Entomopathogens help regulate natural insect populations, and their use in pest management is the subject of active research. Classical and molecular techniques are currently available to elucidate disease etiology and pathogenesis. Here, we performed an Azan trichrome stain for light microscopic visualization of pathogens in the larval tissues of two Neotropical lepidopterans.
Epinotia aporema larvae exhibiting symptoms of microsporidiosis were fortuitously detected in a rearing facility (IMyZA-INTA, Argentina), while Helicoverpa gelotopoeon specimens were experimentally infected with an Argentinean isolate of Helicoverpa armigera nucleopolyhedrovirus (HearNPV). Caterpillars were fixed in Duboscq-Brasil and paraffin embedded. Microtome sections (5μm-thick) were deparaffinized and stained2 for observation under a Nikon Eclipse 80i microscope equipped with a Nikon DS-U3 digital camera.
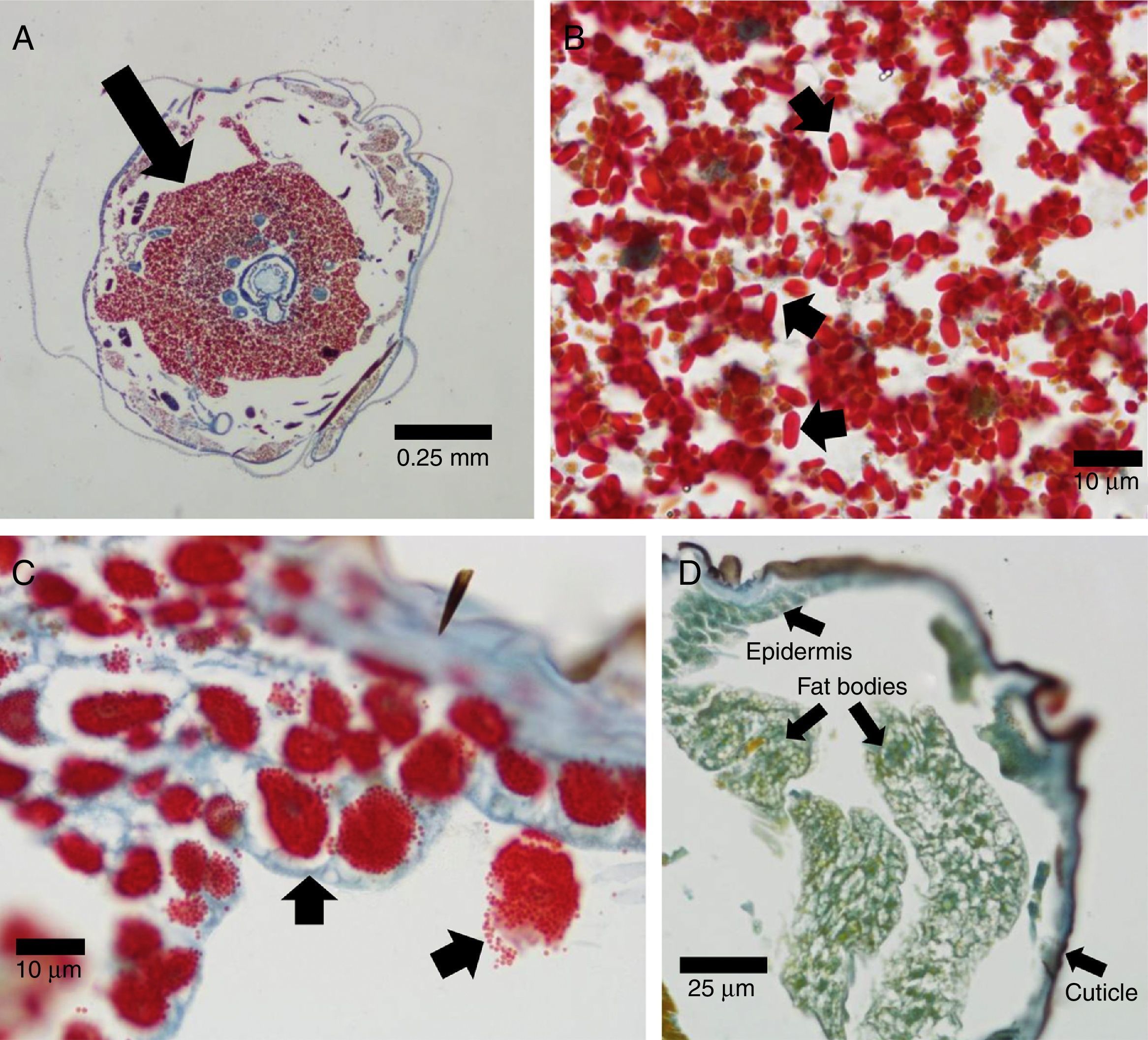
Diseased E. aporema samples revealed the occurrence of microsporidians occupying a large portion of the body cavity (Fig. 1A). Oval spores were stained bright red (Fig. 1B). No microsporidians were identified in the healthy controls (not shown). This confirmed the preliminary diagnosis and is, to our knowledge, the first report of microsporidia in E. aporema. Their moderate pathogenicity, the wide distribution across the insect body, the presence of infected pupae and adults, and the observation of only one type of spores, suggest that these microsporidia belong to the genus Nosema. Further molecular studies are needed to corroborate this issue. These microbial pathogens are widespread among Lepidoptera, causing a serious problem for insect mass rearing3. With regard to H. gelotopoeon, cytological examinations evidenced numerous polyhedral occlusion bodies, also displaying a bright red color, within the nuclei of HearNPV-infected cells (Fig. 1C). The epidermis, fat bodies and tracheal system were colonized, thus indicating the polyorganotropic nature of the virus isolate. No polyhedra were found in the negative controls (Fig. 1D). Our findings complement a previous electron microscopy work on this new baculovirus isolate1.
Azan staining technique applied to transversal sections of lepidopteran larvae. (A) Heavily infected E. aporema specimen showing proliferation of microsporidia (arrow). 4× objective lens. (B) Microsporidia spores (arrows). 100× objective lens. (C) Enlarged nuclei containing polyhedral occlusion bodies (arrows) in H. gelotopoeon-infected cells of epidermis and fat bodies. 100× objective lens. (D) H. gelotopoeon healthy control, where staining ranged from yellowish-green (with darker green nuclei) to blue, depending on the tissue. 40× objective lens. A lower magnification is presented in order to show that the characteristic staining of viral infection is absent across the different tissues.
Overall, the Azan staining technique applied to microtome sections proved to be a useful accessory tool to identify and localize different types of pathogens or cytopathic effects within lepidopteran larvae.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestsThe authors declare that they have no conflicts of interest.
This work was funded by CONICET through grant PIP N° 11420110100306. We are grateful to Dr. Fernando Delgado (Instituto de Patobiología – INTA) for providing access to light-microscopy equipment used to take photographs.