Bovine respiratory disease (BRD) is one of the most frequent clinical concerns in weaned calves after their arrival at the feedlot. This work reports the first local isolation of Mycoplasma bovis from feedlot calves with pneumonia and polyarthritis in Argentina. Twenty four out of 545 calves showed progressive, subacute to chronic respiratory distress, coughing, and fever. Thirty percent of the affected calves also showed lameness and swelling of elbow or carpal, and knee or tarsal joints. Five necropsies were performed and severe multifocal to coalescent pulmonary nodules, containing white-yellowish caseous exudate encircled by fibrous tissue, and fibrinonecrotic arthritis and tenosynovitis were detected. Mycoplasma was isolated from lung and joint samples. The 16S-23S rRNA ITS consensus sequence obtained from these isolates showed 100% similarity with the same region of M. bovis strains. Since there are no commercially available vaccines in the region for the prevention and control of M. bovis pneumonia and arthritis, surveillance is a priority to reduce the source of disease to naïve animals.
La enfermedad respiratoria bovina es uno de los problemas sanitarios más frecuentes en terneros recién destetados luego de su arribo a los corrales de encierre (feedlots). Este trabajo describe el primer aislamiento local de Mycoplasma bovis de terneros de feedlot con neumonía y poliartritis en Argentina. Se vieron afectados 24 de 545 terneros; mostraron enfermedad respiratoria progresiva, subaguda a crónica, tos y fiebre. De los terneros afectados, el 30% presentó, además, problemas en articulaciones carpales o tarsales. Se realizaron 5 necropsias de terneros afectados y se observaron nódulos pulmonares multifocales a coalescentes, que contenían un exudado caseoso blanco-amarillento, rodeados de tejido fibroso, artritis y tenosinovitis fibrinonecrótica. Se aisló Mycoplasma de muestras de pulmón y articulación. La secuencia consenso del gen codificante del ARNr 16S-23S rRNA obtenido de estos aislamientos mostró un 100% de similitud con la misma región de cepas de M. bovis. Teniendo en cuenta que no hay vacunas disponibles comercialmente en la región para la prevención y el control de neumonías y poliartritis por M. bovis, es importante realizar una vigilancia epidemiológica a fin de reducir las fuentes de infección para animales susceptibles.
Bovine respiratory disease (BRD) is one the most frequently occurring clinical concerns in weaned calves after their arrival at the feedlot19, causing important economic losses in the beef industry22. Infectious pneumonia usually has complex causes, involving two or more microorganisms and is commonly predisposed by environmental factors27. Viruses most frequently associated with BRD are bovine herpesvirus type 1 (BoHV1), bovine parainfluenza virus type 3 (BPIV-3), bovine respiratory syncytial virus (BRSV), and bovine viral diarrhea virus (BVDV). Secondary bacterial infections are usually associated with Mannheimia haemolytica, Pasteurella multocida and Histophilus somni. Other bacteria frequently detected in BRD are mycoplasmas, especially Mycoplasma bovis5,9,10,18.
M. bovis is most commonly recognized as a cause of pneumonia and arthritis in calves and mastitis in dairy cattle in North America and Europe6,10,15. Margineda et al.14 reported for the first time the presence of M. bovis as a cause of pneumonia in feedlot cattle and dairy calves in Argentina. Nevertheless, the prevalence, morbidity, mortality, and economic relevance of M. bovis pneumonia in the BRD complex in Argentina are still unknown.
Although M. bovis has been previously isolated from other clinical presentations of dairy cattle7 and M. bovis-pneumonia has been previously diagnosed in Argentina14, this study reports the first local isolation of M. bovis from feedlot calves with pneumonia and polyarthritis in Buenos Aires province, Argentina.
Materials and methodsClinical history of the herdThe outbreak occurred in a feedlot in Carlos Tejedor department (35°11’01″S 62°36’16″W), Buenos Aires province, Argentina. During December 2018 until January 2019, 545 early-weaned calves weighing 45–55kg arrived at the feedlot, from three farms. Upon arrival, calves were twice treated with tilmicosin (metaphylaxis, days 0 and 21 post-arrival) and immunized using a commercial polyvalent vaccine against BoHV1, P. multocida, Moraxella bovis, Clostridium chauvoei and Clostridium perfringens (days 0, 21 and 42 post-arrival). The diet consisted of cracked corn, soybean expeller, wheat bran and a commercial vitamins-minerals premix. Calves were allocated in three different lots: 200 in lot A, 143 in lot B and 202 in lot C.
Post mortem examination and tissue samplingAfter clinical examination, respiratory signs and polyarthritis were observed in affected calves. The five most severely affected calves were euthanized in accordance with the regulations of INTA's Animal Ethics Committee and necropsied accordingly. Samples from the central nervous system, heart, liver, spleen, kidney, muscle, lung, mediastinal lymph nodes and synovial capsules were collected and fixed in 10% neutral buffered formalin for histopathological and immunohistochemistry (IHC) examination. Furthermore, lung, synovial fluid and capsule samples were collected for microbiological examination.
Histopathology and Mycoplasma bovis immunohistochemistryFormalin fixed tissues were paraffin embedded, sectioned at 4–5μm and stained with hematoxylin and eosin (HE) for histologic examination. Formalin fixed and paraffin-embedded lung and synovial capsules were examined using IHC for the detection of M. bovis as previously described11, using mouse anti-M. bovis monoclonal antibody (Millipore MAB970) at 1:100 dilution. Positive and negative controls were included14. No other Mycoplasma spp. were tested by IHC in this study.
MicrobiologyLung and synovial fluid samples were inoculated onto Mycoplasma Base Medium with Selective Mycoplasma supplement – MM (Oxoid Ltd., Wad Road, Basingstoke, UK) and Columbia Blood Agar – CBA (Oxoid Ltd., Wad Road, Basingstoke, UK) with 7% bovine blood and MacConkey agar – MC (Oxoid Ltd., Wad Road, Basingstoke, UK). All plates were incubated at 37°C, MM under 5% CO2, CBA under 10% CO2 and MC under aerobiosis, and examined at 96, 48 and 24h, respectively. Genera were classified in accordance with the Bergey's Manual of Systematic Bacteriology4. Lung smears were heat-fixed and stained using Ziehl – Neelsen (ZN) methods to detect acid-fast bacteria (AFB).
PCR and sequencingMolecular detection of Mycoplasma was performed for both, clinical samples and to confirm the presence of the agent after culture. Briefly, DNA was extracted using the Puri-Prep S commercial kit (Inbio Highway, Argentina) according to the manufacturer's instructions. For Mycoplasma detection, a nested PCR targeting the 16S-23S rRNA intergenic spacer region (ITS) was performed under the conditions reported by Tang et al.26, using primers previously reported12,16. To identify the mycoplasma species, the obtained PCR products were purified (Puriprep-GP Kit, Inbio Highway), quantified and sequenced (ABI 3130xl; Applied Biosystems) using the inner primers described by Harasawa et al12. The sequences were curated using the BioEdit software and aligned using Clustal Omega software. Since all the sequences were identical, a unique consensus sequence was obtained and then aligned against the database using nucleotide BLAST (http://www.ncbi.nlm.nih.gov/blast), excluding uncultured and environmental sample sequences.
VirologyLung samples were homogenized in Eagle's minimum essential medium (MEM) (Gibco, 4150034; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum, and inoculated on Madin-Darby Bovine Kidney (MDBK) cells. Cell cultures were incubated at 37°C and 5% CO2 for 5 days and examined daily for evidence of cytopathic effect. After four consecutive passages, cultures were tested for BoHV-1, BPIV-3, BRSV and BVDV by direct fluorescent antibody tests.
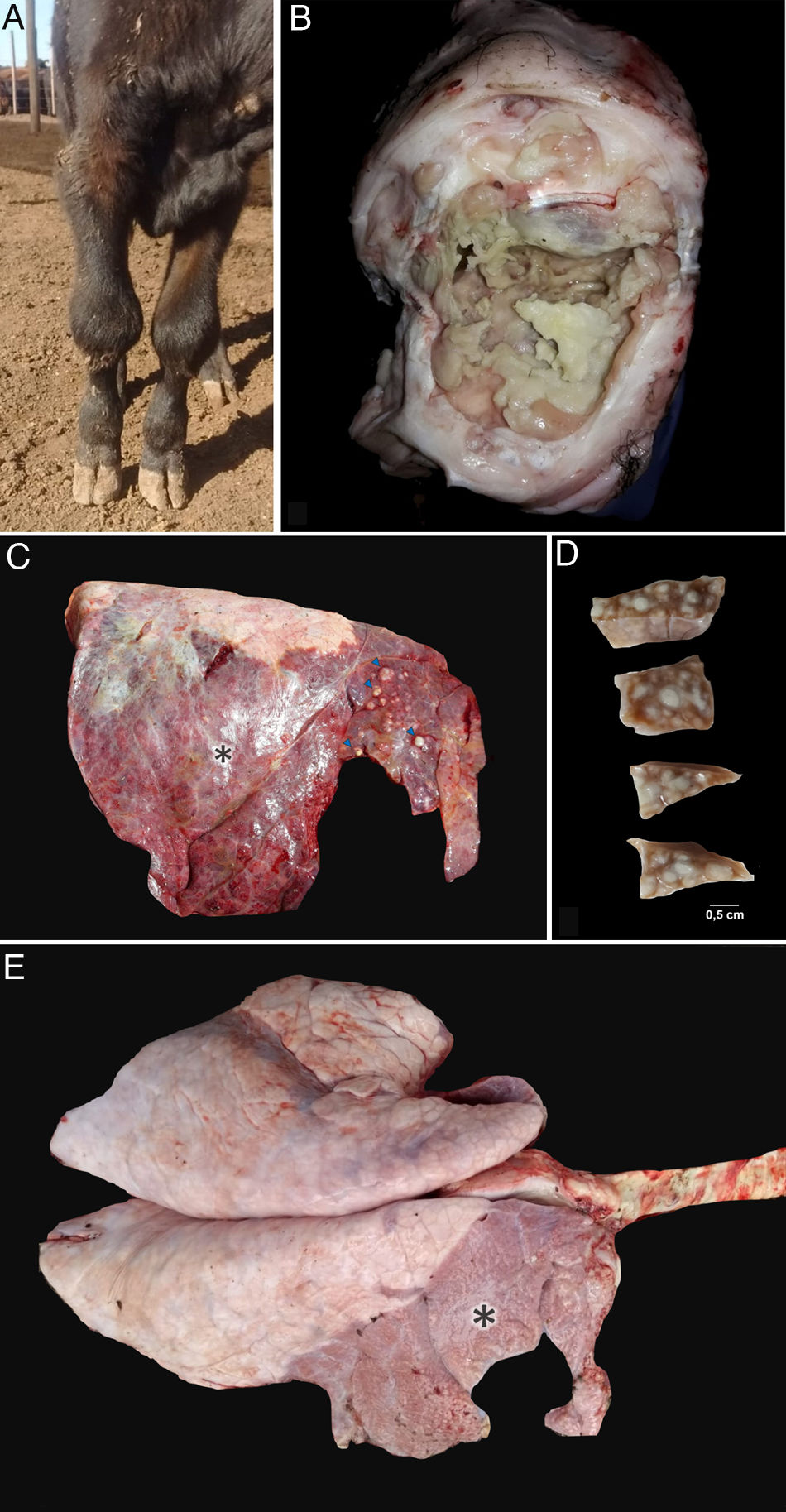
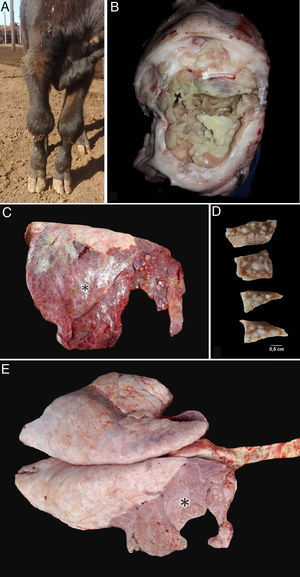
ResultsClinical cases were initially reported in lots A (9 cases) and B (13 cases). The first two cases occurred during December 2018, 1 in January, 9 during February and 10 during March 2019. All the affected calves (24 out of 545; morbidity=4.4%) showed progressive, subacute to chronic respiratory distress, coughing, hyperpnea, poor body condition, lethargy, dehydration and fever (39.5–41.3°C). Thirty percent of the affected calves also showed lameness, grinding noise when they walk, and pain in one or more joints associated with visible swelling of the affected front (Fig. 1a) and hind leg joints (elbow or carpal, and knee or tarsal joints, respectively).
Post mortem findings in necropsied calves. (a) Bilateral swollen carpal joints of a calf with mycoplasma arthritis. (b) Necropsy #3. Fibrinonecrotic carpal arthritis and tenosynovitis in calf with Mycoplasmosis. (c) Necropsy #4. Focally extensive subacute to chronic pneumonia affecting approximately 90% of the right lung (apical, cranial and caudal lobes) of a calf with mycoplasma pneumonia. Multifocal caseonecrotic nodules are present in the cranial lung lobe (blue arrows). (d) Necropsy #1. Multifocal caseonecrotic nodules in the lung parenchyma. Formalin fixed tissue. (e) Necropsy #1. Focally extensive subacute to chronic pneumonia mainly affecting the whole right apical and cranial lobes and the cranial region (20% approximately of parenchyma) of the right caudal lobe of a calf with mycoplasma pneumonia (*).
Post mortem examinations were performed in five affected calves. Fibrinonecrotic arthritis and tenosynovitis were detected in the affected joints (Fig. 1b). The lungs of the five calves showed cranioventral consolidation affecting 20 to 50% of the pulmonary parenchyma. All the examined lungs showed multifocal to coalescent white nodules, some of them protruding above the pleural surface (Fig. 1c). On section, white nodules ranged 0.3–2.5cm and contained white-yellowish caseous exudate, encircled by fibrous tissue (Fig. 1d). Pleural fibrosis was observed in one of the examined animals (Fig. 1e) and chronic pleural adhesions in two calves. No other gross lesions were observed in the affected necropsied calves.
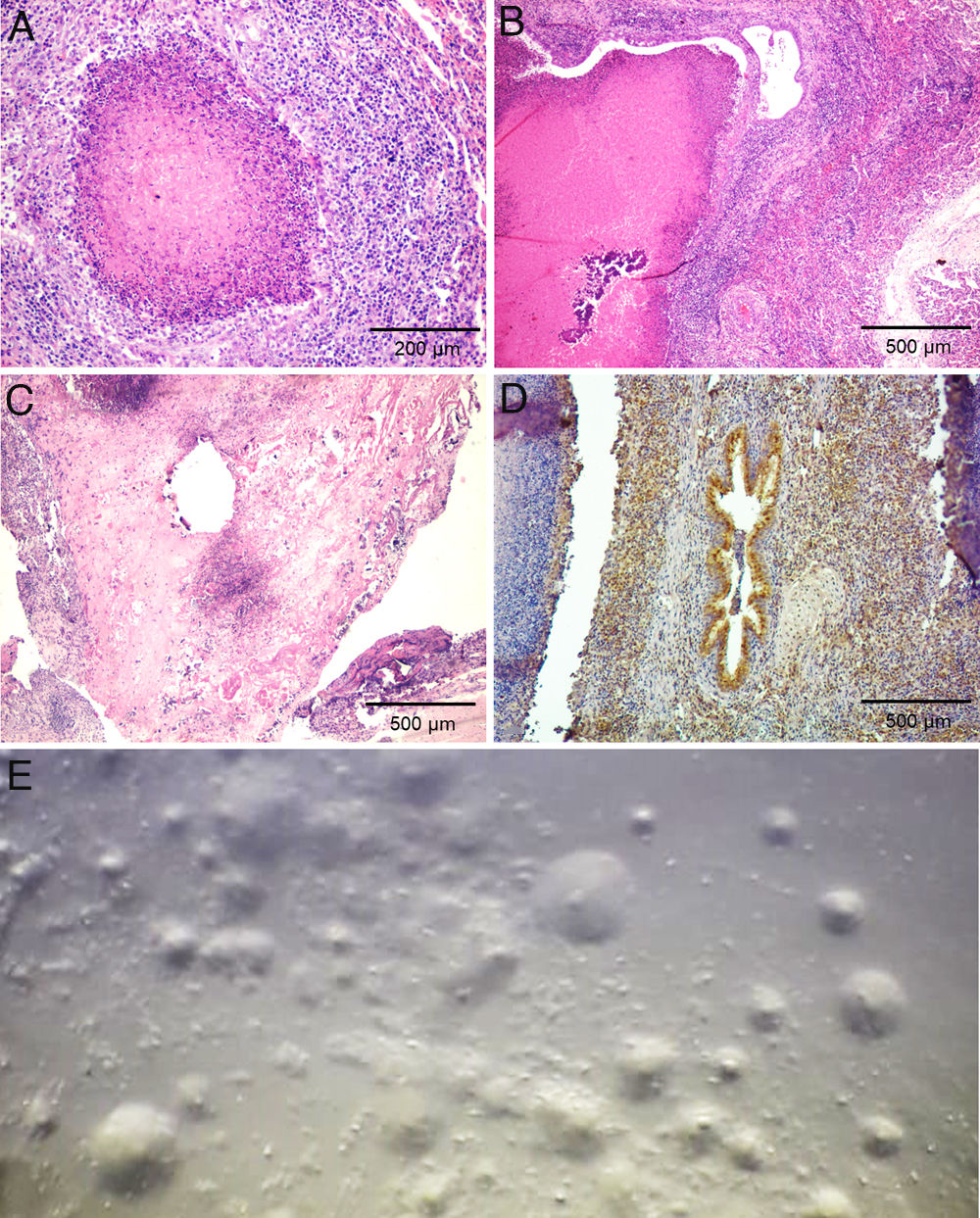
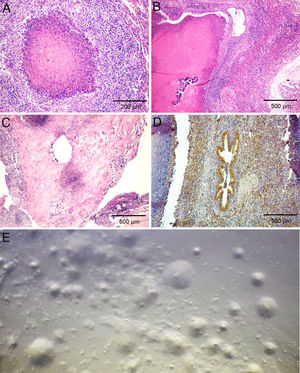
Histologically, multifocal necrotic areas in the pulmonary parenchyma were observed. These foci contained many necrotic inflammatory cells that retained their cellular outlines but had intensely eosinophilic cytoplasm and nuclei lysis (Fig. 2a and b). These foci were delineated by a band of neutrophils and macrophages, encircled by a layer of fibroblasts macrophages, lymphocytes, and plasma cells. The smaller bronchioles contained an accumulation of necrotic leukocytes in the bronchiolar lumen and the epithelium was discontinuous. The bronchiolar walls were thickened by edema and infiltrate of lymphocytes with fewer neutrophils and macrophages. Similar foci of caseous necrosis that contained recognizable necrotic leukocytes were occasionally present in the alveoli. Macrophages and scarce plasmocytes infiltrated the interlobular septa. Follicular hyperplasia was observed in mediastinal lymph nodes. In joint capsule, fibrin admixed with many neutrophils was adherent to the synovium, with areas of hyperplasia alternating with areas of necrosis or denudation of synoviocytes. The subsynovial stroma had severe infiltration of many neutrophils, macrophages, lymphocytes, and plasma cells; and prominent fibroblast hyperplasia (Fig. 2c). No other microscopical lesions were detected in the affected calves.
Laboratory findings. (a) Necropsy #4. Lung. Necrotic focus delineated by a band of neutrophils and macrophages, encircled by a layer of fibroblasts, macrophages, lymphocytes, and plasma cells. Hematoxylin and eosin, 100×. (b) Necropsy #3. Lung. Large area of caseous necrosis with mineralization surrounded by inflammatory infiltrate characterized by fibroblast, macrophages and lymphocytes, mainly, typical of Mycoplasma pneumonia. Macrophages and scarce plasmocytes were infiltrating the interlobular septa. Hematoxylin and eosin, 40×. (c) Necropsy #4. Carpal joint. Necrotic foci or denudation of synoviocytes with severe infiltration of neutrophils, macrophages, lymphocytes, and plasma cells; and prominent fibroblast hyperplasia. Hematoxylin and eosin, 40×. (d) Necropsy #3. Lung. Immunohistochemistry labeling of M. bovis in the lung was observed mainly in bronchioles containing caseous debris. (e) Typical Mycoplasma fried-egg-shaped colonies were observed in the lung culture collected during necropsy #1.
Immunohistochemistry showed abundant M. bovis antigen in the lungs and joint capsule of calves. In lungs, the positive staining was observed mainly at the margin of the necrotic lesions, and to a lesser extent at the center of the necrotic foci. In bronchioles containing caseous debris, the antigen was present within the debris and adjacent to bronchiolar epithelial cells (Fig. 2d). M. bovis antigen was identified in synovial and subsynovial stroma within the debris and adjacent neutrophils and macrophages. Staining for M. bovis antigen was not visible in the sections of negative controls.
Typical Mycoplasma fried-egg-shaped colonies were observed in all the lung samples from the five calves (Fig. 2e). In none of the synovial fluid samples compatible-Mycoplasma colonies were observed. Lung and synovial fluid sampled during the necropsies of calves #1, #2, #3 and #4 were negative for the isolation of aerobic and microaerophilic bacteria using routine diagnostic procedures. Trueperella pyogenes and H. somni were isolated from calf #5 lung sample. No AFB were observed in the ZN staining of lung smears.
The lung samples from all calves and synovial samples from calves #1, #3, and #4 rendered PCR positive results. The presence of the agent was also confirmed in the lung cultures. The 16S-23S rRNA ITS consensus sequence obtained showed 100% similarity with the same region of M. bovis strains NADC59 (CP042939.1), MJ1 (CP042938.1), KG4397 (AP019558.1), NADC61 (CP022599.1), NADC67 (CP022596.1), NADC62 (CP022595.1), NADC58 (CP022594.1), NADC57 (CP022593.1), NADC56 (CP022592.1), NADC55 (CP022591.1), NADC54 (CP022590.1), NADC18 (CP022589.1), MJ4 (CP022588.1), MJ3 (CP022587.1), MJ2 (CP022586.1), JF4278 (LT578453.1), Ningxia-1 (CP023663.1), 08M (CP019639.1), 72242 (KX687011.1), 393B08 (KX687010.1), 268B07 (KX687009.1), HB0801-P115 (CP007589.1), NM2012 (CP011348.1), 1982-M6152 (CP058969.1), 2019-043682 (CP058968.1), PG45 (CP002188.1), 70-213 (AY779747.1) and ATCC 25523 (AY729934.1).
The lung samples tested were negative for BoHV-1, BPIV-3, BRSV and BVDV.
DiscussionBovine respiratory disease causes important economic losses in the beef industry19 and is described as multifactorial with different etiological agents involved9,10. Although M. bovis is frequently detected in association with BRD worldwide9,10,15, only one description of the disease is available in Argentina14.
This report describes an outbreak and the first isolation of M. bovis in feedlot calves with chronic pneumonia and polyarthritis in Argentina. Clinical signs observed in the animals are similar to previous reports: subacute to chronic respiratory distress with fever and severe lameness resulting from polyarthritis (mainly affecting carpal and tarsal joints), also known as “pneumonia-arthritis syndrome”1,10. Failure of antibiotic treatment and retarded growth are other characteristics of the disease24, as was observed in this outbreak. Based on the information recorded during the occurrence of the outbreak, 4.4% of the exposed calves were affected. However, the incidence of mycoplasma pneumonia can be as high as 100%21. Nevertheless, before the disease was confirmed in this feedlot, some of the calves in the affected lots (A and B) were moved to different lots with other animals. Then, Mycoplasma-like disease was observed in these animals (as reported by the veterinary practitioner), suggesting rapid transmission of the agent to susceptible animals20. Therefore, the exact epidemiological rates of this outbreak are actually unknown. Certain animals may act as reservoirs of Mycoplasma in the respiratory tract without developing the clinical disease28 and probably, reservoir calves may have been introduced in December or January, providing the source for infection to in-contact calves, as was previously reported3. No previous history of these calves was available to explain this issue. Tilmicosin treatment of these calves was probably not efficient to reduce their reservoir status, since this is not recommended as effective for M. bovis therapy. Moreover, enrofloxacin, florfenicol and spectinomycin would be better options as metaphylactic antibiotic treatment6.
Post mortem diagnosis during BRD should be carried out in untreated animals in the initial stages of the clinical disease. Therefore, the diagnosis of BRD due to M. bovis sometimes have some difficulties, since chronically affected calves have probably been already treated with a variety of antimicrobials8. Nevertheless, pathological changes associated with Mycoplasma pneumonia are characteristic and can provide useful information. Mycoplasma pneumonia is characterized as subacute or chronic suppurative bronchopneumonia with multiple foci of caseous necrosis1,6,10,21, as observed during the five necropsied calves in this outbreak. With regard to histopathology, foci of acute coagulative necrosis surrounded by a densely basophilic border of necrotic leukocytes (“oat cells”) are also morphologically distinctive from other bacterial etiologies of BRD10,21.
Bovine respiratory disease is usually caused by multiple microorganisms and their identification in tissue samples from an affected calf should be carefully interpreted13,18. T. pyogenes and H. somni were isolated from one of the sampled lungs. These bacteria could be responsible for BRD. However, the clinical history and the pathological findings resemble “pneumonia-arthritis syndrome” previously associated with M. bovis1,10.
Molecular diagnostics have substituted classic diagnostic procedures such as culture for Mycoplasma spp and other fastidious microorganisms, providing very specific, sensitive and rapid tests8. During this work, PCR was applied as a screening test, and then, the tissue samples were cultured and Mycoplasma was isolated. Although previous reports mentioned similar isolation success in lung and synovial samples2, only the lung samples were positive for Mycoplasma isolation in this study. Previously reported DNA amplicons were then sequenced and 100% nucleotide identity was observed with M. bovis reference strains, confirming the etiological agent involved during this outbreak. Nevertheless, the bacteriological results should be interpreted in conjunction with the presence of pathological changes associated with this infection, since M. bovis can be part of the microbiota of the healthy bovine upper respiratory tract.
In accordance with Margineda et al.14, this work should alert practitioners about the presence of Mycoplasma infections as the cause of BRD in Argentina, specifically considering that other species of Mollicutes causing arthritis and pneumonia such as U. diversum, M. bovigenitalum, M. bovirhinis, M. alkalescens and M. leachii have been reported in Argentina17,23,25.
Since there are no commercially available vaccines in the region for the prevention and control of M. bovis pneumonia and arthritis, and the disease caused by M. bovis is refractory to delayed antimicrobial therapy, surveillance is a priority to reduce the source of disease to naïve animals. Removal of clinically affected animals and quarantine of the affected lots is useful to reduce the dissemination of mycoplasmosis to unaffected lots.
This work reports the first local isolation of M. bovis from feedlot calves with pneumonia and polyarthritis in Argentina. Further work should be done in order to broaden the regional information about the clinical prevalence of this pathogen.
Ethical disclosuresAffected calves were euthanized according to the regulations of the Animal Ethics Committee of INTA.
FundingThis study was financially supported by Red Nacional de Laboratorios de Diagnóstico Veterinario (RIST.I111; INTA, Argentina); Ministerio de Ciencia, Tecnología e Innovación, Argentina (PICT 0442/2015 and PICT 02148/2018); Universidad Nacional de Rio Cuarto, Argentina (PPI 2016–2018, 188/2016) and Innovaciones Tecnológicas Agropecuarias S.A.
Authors’ contributionsGermán Cantón conceived of the presented manuscript. Germán Cantón, Ignacio Llada, Facundo Urtizbiría and Sofía Fanti performed the post mortem examination and sampling of the animals. Germán Cantón, Ignacio Llada, Carlos Margineda, Valeria Scioli and Eleonora Morrell carried out the histopathological analysis. Carlos Margineda performed the inmmunohistochemical analysis of the tissue samples. María Andrea Fiorentino, Enrique Louge Uriarte, Erika Sticotti and Pablo Tamiozzo carried out the microbiological examination of the specimens. All authors discussed the results and contributed to the final manuscript.
Conflicts of interestThe authors declare that they have no conflicts of interest.
We acknowledge Dr. Fernando Ibañez, Susana Pereyra, Jorgelina Lomónaco and Paula Nievas for technical assistance.