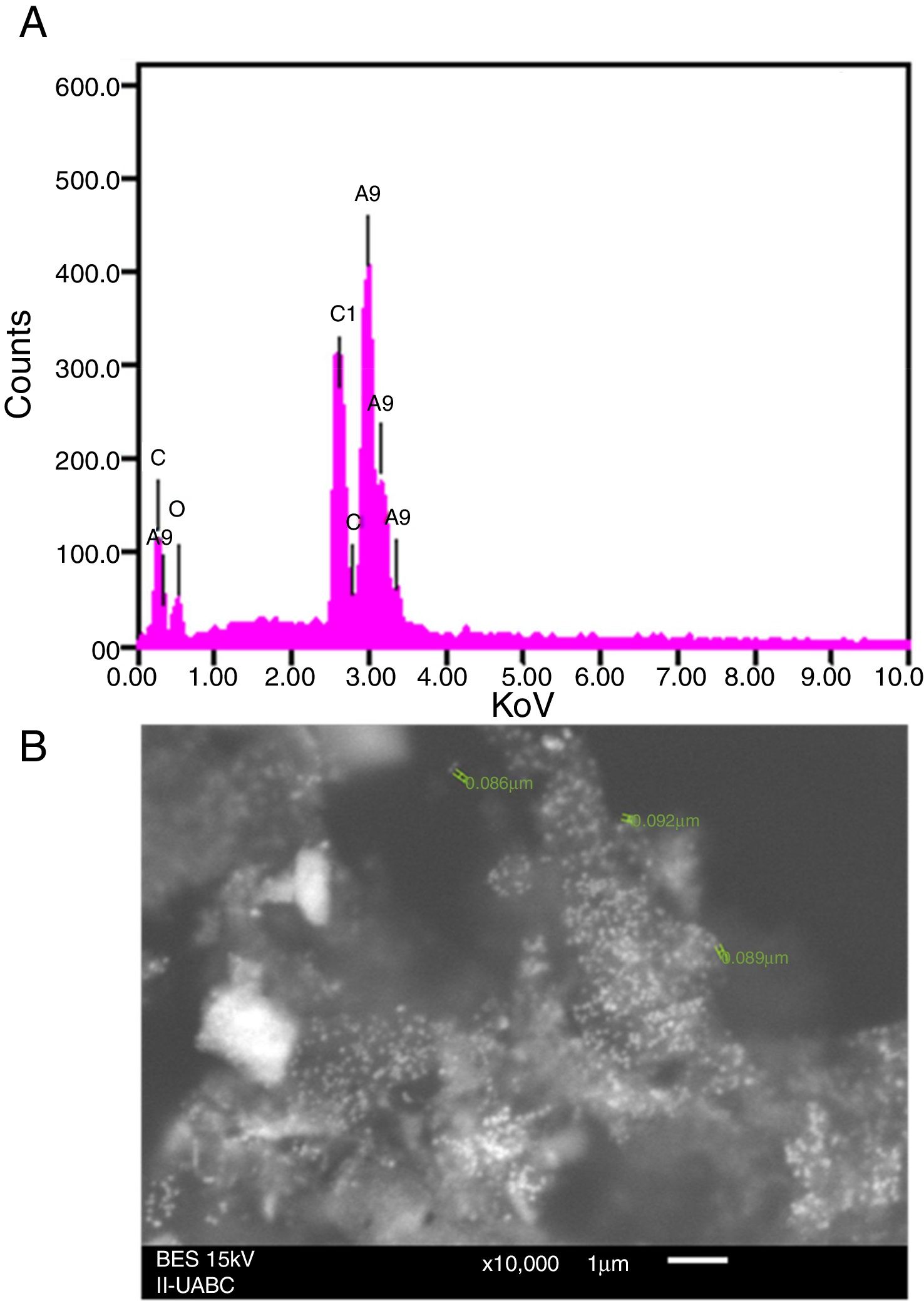
Scanning electron microscopy (SEM) and energy dispersive X-ray (EDS)The presence of AgNP from J. spicigera was confirmed by EDS, and the surface characterization was performed by the SEM analysis (JEOL 6010L; JEOL, Tokyo, Japan) with an accelerating voltage of 10kV and a STEM support according to Arrieta et al.4.
Zeta potential and dynamic light scattering (DLS)The hydrodynamic sizes and the zeta potential of biosynthesized AgNP in solution were analyzed using a Nanotrac Wave Instrument (Microtrac). Three milliliters of sample were transferred into the clear disposable zeta cell for zeta potential measurement. Measurements were made by dynamic light scattering (DLS) in the range of 0.1–1000μm at 25°C, using a laser wavelength of 780nm and a scattering angle of 90°. DLS data were analyzed by the Microtrac FLEX operating software27.
Antibacterial activity of AgNPThe effect of antibacterial activity of biosynthesized AgNP was determined using the disk diffusion method. Three types of bacteria, Bacillus cereus, Klebsiella pneumoniae, and Enterobacter aerogenes were tested. Bacteria cultures were grown in nutritious broth medium at 37°C. After 24h of growth, each microorganism was inoculated on the surface of nutrient agar plates at a concentration of 105 cells/ml. Subsequently, sterile paper disks (6mm in diameter) were placed on the surface of each inoculated plate. The plates were divided into two groups; in the first group, 30μl of leaf extract and sterile distilled water were applied to the disks as positive and negative control, respectively. In the second group, sterile paper disks were placed on the agar plates, and 30μl of 100mg/ml (w/v) samples were applied to the disks. All the plates were incubated at 32°C for 24h. After this period, the zone of inhibition which appeared as a clear area around the disks could be observed.
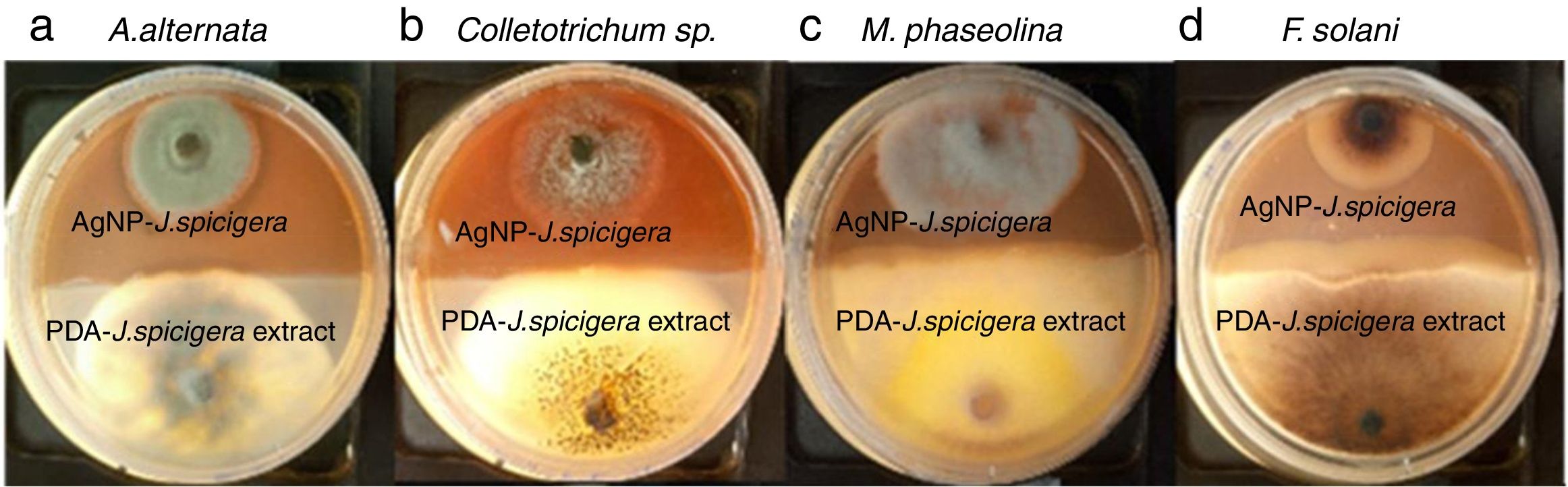
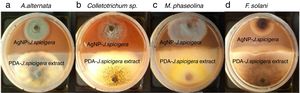
Effect of AgNP against phytopathogenic fungiAntifungal activity of AgNP was performed by the dual culture technique in individual culture plates. The plates were divided into two quadrants; the first quadrant was prepared with 15ml of PDA-AgNP (100mg/ml) and the second quadrants were only prepared with PDA medium. After an agar disk (6mm) was taken from 4-day-old PDA culture plates of each fungus (Colletotrichum sp., Fusarium solani, Alternaria alternate and Macrophomina phaseolina) and placed at the periphery of the PDA-AgNP plates. Another agar disk of the same size of each fungus was also placed at the periphery but on the opposing end of the same Petri dish (PDA+J. spicigera extract). The plates were incubated at 27°C for 9 days and the zone of inhibition was recorded.
Statistical analysisDifferences between the treatments were evaluated using one-way analyses of variance and the Tukey's test (p≤0.05), and SAS Version 9.0 (SAS Institute, 2002) was used.
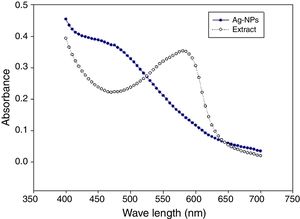
ResultsBiosynthesis of AgNP-Justicia spicigeraThe UV-visible spectroscopy is an important preliminary technique to ascertain the formation and stability of metal nanoparticles in aqueous suspension. Thus, the formation of AgNP by reduction of aqueous Ag+ during exposure to the aqueous extract of J. spicigera was followed and characterized by UV-visible spectroscopy.
As shown in Figure 1, the surface plasmon resonance of the AgNP was centered at approximately 487nm.
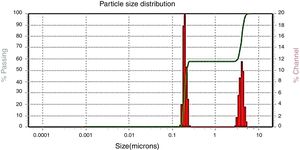
Zeta potential and dynamic light scattering (DLS)The average hydrodynamic size and zeta potential of the AgNP was determined by DLS as shown in Figure 2. The sample was a mixture of AgNP of different sizes; DLS intensity analysis gave two broad peaks and was weighted toward the larger particles (z-average size of 4.04μm and 192nm). These results suggest that DLS measurement may not be accurate for polydisperse samples due to its nature to respond toward larger particles. In addition, the zeta potential was found to be 0.2mV for synthesized AgNP indicating less stability and thus, a tendency to agglomerate and form large particles.
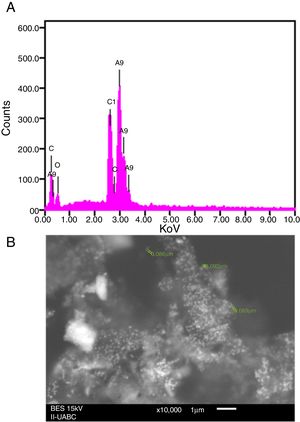
SEM and EDS analysisEDS analysis gives qualitative as well as quantitative status of elements that may be involved in the realization of nanoparticles9. In this sense, the elemental profile of synthesized nanoparticles using J. spicigera shows higher counts at 3keV due to silver, confirming the formation of silver nanoparticles (Fig. 3A). The elemental profile also showed the presence of oxygen (11.41%), carbon (64.35%) and chlorine (6.10%) respectively (Fig. 3). On the other hand, the SEM provided further insight into the morphology and size details of the silver nanoparticles. In the present study the SEM analysis showed a particle size between 86 and 100nm with spherical morphology (Fig. 3B).
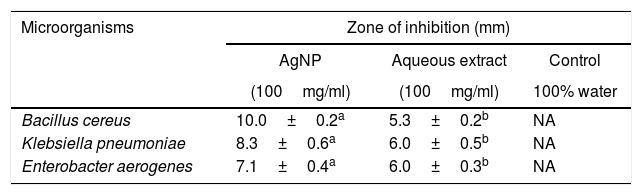
Antimicrobial activity of synthesized silver nanoparticlesTo investigate the antibacterial activity of the synthesized AgNP from J. spicigera against B. cereus, K. pneumoniae, and E. aerogenes, the concentration of AgNP (100mg/ml) was used. Our results showed that the antibacterial activity of disks prepared with AgNP (100mg/ml) doses was more effective in comparison with the impregnated disk with control (J. spicigera leaf extract) (Table 1).
Inhibition of different bacteria by AgNP from Justicia spicigera
| Microorganisms | Zone of inhibition (mm) | ||
|---|---|---|---|
| AgNP | Aqueous extract | Control | |
| (100mg/ml) | (100mg/ml) | 100% water | |
| Bacillus cereus | 10.0±0.2a | 5.3±0.2b | NA |
| Klebsiella pneumoniae | 8.3±0.6a | 6.0±0.5b | NA |
| Enterobacter aerogenes | 7.1±0.4a | 6.0±0.3b | NA |
Results are expressed as mean±standard deviation of values from triplicate experiments. Values with the same letter within each line are equal according to the Tukey test at p≤0.5. NA: no antibacterial activity was found.
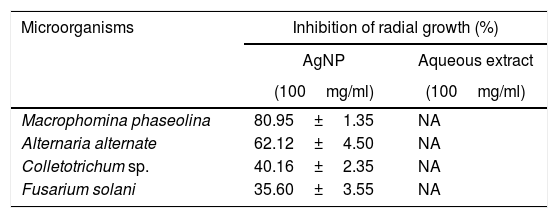
The inhibition effect of AgNP from J. spicigera was examined in PDA. A significantly higher mycelial growth inhibition (p≤0.05) was observed on PDA-AgNP medium against M. phaseolina and A. alternate with 79.77% and 60.10% of radial growth inhibition, respectively, when compared to the control after nine days of incubation (Table 2 and Fig. 4). However, in Colletotrichum sp., and F. solani a lower mycelial growth inhibition (40% and 35%, respectively) was observed after nine days of incubation.
Mycelial growth inhibition by AgNP from Justicia spicigera
| Microorganisms | Inhibition of radial growth (%) | |
|---|---|---|
| AgNP | Aqueous extract | |
| (100mg/ml) | (100mg/ml) | |
| Macrophomina phaseolina | 80.95±1.35 | NA |
| Alternaria alternate | 62.12±4.50 | NA |
| Colletotrichum sp. | 40.16±2.35 | NA |
| Fusarium solani | 35.60±3.55 | NA |
Results are expressed as mean±standard deviation of values from triplicate experiments. NA: no antifungal activity was found with the concentrations used in this experiment.
Nowadays, synthesizing metal nanoparticles using plants has been extensively studied and has been recognized as a green and efficient way for further exploiting plants as convenient nanofactories21,23. In this study the biosynthesis of silver nanoparticles from J. spicigera showed a reddish brown color in the aqueous solution as a result of a bioreduction mechanism of metal nanoparticles by secondary metabolites (for e.g., phenolic acid, flavonoids and alkaloids) present in the aqueous extract of J. spicigera. Similarly, Abdelmoteleb et al.2 and Pulit-Procial et al.19,20 reported that the silver nanoparticles from Pluchea sericea exhibited striking colors, from light yellow to brown in the aqueous solution due to the increasing number of AgNP formed as a result of the reduction of Ag ions by secondary metabolites present in the aqueous solution. Therefore, the secondary metabolites such as flavonoids and phenols present in the aqueous extract of J. spicigera may be responsible for stabilizing the synthesized nanoparticles; however, the probable mechanism is unclear and further investigation are required. In this sense, Sahadevan et al.22 reported that flavonoids present in leaf extract are responsible for reducing silver ions and capping for Ag-nanoparticles. On the other hand, the interaction, AgNP and extract of J. spicigera by DLS analyses showed a major particle size distribution peak at 4.04μm and a second peak at 192nm, which represented the existence of interaction between Ag ions and biomolecules from J. spicigera, which resulted in their further aggregation. This result is consistent with that previously reported by Yu et al.26 who found that the formation of new peaks of enlarged sizes indicated the aggregations of different components after mixing the silver solution with apple extract. As expected, the SEM measured size (89nm) is considerably shorter than the DLS size. According to Erjaee et al.8, these differences possibly reflect the fact that SEM only measures a number-based size distribution of the physical size while DLS measures the hydrodynamic diameter of the particle, plus biomolecules that are attached to the surface of AgNP in the solution. On other hand, Kumari et al.14 found that the DLS mean from AgNP of Cordia dichotoma was approximately 80% (90.42nm) higher than the TEM mean size of 10nm. In this sense, it is important to consider that the enlargement of size distributions induces errors to DLS particle sizing measurements when compared to the TEM analyses. Moreover, it is known, that metallic Ag-nanocrystals show a typical optical absorption peak at 2.9 to 3.2keV due to their surface plasmon resonance8. In this context, in the present study the elemental analysis of AgNP reveals the highest proportion of silver (Ag) followed by peaks carbon and oxygen atoms that suggested the presence of organic molecules on the surface of silver nanoparticles, which might have come from the plant leaf extract. In our study, the antibacterial activity of AgNP from J. spicigera is not completely clear; however, recent studies mention that bactericidal efficiency could be the result of an electric charge on the surface of AgNP, oxidative stress induction, metal ion release, or non-oxidative mechanisms that can occur simultaneously1. Additionally, certain studies have proposed that AgNP prompt neutralization of the surface electric charge of the bacterial membrane and change its penetrability, ultimately leading to bacterial death25. Bose and Chatterjee6 also found that AgNP from Justicia adhatoda, significantly increase cell permeability and affect proper transport through the plasma membrane. Therefore, further studies are needed to confirm the potential of AgNP from J. spicigera in the biocontrol of the evaluated bacteria. Silver nanoparticles from different sources have a biotechnological potential against fungi due to alterations and structural changes in hyphae, cell wall and germination, caused by the applications of nanoparticles10. However, in the current study, this effect in Colletotrichum sp., and F. solani was not registered. In this sense, the AgNP tolerance observed in Colletotrichum sp., and F. solani can be attributed to their complex multicellular organization15. In this sense, authors such as Harris9 and Villamizar-Gallardo et al.24 explain that this tolerance can be attributed to the fact that F. solani produces macroconidia which, by presenting complex cellular organization, probably hinders the internal transport of AgNP, thus decreasing their fungicidal effect.
ConclusionsIn the present study the bioreduction of silver nitrate solution using J. spicigera leaves extract offers environmental friendly and simple method for green synthesis of silver nanoparticles. These nanoparticles showed effective antibacterial and antifungal activity against B. cereus, K. pneumoniae, E. aerogenes, A. alternata and M. phaseolina. Future studies are required for evaluating the effect of application of these nanoparticles under field conditions.
Conflict of interestThe authors declare that they have no conflicts of interest.
The authors are particularly gratefully to Conacyt (219750), SEFOA and Tecnológico Nacional de México.