The enzymatic characterization of vibrios has been used as a virulence indicator of sanitary interest. The objective of this study was to determine the enzymatic profile of Vibrio parahaemolyticus strains (n=70) isolated from Crassostrea rhizophorae oysters. The strains were examined for the presence of gelatinase (GEL), caseinase (CAS), elastase (ELAS), phospholipase (PHOS), lipase (LIP), amilase (AML) and DNase. All enzymes, except elastase, were detected in more than 60% of the strains. The most recurrent enzymatic profiles were AML + DNase + PHOS + GEL + LIP (n=16; 22.9%) and AML + CAS + DNase + PHOS + GEL + LIP (n=21; 30%). Considering the fact that exoenzyme production by vibrios is closely related to virulence, one must be aware of the bacteriological risk posed to human health by the consumption of raw or undercooked oysters.
La caracterización enzimática de los vibrios se ha utilizado como un indicador de virulencia de interés sanitario. El objetivo de este estudio fue determinar el perfil enzimático de 70 cepas de Vibrio parahaemolyticus aisladas a partir de ostras Crassostrea rhizophorae. Se investigó la presencia de gelatinasa (GEL), caseinasa (CAS), elastasa (ELAS), fosfolipasa (PHOS), lipasa (LIP), amilasa (AML) y DNasa. Todas las enzimas se detectaron en más de 60% de las cepas, excepto la elastasa. Los perfiles enzimáticos más recurrentes fueron AML + DNasa + PHOS + GEL + LIP (n=16; 22,9%) y AML + CAS + DNasa + PHOS + GEL + LIP (n=21; 30%). Teniendo en cuenta el hecho de que la producción de exoenzimas por vibrios está estrechamente relacionada con la virulencia, se alerta acerca del riesgo bacteriológico para la salud humana asociado al consumo de ostras crudas o poco cocidas.
The indigenous microbiota of oysters is known as a source of etiological agents of bacterial diseases associated with shellfish consumption. Among the autochthonous microorganisms of marine and estuarine mollusks, the Vibrio parahaemolyticus is often considered one of the main causes of oyster-associated gastroenteritis in human beings. The ability to induce gastroenteric disturbs is associated to the enzymatic expression - mainly hemolysin production14.
Besides hemolysin, other enzymes are associated with the pathogenesis of this bacterium. Gelatinase production was already recognized as a virulence factor involved in bacteremia cases in humans beings13. Vibrios isolated from sea water producing DNase, amylase, gelatinase, chitinase and elastase showed positivity in the mouse lethality test and fluid accumulation in the suckling mice model11.
Caseinolytic activity has been related to lethal bacterial toxicity4 and gelatonilytic enzymes are recognized to hydrolyze collagen, hemoglobin and other small amounts of biologically active peptides13. Bacterial elastase is able to cause dermonecrosis, destruction of tissues, edema, and ulceration5.
Bacterial lipolytic activity is involved in nutrient acquisition through the degradation of membrane lipids, which may cause harm to the host10. Phospholipases are associated with virulence in bacterial diseases and may act as hemolysins3. Extracellular DNase acts as endonuclease and contributes to DNA hydrolysis11.
Due to the potential virulence mentioned above, exoenzyme expression has been used as an indicator of health risk in bacteria isolated from clinical sources, enviroment and food1,6,11. Thus, the objective of this study was to determine the enzymatic profile of V. parahaemolyticus strains isolated from fresh and frozen Crassostrea rhizophorae oysters by detecting the following exoenzymes: gelatinase, caseinase, elastase, lipase, phospholipase, amylase and DNase.
Seventy strains of V. parahaemolyticus isolated from soft tissues with the intervalvar liquids of C. rhizophorae oysters marketed both as fresh (n=30; Nos. 1 to 30) and frozen (n=40; Nos. 31 to 70) in Fortaleza-Brazil in 2010 were selected in this study. We used the following ATCC standard strains as controls: Pseudomonas aeruginosa ATCC 27853, Escherichia coli ATCC 25922, and Staphylococcus aureus ATCC 25923. All strains (n=70) were obtained from the bacterial collection of the Laboratory of Environmental Microbiology and Seafood, from the Sea Sciences Institute of the Federal University of Ceará (LABOMAR-UFC), presenting the phenotypic profile described by Noguerola and Blanch9 as compatible for the V. parahaemolyticus species: sucrose (—), arginine hydrolysis (—), lysine decarboxylation (+), ornithine decarboxylation (+), indole (+), oxidase (+), ONPG (—), mannitol (+), Voges Proskauer (—), D-glucosamine (+), growth at 0% NaCl (—), growth at 8% NaCl (+), 10 μg ampicillin resistance (+), 10 μg resistance to O129 (+) and 150μg resistance to O129 (—).
For exoenzyme detection, the strains (n=70) were maintained in tryptone soy agar (TSA) containing 1% NaCl. Gelatinase (GEL), caseinase (CAS) and elastase (ELAS) production was evaluated in TSA supplemented with 0.5% (w/v) gelatin7, agar milk15 and agar Noble containing 0.3% elastin (Sigma E1625) (w/v)12, respectively, with incubation at 35°C for 48h. All media were supplemented with 1% NaCl. The visualization of gelatin hydrolysis was made by the application of saturated ammonium sulfate — to precipitate unhydrolyzed gelatine — in the agar surface. The positive reaction to gelatinase, caseinase and elastase was characterized by the presence of clear halos around the bacterial growth. As a control, we used Pseudomonas aeruginosa strain ATCC 27853 (positive control) and Escherichia coli strain ATCC 25922 (negative control).
For lipase (LIP) and phospholipase (PHOS) production tests, TSA was used (1% NaCl) supplemented with 1% (v/v) Tween 80 and 5% (v/v) egg yolk emulsion, respectively7, with incubation at 35°C for 48h. Opaque halos around the inoculum site were considered as a positive reaction for lipase and phospholipase. P. aeruginosa strain ATCC 27853 was used as positive control for the lipase test and the standard Staphylococcus aureus ATCC 25923 was the positive reference for the phospholipase test. E. coli strain ATCC 25922 was used as negative control on both assays.
Amylase (AML) was detected in nutrient agar (1% NaCl), supplemented with 0.1% (p/v) soluble starch11. To detect DNase, DNase agar (1% NaCl) containing 0.01% (w/v) of toluidine blue was used. In both assays, incubation was performed at 35°C for 48h. The bacterial colonies that produced clear halos with iodine solution were considered amylase-positive. A pink halo around the inoculum streak was considered a positive reaction for DNase.
All strains (n=70) were DNase positive. Amylolytic activity was detected in 100% and 97.5% of strains isolated from fresh and frozen oysters, respectively.
Frozen oyster strains showed the following levels of proteolytic, lipolytic and phospholipolytic activity: 100% GEL+, 50% CAS+, 80% LIP+ and 67.5% PHOS+. Similar levels were found in isolates from fresh oysters: 86.7% GEL+, 50% CAS+, 80% LIP+ and 73.3% PHOS+. Elastase was not detected in any strain.
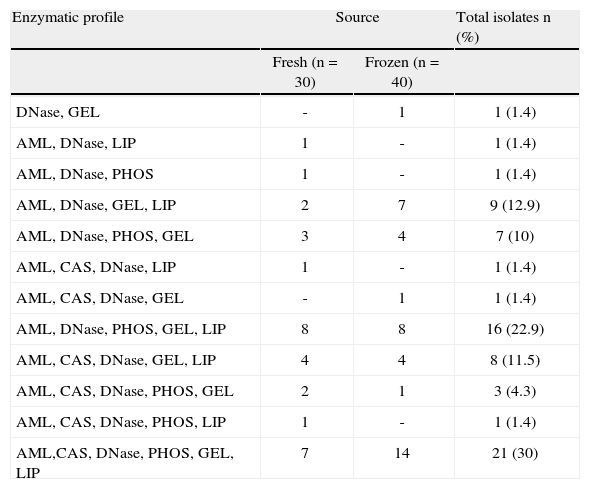
Among the isolates from fresh oysters, 10 enzymatic profiles were detected, the most recurrent being AML, DNase, PHOS, GEL, LIP. On the other hand, the strains from frozen oysters showed 8 enzymatic profiles, being the most prevalent: AML, CAS, DNase, PHOS, GEL, LIP (table 1).
Enzymatic profiles of Vibrio parahemolyticus strains isolated from Crassostrea rhizophorae.
| Enzymatic profile | Source | Total isolates n (%) | |
| Fresh (n=30) | Frozen (n=40) | ||
| DNase, GEL | - | 1 | 1 (1.4) |
| AML, DNase, LIP | 1 | - | 1 (1.4) |
| AML, DNase, PHOS | 1 | - | 1 (1.4) |
| AML, DNase, GEL, LIP | 2 | 7 | 9 (12.9) |
| AML, DNase, PHOS, GEL | 3 | 4 | 7 (10) |
| AML, CAS, DNase, LIP | 1 | - | 1 (1.4) |
| AML, CAS, DNase, GEL | - | 1 | 1 (1.4) |
| AML, DNase, PHOS, GEL, LIP | 8 | 8 | 16 (22.9) |
| AML, CAS, DNase, GEL, LIP | 4 | 4 | 8 (11.5) |
| AML, CAS, DNase, PHOS, GEL | 2 | 1 | 3 (4.3) |
| AML, CAS, DNase, PHOS, LIP | 1 | - | 1 (1.4) |
| AML,CAS, DNase, PHOS, GEL, LIP | 7 | 14 | 21 (30) |
GEL: gelatinase, AML: amylase, LIP: lipase, PHOS: phospholipase, CAS: caseinase.
The enzyme profiles were similar for isolates from fresh and frozen oysters (table 1), which suggests that the use of ice for bivalve storage may not contribute to the loss of exoenzymatic expression capacity by V. parahaemolyticus. In accordance with these findings, Feller et al.2 demonstrated that the bacterial secretion of lipase, α-amylase, protease and β-lactamase may occur in temperatures ranging from −2 to 4°C.
The virulence factors described in this study - caseinase, gelatinase, lipase against Tween 80, phospholipase against egg yolk, DNase and amylase — were also found in vibrios isolated from marine environment. Stronger phospholipase and caseinase activities were detected in vibrios isolated from marine shrimp (Penaeus monodon) and associated with bacterial pathogenicity7. Zhang and Austin15 detected phospholipase, lipase, caseinase and gelatinase activity in vibrios isolates from diverse sources (shrimp, shark, fish, tank water). These isolates were able to cause the following symptoms in fish: severe necrosis and sloughing of mucosal intestine cells, focal congestion of the liver, hemosiderosis in the spleen, and hyaline degeneration of some kidney tubules with severe glomerular damage15.
Multiple enzymatic profiles were detected in 100% of the isolates (table 1), presenting the following dominance pattern in their enzymatic activities: DNase > amylase > gelatinase > lipase > phospholipase > caseinase. Similar results were observed by Medji et al.8, who detected concomitant enzymatic activity of DNase caseinase, amylase, lipase and lecithinase in marine vibrios isolated from fish.
In this study, the ability to hydrolyze elastin was the only virulence factor that was not detected. However, the production of elastolytic protease by other Vibrio species has already been described6.
Our results suggest that oysters (both fresh and frozen) can be vehicles of V. parahaemolyticus having the ability to express exoenzymes associated with bacterial pathogenesis. Thus, in case of human consumption, prior cooking is strongly recommended.
Conflicts of interestThe authors declare that they have no conflicts of interest.
To CNPq for financial support.